Abstract
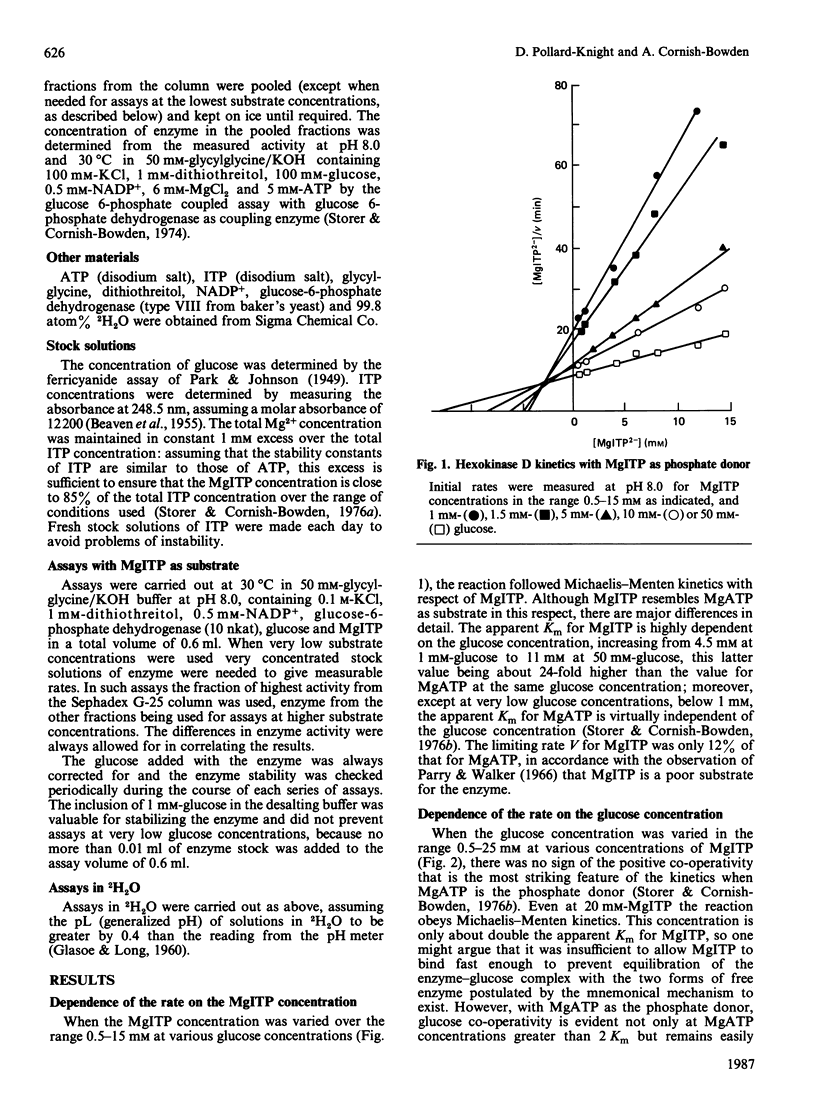
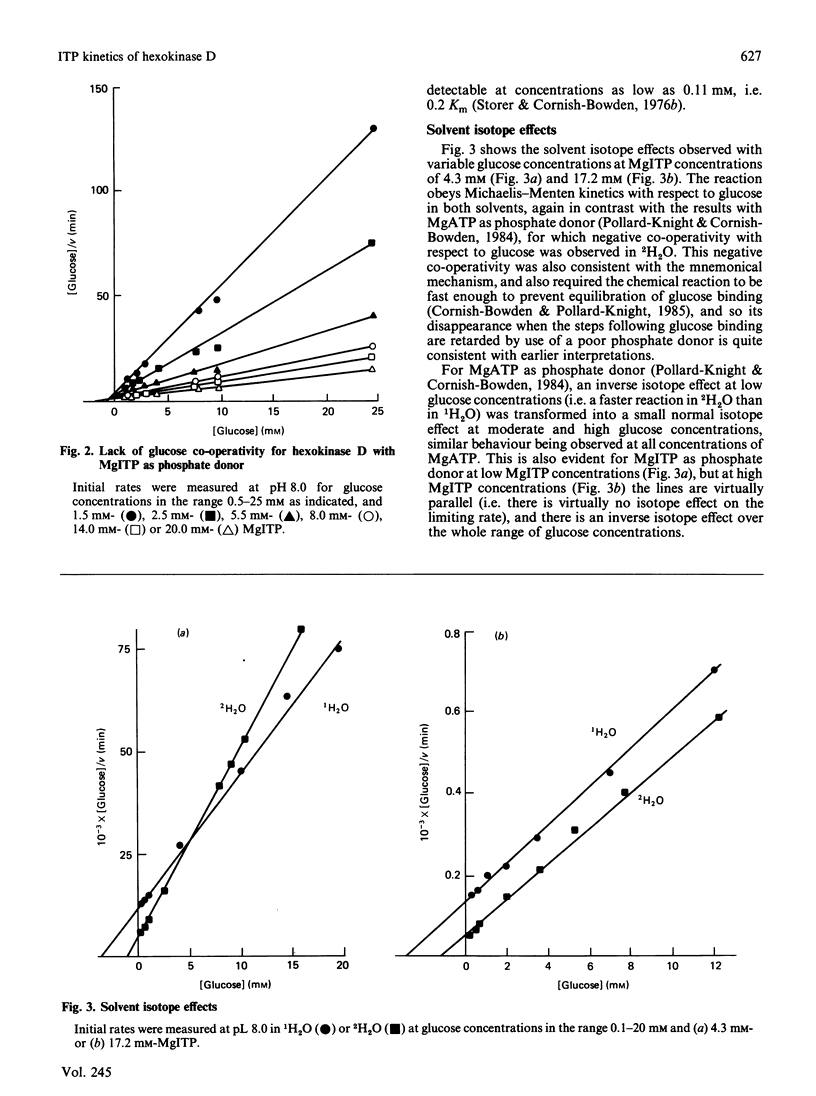
When ATP, the normal phosphate donor for hexokinase D ('glucokinase'), is replaced by ITP, the positive co-operativity with respect to glucose disappears. This may be rationalized in relation to kinetic models for hexokinase D co-operativity, which assume that with the normal substrates the chemical reaction and subsequent release of products occur so rapidly that binding of substrates cannot approach equilibrium and is therefore not constrained by the thermodynamic requirement that the Hill coefficient for substrate binding cannot exceed the number of binding sites. ITP is a much poorer substrate than ATP, however: its Km value at high glucose concentrations is 24 times the value for ATP, whereas the value of the limiting rate V is decreased about 8-fold. Consequently it is no longer possible for the ternary complex to be converted into products rapidly enough to generate kinetic co-operativity. The negative co-operativity with respect to glucose observed in 2H2O with ATP as phosphate donor also disappears when ITP is used instead of ATP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainslie G. R., Jr, Shill J. P., Neet K. E. Transients and cooperativity. A slow transition model for relating transients and cooperative kinetics of enzymes. J Biol Chem. 1972 Nov 10;247(21):7088–7096. [PubMed] [Google Scholar]
- Connolly B. A., Trayer I. P. Reaction of rat hepatic glucokinase with substrate-related and other alkylating agents. Eur J Biochem. 1979 Sep;99(2):299–308. doi: 10.1111/j.1432-1033.1979.tb13257.x. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Pollard-Knight D. Solvent isotope effects on the hexokinase D reaction: evidence for the mnemonical interpretation of the kinetic co-operativity. Arch Biol Med Exp (Santiago) 1985 Dec;18(3-4):293–300. [PubMed] [Google Scholar]
- Cornish-Bowden A., Storer A. C. Mechanistic origin of the sigmoidal rate behaviour of rat liver hexokinase D ('glucokinase'). Biochem J. 1986 Nov 15;240(1):293–296. doi: 10.1042/bj2400293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas M. L., Rabajille E., Niemeyer H. Fructose is a good substrate for rat liver 'glucokinase' (hexokinase D). Biochem J. 1984 Sep 1;222(2):363–370. doi: 10.1042/bj2220363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas M. L., Rabajille E., Niemeyer H. Suppression of kinetic cooperativity of hexokinase D (glucokinase) by competitive inhibitors. A slow transition model. Eur J Biochem. 1984 Nov 15;145(1):163–171. doi: 10.1111/j.1432-1033.1984.tb08536.x. [DOI] [PubMed] [Google Scholar]
- Ferdinand W. The interpretation of non-hyperbolic rate curves for two-substrate enzymes. A possible mechanism for phosphofructokinase. Biochem J. 1966 Jan;98(1):278–283. doi: 10.1042/bj0980278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- Hohnadel D. C., Cooper C. The effect of structural modifications of ATP on the yeast-hexokinase reaction. Eur J Biochem. 1972 Nov 21;31(1):180–185. doi: 10.1111/j.1432-1033.1972.tb02517.x. [DOI] [PubMed] [Google Scholar]
- Holroyde M. J., Allen M. B., Storer A. C., Warsy A. S., Chesher J. M., Trayer I. P., Cornish-Bowden A., Walker D. G. The purification in high yield and characterization of rat hepatic glucokinase. Biochem J. 1976 Feb 1;153(2):363–373. doi: 10.1042/bj1530363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Buc J., Navarro A., Ricard J. Regulatory behavior of monomeric enzymes. 2. A wheat-germ hexokinase as a mnemonical enzyme. Eur J Biochem. 1974 Nov 1;49(1):209–223. doi: 10.1111/j.1432-1033.1974.tb03826.x. [DOI] [PubMed] [Google Scholar]
- Niemeyer H., de la Luz Cárdenas M., Rabajille E., Ureta T., Clark-Turri L., Peñaranda J. Sigmoidal kinetics of glucokinase. Enzyme. 1975;20(6):321–333. doi: 10.1159/000458957. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Parry M. J., Walker D. G. Purification and properties of adenosine 5'-triphospae-D-glucose 6-phosphotransferase from rat liver. Biochem J. 1966 May;99(2):266–274. doi: 10.1042/bj0990266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson G. Mechanistic origin of the kinetic cooperativity of hexokinase type L1 from wheat germ. Eur J Biochem. 1986 Jan 2;154(1):167–170. doi: 10.1111/j.1432-1033.1986.tb09374.x. [DOI] [PubMed] [Google Scholar]
- Pettersson G. Mechanistic origin of the sigmoidal rate behaviour of glucokinase. Biochem J. 1986 Jan 15;233(2):347–350. doi: 10.1042/bj2330347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard-Knight D., Cornish-Bowden A. Mechanism of liver glucokinase. Mol Cell Biochem. 1982 Apr 30;44(2):71–80. doi: 10.1007/BF00226892. [DOI] [PubMed] [Google Scholar]
- Pollard-Knight D., Cornish-Bowden A. Solvent isotope effects on the glucokinase reaction. Negative co-operativity and a large inverse isotope effect in 2H2O. Eur J Biochem. 1984 May 15;141(1):157–163. doi: 10.1111/j.1432-1033.1984.tb08170.x. [DOI] [PubMed] [Google Scholar]
- Ricard J., Meunier J. C., Buc J. Regulatory behavior of monomeric enzymes. 1. The mnemonical enzyme concept. Eur J Biochem. 1974 Nov 1;49(1):195–208. doi: 10.1111/j.1432-1033.1974.tb03825.x. [DOI] [PubMed] [Google Scholar]
- SALAS J., SALAS M., VINUELA E., SOLS A. GLUCOKINASE OF RABBIT LIVER. J Biol Chem. 1965 Mar;240:1014–1018. [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Kinetic evidence for a 'mnemonical' mechanism for rat liver glucokinase. Biochem J. 1977 Jul 1;165(1):61–69. doi: 10.1042/bj1650061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Kinetics of rat liver glucokinase. Co-operative interactions with glucose at physiologically significant concentrations. Biochem J. 1976 Oct 1;159(1):7–14. doi: 10.1042/bj1590007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. The kinetics of coupled enzyme reactions. Applications to the assay of glucokinase, with glucose 6-phosphate dehydrogenase as coupling enzyme. Biochem J. 1974 Jul;141(1):205–209. doi: 10.1042/bj1410205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel J., Koshland D. E., Jr The effect of NAD+ on the catalytic efficiency of glyceraldehyde-3-phosphate dehydrogenase from rabbit muscle. Biochim Biophys Acta. 1970 Feb 11;198(2):183–191. doi: 10.1016/0005-2744(70)90050-1. [DOI] [PubMed] [Google Scholar]
- Tippett P. S., Neet K. E. An allosteric model for the inhibition of glucokinase by long chain acyl coenzyme A. J Biol Chem. 1982 Nov 10;257(21):12846–12852. [PubMed] [Google Scholar]


