Abstract
Background
The pathogenesis of chronic active Epstein–Barr virus (EBV) disease (CAEBV) is complex, involving infection, inflammation, and in some cases malignancy. EBV reactivates in some patients, who develop a chronic disease with infectious mononucleosis-like symptoms. On occasion, it might be confused with autoimmune hepatitis (AIH), based on the similar symptoms and elevated liver enzymes. We aimed to describe a similar case to remind reader of this disease.
Case Presentation
: A 14-year-old girl was diagnosed with AIH based on biopsy and biomarker findings 1 year prior to admission and initially presented with elevated liver enzymes, portal hypertension, and parenchymal liver disease. Despite steroid administration, her condition fluctuated, and she was admitted to the hospital several times. She underwent a renal biopsy because of massive ascites, hypoalbuminemia, and increased renal echogenicity. An evaluation for impaired liver function showed positivity for EBV IgM, IgG, and early antigen (EBEA) antibodies. A PCR-based assay showed 5265 copies/mL of EBV DNA in peripheral blood. Epstein–Barr encoding region (EBER) in situ hybridization revealed abundant positive cells. Immunohistochemistry for CD56 also showed abundant positive cells, and CAEBV was diagnosed on this basis. Chemotherapy, hematopoietic stem cell transplantation (HSCT), and liver transplantation were proposed but her family refused.
Conclusions
CAEBV should be considered when diagnosing AIH or in cases of treatment-refractory AIH.
Keywords: Chronic active Epstein–Barr virus infection, Teen age, Girl, Autoimmune hepatitis
Background
Epstein–Barr virus (EBV), a double-stranded DNA virus also known as human herpesvirus 4, is a member of the human herpes virus family. Because HBV establishes a latent infection in B cells, patients remain infected for life. EBV infections in children and young adults can lead to infectious mononucleosis, with symptoms of fever, sore throat, lymphadenopathy, hepatomegaly, splenomegaly, lymphocytosis, and liver dysfunction. EBV reactivates in some patients, who develop a disease that does not resolve and has a chronic course and infectious mononucleosis-like symptoms [1]. Kimura et al. [2]. proposed several subtypes of EBV-T/NK-lymphoproliferative disorder (LPD) in 2012 by analyzing 108 patients with hydroa vacciniforme-like lymphoproliferative disorder, severe mosquito bite allergy, EBV-hemophagocytic lymphohistiocytosis (HLH), or chronic active EBV disease (CAEBV). The last was also associated with systemic inflammation. The diagnosis of CAEBV is hampered by its rarity. Management is challenging because of delayed diagnosis and the lack of treatment guidelines. We present a rare case of a teenage girl with CAEBV that initially mimicked autoimmune hepatitis (AIH).
Case report
A 14-year-old girl was admitted to our hospital as a result of amenorrhea for 3 months, body-weight loss, epistaxis, and bilateral flexor itching when she was 12 years 11 months of age. There was no relevant family or psychosocial factor, and no history of genetic disorders. Physical examination revealed hepatosplenomegaly and petechiae on her wrists and legs. The laboratory data showed leukopenia (2400/µL), thrombocytopenia (147 × 103/µL), elevated liver parameters (aspartate transaminase 236 U/L, alanine aminotransferase 153 U/L, γ-glutamyltransferase 219 U/L, total bilirubin level 0.7 mg/dL, direct bilirubin 0.4 mg/dL, erythrocyte sedimentation rate 23 mm/h, and hypoalbuminemia 2.68 g/dL). Abdominal sonography disclosed marked hepatosplenomegaly and suspect liver cirrhosis with portal hypertension. A computed tomography (CT) angiogram revealed portal hypertension with parenchymal liver disease (Fig. 1) and bilateral pleural effusion with pericardial effusion. The results of hormone studies and a gynecologic ultrasound were normal. A common viral hepatitis study yielded no relevant findings. The ceruloplasmin level was in the normal range and there was no Kayser–Fleischer ring. The patient was positive for antinuclear and anti-smooth muscle antibodies and negative for anti-mitochondrion antibodies and showed elevated immunoglobulin G and A levels, indicative of AIH. A liver biopsy demonstrated moderate fatty changes (50%) with focal bridging fibrosis and marked pericentral fibrosis, compatible with non-alcoholic steatohepatitis. Prednisolone and ursodeoxycholic acid were prescribed because of suspicion of AIH and cholestasis. Proteinuria, fatigue, bilateral calf pain, and lower limb weakness were noted when the patient was 14 years of age. At that time, she was admitted for evaluation for systemic lupus erythematosus (SLE) but did not fulfill the criteria.
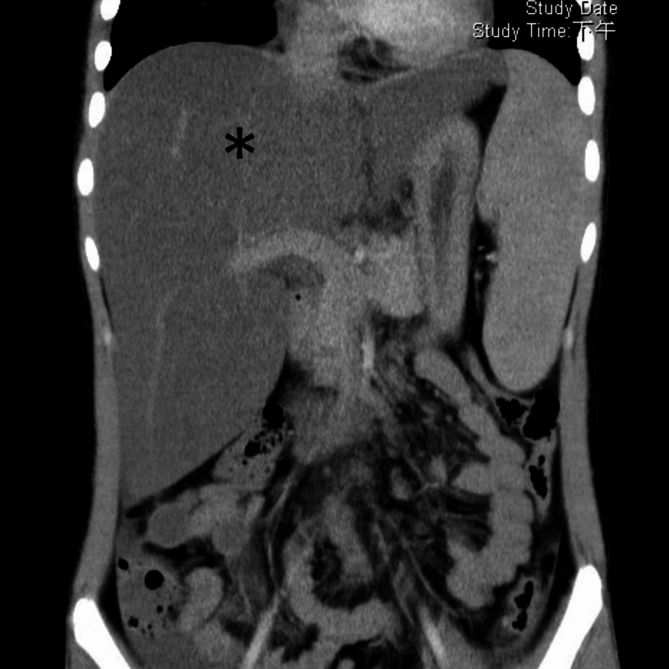
Fig. 1.
Computed tomography showed diffuse hypodense infiltration (asterisk) with marked enlargement of the liver, associated with splenomegaly, a prominent portal vein, peri-splenic/peri-gastric collaterals, and ascites, which correlated with portal hypertension and hepatic steatosis
About 1 year after her first hospitalization, the patient was admitted for sudden-onset involuntary movement of the extremities with recent tongue ulceration and non-blanchable, non-pruritic macules on both ankles and ascending to her thighs. She had experienced progressive hair loss in the prior few months. Electroencephalography revealed no relevant finding. Five months later, the patient suffered from bilateral lower leg swelling, chest pain, and dyspnea for several weeks. She had recently complained of vertigo, poor appetite, sleepiness, headache, and bilateral periocular edema. The evaluation for SLE was repeated; the patient’s C3, C4, and anti-dsDNA levels were within the normal ranges. Cardiac sonography revealed minimal pericardial effusion. Renal sonography demonstrated a hyperechoic change with enlargement of both kidneys, suggesting renal parenchyma disease with massive ascites. A renal biopsy showed moderate-to-marked tubulitis accompanied by marked mononuclear cell infiltration in the interstitium, suggesting tubulointerstitial nephritis. The patient did not meet the criteria for SLE.
The patient was subsequently readmitted because of hypoalbuminemia, dyspnea on exertion, and fever. A chest X-ray showed cardiomegaly with bilateral pulmonary edema, and cardiac sonography revealed adequate heart function with pericardial effusion of 9.8 mm. Her symptoms improved after an albumin infusion and furosemide administration. However, after discharge, she had intermittent fever and lower-limb edema with dyspnea on exertion. Based on her hyperbilirubinemia and elevated liver parameters and portal hypertension, hepatosplenomegaly, and moderate ascites on abdominal sonography, we performed a repeat liver biopsy. At this time, laboratory data showed leukopenia (2300/µL), neutrophil count 1580/µL, lymphocyte count 513/µL, hemoglobin 10.6 g/dL, platelet count 167 × 103/µL, total bilirubin 1.8 mg/dL, aspartate transaminase 154 U/L, alanine aminotransferase 86 U/L, γ-glutamyltransferase 251 U/L, ferritin 541.3 ng/mL, IgG 2550 mg/dL, IgA 915 mg/dL and IgM 79.4 mg/dL. The results indicated severe periportal or periseptal interface hepatitis (piecemeal necrosis), confluent necrosis, focal (spotty) lytic necrosis, apoptosis, focal inflammation, and portal inflammation. Panendoscopy to evaluate the portal hypertension showed esophageal varices, which were ligated.
Unfortunately, desaturation and hypotension were noted immediately after the procedure. A chest X-ray demonstrated pulmonary edema and cardiomegaly in progress. Cardiac sonography showed a moderate to large amount of pericardial effusion and a small aneurysm in the proximal right coronary artery. To prevent cardiac tamponade, the patient was transferred to the pediatric intensive care unit (PICU).
In the PICU, methylprednisolone pulse therapy was initiated for suspected vasculitis and autoimmune disease. We consulted a cardiovascular surgeon for pericardial drain placement. Propranolol, ursodeoxycholic acid, and silymarin were prescribed for the elevated liver parameters and portal hypertension, and colchicine and ibuprofen for suspected serositis. Further evaluation for impaired liver function showed that the patient was positive for EBV IgM, EBV IgG, and EBEA antibodies. A PCR-based assay showed 5265 copies/mL EBV DNA in peripheral blood. The diagnosis of CAEBV was confirmed by the detection of abundant positive cells by EBER in situ hybridization of initial and latest liver biopsies. Immunohistochemistry for CD56 also revealed abundant positive cells. EBV DNA PCR of previous blood and pericardial effusion samples yielded positive results. The EBV-related antibody titers were EBV VCA IgG 750 U/mL, EBV VCA IgM 99 U/mL, EBV EA 150 U/mL, and EBNA 600 U/mL, all via fluorescent antibody method. A peripheral blood smear showed EBV DNA-coding NK-cell predominance (cell sorting for NK and T cell, followed by sequencing), confirming the diagnosis of CAEBV with NK-LPD. Step 1 therapy was started at that time. Chemotherapy, hematopoietic stem-cell transplantation (HSCT), and liver transplantation were proposed because the disease was progressing, but the patient refused.
Characteristics, presentation, diagnosis, and treatment
Because of its rarity, the incidence of CAEBV is unknown. A Japanese study group estimated an annual incidence of 23.8 cases/year in Japan [3]. Notably, T- and NK-cell CAEBV are common in Native Americans and East Asians; B-cell CAEBV was found in 73% of the available tissues in one study in America [4].
CAEBV is considered to be a disease of childhood. The age of onset is younger in Asia than in the United States (mean 11.3 and 19.0 years, respectively) [4, 5]. Fever, hepatomegaly, splenomegaly, and hepatitis are the typical symptoms of CAEBV in Asia, whereas lymphadenopathy is the most common symptom in the United States [4, 5]. Hypogammaglobulinemia and pancytopenia have not been reported in Asian CAEBV cases but are present in > 40% of those in the United States [4, 5].
Several definitions and criteria for CAEBV have been suggested [2, 6, 7]. CAEBV is defined as sustained or recurrent IM-like symptoms for > 3 months, an increased EBV load in peripheral blood or tissue lesions, EBV infection of T or NK cells in peripheral blood or affected tissues, and the exclusion of an underlying immunodeficiency [6]. Early diagnosis of CAEBV is crucial to prevent life-threatening events like sepsis, HLH, systemic lymphoma, and organ failure.
Treatment of CAEBV is problematic because it is both an inflammatory disease and a neoplasm [8]. Immunosuppressive agents, interferon, intravenous immunoglobulins, antiviral therapy, and conventional chemotherapy have merely a transient or poor effect. Only allogeneic HSCT has a measurable effect on the survival rate (15-year survival rate of 60.6% compared to 25.7% without HSCT) [2], although there is a high risk of transplantation-related complications [1].
Discussion
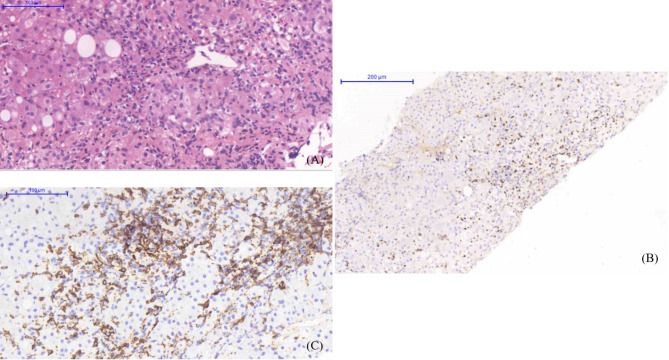
CAEBV seldom presents with only liver manifestations. In our case, the patient had an unusual initial presentation of portal hypertension and liver parenchymal disease, indicating that the infection and inflammation had begun long before her first admission. These symptoms prompted a liver biopsy. Although one of the diagnostic criteria, the role of pathological features in CAEBV is unclear [9]. In mild cases, liver pathology may show preserved hepatic structures with mild inflammatory infiltration of small and intermediate lymphocytes in the portal and sinusoidal areas, similar to viral hepatitis [9, 10]. In a case series in China, liver specimens showed moderate-to-severe edematous degeneration, mild-to-severe foamy steatosis, mild-to-moderate puncta, and focal necrosis; some had interface inflammation and fusion necrosis. Mild cholestasis was detected in hepatocytes and capillary bile ducts and some patients developed an inflammatory sarcoma [11]. To confirm the presence of EBV in proliferating lymphocytes, a histologic diagnosis should be made based on EBER in situ hybridization [9]. In our case, the immunophenotype was determined by double staining for EBER and CD56 (Fig. 2).
Fig. 2.
Hematoxylin-and-eosin staining of the liver biopsy showed fatty changes, bridging fibrosis, and marked pericentral fibrosis, compatible with non-alcoholic steatohepatitis (A). EBER in situ hybridization (B) and IHC for CD56 (C) revealed abundant positive cells in the portal tracts and hepatic lobules
Several case reports have linked EBV infection to AIH, although the mechanism is unclear [12]. Also, the link between CAEBV and AIH is unknown; indeed, our first impression of this case was AIH or another autoimmune disease with elevated liver parameters or liver involvement. In a similar case, a 22-year-old girl with fever, liver enzyme elevation, and pancytopenia was diagnosed with AIH 6 years prior, based on liver biopsy and laboratory data. Biomarkers of EBV were detected and CAEBV was indicated by in situ hybridization of a liver biopsy specimen. Ten months after diagnosis, the patient died from hemophagocytosis and liver failure [13]. Another case is that of a 50-year-old woman who had developed SLE at 4 years of age, who was diagnosed with AIH 5 years prior based on fever and a liver disorder. She presented with a fever and respiratory distress. Chest CT showed ground-glass opacities in both lung fields. She experienced black stools after tapering of the steroid dose; upper gastrointestinal endoscopy revealed multiple ulcers in the gastric antrum. EBER in situ hybridization of liver and gastric mucosal specimens showed EBER-positive lymphocytes. Malignant lymphoma developed despite treatment with cyclophosphamide, doxorubicin, vincristine, and prednisolone. The patient died from hepatic failure before HSCT [14]. In both cases, a clinical condition mimicking AIH delayed the definite diagnosis of CAEBV and thereby contributed to the fatal outcome. All of the above cases were in females, so the sex distribution of CAEBV subtypes with prominent liver involvement warrants further investigation. We initially believed that the patient had AIH. Because she had long suffered from the symptoms of CAEBV, the patient decided to receive palliative treatment only and was subsequently lost to follow-up and died at home.
The confusing manifestations of CAEBV hamper its diagnosis. We recommend a comprehensive workup for EBV infection before diagnosing AIH or treatment-refractory AIH, which may be a manifestation of CAEBV.
Acknowledgements
We thank the patient and her parents for providing the vital information, as well as our nursing staff and medical teams for her clinical care.
Abbreviations
- EBV
Epstein–Barr virus
- CAEBV
Chronic active Epstein–Barr virus disease
- AIH
Autoimmune hepatitis
- EBEA
EBV IgM, IgG, and early antigen
Author contributions
H-C. C., C-Y. W., S-F. H., and S-H. C. carried out diagnosis and management of the patients.W-Y. T. and C-C. C performed writing of the manuscript. C-C. C. performed critical evaluation and revision of the manuscript. All authors read and approved the final manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The ethic approval of this study has been approved by the Institutional Review Board of Chang Gung Memorial Hospital (No. 202300634B0), which allowed the collection of delinked medical records. All methods were performed in accordance with the ethical standards as laid down in the Declaration of Helsinki and its later amendments. A written informed consent was obtained from a parent and/or legal guardian.
Consent for publication
Written informed consent was obtained from the patient’s legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Competing interests
The authors declare no competing interests.
Consent statement
Patient consent was obtained from the individual and her legal guardian/next of kin.
Disclosure statement
No authors’ financial ties to products in the study or potential/perceived conflicts of interest.
Statement of financial support
No financial assistance was received in support of the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kimura H, Cohen JI. Chronic active Epstein–Barr Virus Disease. Front Immunol. 2017;8(1867). [DOI] [PMC free article] [PubMed]
- 2.Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, et al. EBV-associated T/NK–cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012;119(3):673–86. [DOI] [PubMed] [Google Scholar]
- 3.Arai A. Chronic active Epstein-Barr virus infection: a bi-faceted disease with inflammatory and neoplastic elements. Immunol Med. 2018;41(4):162–9. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JI, Jaffe ES, Dale JK, Pittaluga S, Heslop HE, Rooney CM, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. 2011;117(22):5835–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura H, Morishima T, Kanegane H, Ohga S, Hoshino Y, Maeda A, et al. Prognostic factors for chronic active Epstein-Barr virus infection. J Infect Dis. 2003;187(4):527–33. [DOI] [PubMed] [Google Scholar]
- 6.Okano M, Kawa K, Kimura H, Yachie A, Wakiguchi H, Maeda A, et al. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol. 2005;80(1):64–9. [DOI] [PubMed] [Google Scholar]
- 7.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arai A. Advances in the study of chronic active Epstein-Barr Virus infection: clinical features under the 2016 WHO classification and mechanisms of development. Front Pead. 2019;7(14). [DOI] [PMC free article] [PubMed]
- 9.Ondrejka SL, Hsi ED. Chronic active Epstein-Barr virus infection: a heterogeneous entity requiring a high index of suspicion for diagnosis. Int J Lab Hematol. 2020;42(Suppl 1):99–106. [DOI] [PubMed] [Google Scholar]
- 10.Ohshima K, Suzumiya J, Sugihara M, Nagafuchi S, Ohga S, Kikuchi M. Clinicopathological study of severe chronic active Epstein-Barr virus infection that developed in association with lymphoproliferative disorder and/or hemophagocytic syndrome. Pathol Int. 1998;48(12):934–43. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Wu H, Gu L, Wu X, Su M, Lin H, et al. Clinicopathologic findings of chronic active Epstein–Barr virus infection in adults: a single-center retrospective study in China. Clin Experimental Med. 2021;21(3):369–77. [DOI] [PubMed] [Google Scholar]
- 12.Rigopoulou EI, Smyk DS, Matthews CE, Billinis C, Burroughs AK, Lenzi M, et al. Epstein-Barr virus as a trigger of autoimmune liver diseases. Adv Virol. 2012;2012:987471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiba T, Goto S, Yokosuka O, Imazeki F, Tanaka M, Fukai K, et al. Fatal chronic active Epstein-Barr virus infection mimicking autoimmune hepatitis. Eur J Gastroenterol Hepatol. 2004;16(2):225–8. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita H, Shimizu A, Tsuchiya H, Takahashi Y, Kaneko H, Kano T, et al. Chronic active Epstein-Barr virus infection mimicking autoimmune hepatitis exacerbation in a patient with systemic lupus erythematosus. Lupus. 2014;23(8):833–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.