Abstract
Peripheral immune activation can have profound physiologic and behavioral effects. One mechanism through which immune activation may affect physiology and behavior is through actions on brainstem neuromodulatory systems, such as serotonergic systems. To test this hypothesis, in Experiment 1, adult male BALB/c mice were implanted with telemetric recording devices and then immunized with Mycobacterium vaccae NCTC 11659 (0.1 mg, s.c.; Days − 28, − 14; N = 36). On Day 1, mice received an acute challenge with M. vaccae (0.1 mg, s.c.) or borate-buffered saline vehicle. Core body temperature and locomotor activity recordings were conducted during a 36 h period beginning 24 h prior to challenge; 12 h following acute challenge, mice were either tested in a 6-min forced swim test, or served as home cage controls (n = 9 per group). In Experiment 2, the protocol was repeated, but with the aim of assessing c-Fos expression in brainstem serotonergic neurons, assessed 90 min following exposure to forced swim (N = 32; n = 8 per group). In Experiment 1, acute M. vaccae challenge in M. vaccae-immunized mice, relative to vehicle-challenged controls, decreased locomotor activity and core body temperature measured 3 h following challenge, as measured by continuous telemetric recordings, and decreased immobility in the forced swim test measured 12 h following challenge. In Experiment 2, acute M. vaccae challenge in M. vaccae-immunized mice decreased home cage locomotion, in alignment with findings in Experiment 1, as measured by video-based behavioral analysis, and, among mice exposed to the forced swim test, increased c-Fos expression in subsets of serotonergic neurons within the dorsal raphe nucleus (DR) measured 13.5 h following challenge. Together, these data are consistent with the hypothesis that acute peripheral immune activation with a heat-killed preparation of M. vaccae transiently induces mild hypothermia in association with suppression of locomotor activity, activates subsets of serotonergic neurons in the DR, and induces antidepressant-like behavioral responses.
Keywords: Actinobacteria, Antidepressant, Body temperature, Hypothermia, M. vaccae, Mycobacterium vaccae
Introduction
Chronic low-grade inflammation is emerging as a core feature of anxiety and affective disorders (Rohleder 2014; Miller and Raison 2016). Importantly, recent prospective human studies support the hypothesis that an enhanced stress-induced inflammatory immune activation plays a causal role in the development of these disorders (Kivimaki et al. 2014; Khandaker et al. 2014; Pervanidou et al. 2007). Further support comes from animal studies showing that acute stress-induced increases in interleukin (IL)-6, a pleiotropic cytokine released in association with inflammatory responses, are predictive for subsequent development of anxiety- and depressive-like symptoms (Hodes et al. 2014). Furthermore, lower numbers of immunoregulatory and anti-inflammatory regulatory T cells (Treg) have been found in depressed patients (Li et al. 2010) and antidepressants have been shown to reduce levels of IL-6 in humans (Hiles et al. 2012) and to increase the number of Treg in mice (Zhang et al. 2013).
Together, these findings support a close relationship between peripheral immune function and anxiety and affective disorders. Previously, we have shown that acute challenge with a heat-killed preparation of the saprophytic bacterium, Mycobacterium vaccae NCTC 11659, which induces long-term anti-inflammatory and immunoregulatory effects, has antidepressant-like behavioral effects in mice (Lowry et al. 2007). The antidepressant-like behavioral effects are associated with activation of a subset of serotonergic neurons in the interfascicular part of the dorsal raphe nucleus (DRI) (Lowry et al. 2007), a subset of serotonergic neurons that projects to forebrain structures controlling cognitive and affective function (Lowry et al. 2008), and increased serotonin and serotonin metabolism in the medial prefrontal cortex measured 12 h following acute challenge with M. vaccae in M. vaccae-preimmunized mice (Lowry et al. 2007).
Interestingly, peripheral administration of lipopolysaccharide (LPS), a component of the cell wall of gram-negative bacteria, also activates DRI serotonergic neurons (Hollis et al. 2006), and, at least under some conditions, can induce antidepressant-like behavioral responses in mice (Renault and Aubert 2006). The effects of LPS on DRI serotonergic neurons were prevented by prior administration of the cyclooxygenase inhibitor, indomethacin, suggesting that one or more components of an LPS-induced inflammatory cascade, as opposed to activation of toll-like receptor 4 (TLR4) alone, was responsible for activation of DRI serotonergic neurons. Although acute challenge with heat-killed M. vaccae, a gram-positive, LPS-negative bacterium, has been shown to increase pulmonary IL-1β, IL-6, and tumor necrosis factor (TNF) mRNA expression following intratracheal administration in M. vaccae-immunized mice, it has no effect on plasma concentrations of IL-1β, IL-6 or TNF, suggesting that it doesn’t induce a systemic immune activation commonly associated with fever (Lowry et al. 2007). Together, these data suggest that peripheral immune activation is sufficient to activate DRI serotonergic neurons, but that systemic inflammation and fever are not necessary.
To further characterize the effects of acute challenge with M. vaccae, we investigated the effects of acute challenge with heat-killed M. vaccae NCTC 11659 on c-Fos expression in brainstem serotonergic neurons, body temperature, locomotor activity, and antidepressant-like behavioral responses in M. vaccae-preimmunized adult male BALB/c mice. As previous studies have shown that i.p. administration of LPS increases c-Fos expression in serotonergic neurons in the DRI and dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray (DRVL/VLPAG), but not the dorsal raphe nucleus, caudal part (DRC), we assessed c-Fos expression in the DRI, DRVL/VLPAG, and DRC following acute challenge with M. vaccae in M. vaccae-immunized mice.
Materials and Methods
Animals and Housing
Adult male BALB/c mice, N = 36 for Experiment 1, N = 32 for Experiment 2 (Harlan Laboratories, Indianapolis, IN, USA; 21–25 g) were singly housed directly following arrival at the animal facility in polycarbonate cages with stainless steel wire bar lids (cage dimensions, 11.5” L × 7.25” W × 5” H, Alternative Design Manufacturing & Supply, Siloam Springs, AR, USA) with aspen chip bedding (Teklad Laboratory Grade Aspen Bedding, Cat No. 7093, Harlan, Madison, WI, USA). Mice were single housed in order to avoid disturbing mice when individuals were moved from long-term housing conditions to the experimental room. In addition, we have found that whole-body heating in rodents is sufficient to activate DRI serotonergic neurons (Hale et al. 2011); consequently, nesting of mice, which may increase cutaneous temperature, would potentially confound any activation of DRI serotonergic neurons following treatment with M. vaccae. Furthermore, single housing was required during telemetric recording, as technological limitations require that animals are single housed. Finally, it is worth noting that pronounced social hierarchy effects are observed on physiologic and behavioral parameters in mice when non-familiar, same-size male conspecifics are group housed (Langgartner et al. 2015). Single housing also has been suggested as an adequate and stress-free housing condition for male mice in a recent statement published by the Society of Laboratory Animal Science (Gesellschaft für Versuchstierkunde, GV SOLAS) (Busch et al. 2014), consistent with studies showing individually housed mice do not show increased stress responses (Arndt et al. 2009). All mice were maintained on a 12 h:12 h LD cycle (lights on at 06:00 a.m.) under standard environmental conditions (23 °C). Standard rodent chow (Cat. No. 2018, Teklad Global 18% Protein Rodent Diet, Harlan) and tap water were provided ad libitum. All studies were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, Eighth Edition (The National Academies Press, 2011) and the Institutional Animal Care and Use Committee (IACUC) at the University of Colorado Boulder approved all procedures. All possible efforts were made to minimize the number of animals used and their suffering.
Surgery
After 8–10 days following arrival at the animal facility, all mice received i.p. implantations of biotelemetry devices (PhysioTel® model transmitters, TA- F10/20; Data Sciences International (DSI), Minneapolis, MN, USA), using sterile technique, to allow continuous recording of core body temperature and locomotor activity. Mice were anesthetized with isoflurane during surgery and placed on a heating pad until fully awake following surgery. In Experiment 1, mice were allowed to recover between 18 and 26 days following surgery prior to other experimental procedures, whereby multiple time-matched cohorts were run separately for subsequent procedures. In Experiment 2, mice were allowed to recover for 9 days following surgery prior to other experimental procedures.
Experimental Designs
Experiment 1
Experiment 1 was a 2 [M. vaccae vs borate-buffered saline (BBS)] × 2 (forced swim test (FST) vs home cage control) design (N = 36). Sample sizes were vehicle/home cage control, n = 9; vehicle/FST, n = 9; M. vaccae/home cage control, n = 9; M. vaccae/FST, n = 9. For an illustration of the experimental design, see Fig. 1a.
Fig. 1.
Experimental timelines for a Experiment 1 and b Experiment 2. Day 1 is defined as the day of the challenge with M. vaccae or vehicle, s.c., whereby Day 1 is defined as the day immediately prior to the challenge. DR dorsal raphe nucleus, FST forced swim test, IHC immunohistochemistry, i.p intraperitoneal, LMA locomotor activity, s.c subcutaneous, Tb core body temperature
Experiment 2
Experiment 2 was a 2 (M. vaccae vs BBS) x 2 (FST vs home cage control) design (N = 32). Sample sizes were vehicle/home cage control, n = 8; vehicle/FST, n = 8; M. vaccae/home cage control, n = 8; M. vaccae/FST, n = 8. For an illustration of the experimental design, see Fig. 1b.
Immunization with a Heat-Killed Preparation of M. vaccae NCTC 11659
All mice were immunized, s.c. (between the scapulae), with 0.1 mg of a heat-killed preparation of whole cell heat-killed M. vaccae NCTC 11659 (10 mg/ml suspension of heat-killed M. vaccae, batch ENG 1, provided by Bio Elpida, Lyon, France, diluted to 1 mg/ml) in 100 µl sterile borate-buffered saline (BBS) on Days − 28 and − 14 at 07:00 p.m. These methods and doses were chosen in agreement with previous studies (Lowry et al. 2007; Reber et al. 2016; Fox et al. 2017). As the M. vaccae antigen is a particulate suspension, sufficient M. vaccae material for 1 day’s injections was freshly diluted immediately prior to each injection from stock material in a single siliconized vessel. Injections were performed using 21 gauge needles to ensure transmission of larger particulates in the suspension.
Habituation to Telemetric Recording Environment
In Experiment 1, on Day − 1 at 7:00 p.m., 24 h before challenge injections, all cages of singly housed mice were moved and placed on receivers for continuous telemetric recording (Physiotel® Receiver, Model No. RPC-1, Data Sciences International (DSI)), in order to habituate mice to this new spatial position within the room; the location of mice within the room (i.e., which telemetric receivers the mice were assigned to) was completely randomized. The testing room was not entered by any investigator during the 24 h habituation period preceding injections, or during the 12 h period following injections. As 8 telemetric recording setups were available, the experiment was conducted using four cohorts of eight mice, and one cohort of four mice.
In Experiment 2, mice were moved into the telemetric recording environment immediately prior to the onset of telemetric recording for each of three 30-min periods, (1) a baseline period from 30 to 0 min, immediately before acute challenge with M. vaccae or vehicle, (2) from 2 h to 2 h, 30 min following challenge, and (3) from 10 h to 10 h, 30 min following challenge. Core body temperature and LMA data were collected every minute throughout each recording period. Only the first 25 min of each recording was analyzed to avoid analysis of the time period when investigators were in the room.
Challenge with M. vaccae NCTC 11659 or Vehicle
On each challenge day at 07:00 p.m., i.e., 1 h after lights off, one-half of the mice were challenged with an acute s.c. administration of 0.1 mg heat-killed M. vaccae NCTC 11659 (identical to preimmunizations) in 100 µl sterile BBS, and the remaining one-half of mice received an s.c. administration of 100 µl sterile BBS vehicle as controls.
Telemetric Recording of Core Body Temperature (Tb) and Locomotor Activity (LMA)
In Experiment 1, core body temperature (Tb) and locomotor activity (LMA) data were collected continuously every minute for a 24 h baseline period before challenge injections (i.e., beginning at the start of the 24 h habituation time period) and a 12 h testing period following injection (activity polling rate 128 Hz; scheduled sampling at 1 min intervals for 5 s periods). Telemetric signals were processed and recorded using the software “Dataquest ART” (version 3.0, DSI). For analysis of the effects of acute challenge with M. vaccae, relative to vehicle-challenged controls, on Tb and LMA, we analyzed a baseline time period from 2 h prior to challenge until challenge with M. vaccae or vehicle (time 0) and a post-challenge time period from time 0 until 11 h after challenge, whereby each individual’s scores were averaged for successive 30-min interval blocks at the start time of each averaged 30-min interval.
In Experiment 2, cages containing individually housed mice were moved to receivers for telemetric recordings at specific time points on the day of the M. vaccae or vehicle challenge only, during three 30-min periods, (1) a baseline period from –30 min to 0 min, immediately before acute challenge with M. vaccae or vehicle, (2) from 2 h to 2 h, 30 min following challenge, and (3) from 10 h to 10 h, 30 min following challenge. Core body temperature and LMA data were collected every minute throughout each recording period. Only the first 25 min of each recording was analyzed to avoid analysis of the time period when investigators were in the room. Cages were returned to the home cage housing room between each intermittent recording period.
Home Cage Behavioral Analysis
In Experiment 2, home cage behavior was recorded using video cameras during three 30-min periods, (1) a baseline period from 30 to 0 min, immediately before acute challenge with M. vaccae or vehicle, (2) from 2 h to 2 h, 30 min following challenge, and 3) from 10 h to 10 h, 30 min following challenge, coinciding with the intermittent measurement of Tb and LMA in these mice. Only the first 25 min of each recording was analyzed to avoid analysis of the time period when investigators were in the room. Digital cameras (Sony Handycam, DCR-HC52, Sony Corporation of America, New York, NY, USA) using mini DV digital tape (Sony DVM-60PRL 60 min Premium Mini DV Tape, TapeStockOnline, Anaheim, CA, USA) were positioned over the mouse cages for a top-down view for video recording, whereby each camera captured four cages at a time. During these 25-min home cage behavior recording time periods, the food hopper along with water bottle were temporarily removed from each home cage and the cage was instead covered by a sheet of transparent perforated Plexiglas® to provide a full view of each cage floor. At least one food pellet was placed directly onto the floor of each home cage for the analysis of feeding-related behavior. All behavioral analysis was performed by an observer blind with respect to treatment groups using Noldus The Observer XT, ver. 10.0 software (Noldus, Leesburg, VA, USA). Behavior was analyzed by calculating the total duration of time each mouse was engaged in each behavior during the entire selected 25-min time period. The behaviors analyzed were (1) locomotion (ambulatory locomotion), (2) rearing, (3) burrowing (moving the bedding), (4) non-ambulatory motor activity (head movements, shifts in body position), (5) grooming, (6) sniffing (of the environment), (7) feeding- related behavior (time spent chewing the food pellet placed in the home cage), and (8) inactivity. Behaviors not analyzed include climbing and drinking-related behavior, due to the temporary removal of the wire bar lid and water bottle. Inter-rater reliability analysis was conducted based on behavioral scoring by two investigators blind to treatment group. The analysis was conducted for individual behaviors at the 10 h to 10 h 25 min time period, using Pearson correlation with all observations. Analysis revealed high inter-rater reliability for all behaviors: (1) locomotion (r = 0.991; p < 0.01); (2) burrowing (r = 0.988; p < 0.01); (3) grooming (r = 0.998; p < 0.01); (4) feeding (r = 0.997; p < 0.01); (5) rearing (r = 0.994; p < 0.01); (6) non-ambulatory motor activity (r = 0.945; p < 0.01); (7) sniffing (r = 0.984; p < 0.01); (8) inactive (r = 0.978; p < 0.01). Based on the high inter-rater reliability of behavioral scoring, only behavioral scores conducted by a single investigator blind to treatment group are presented.
Forced Swim Test
In Experiments 1 and 2, the forced swim test (FST) was conducted as described previously (Lowry et al. 2007) with the exception that mice were only exposed to a single swim session, 12 h following challenge, and mice in Experiment 2 were intermittently transferred to the telemetric recording room, as described above. Thus, 12 h following the challenge (i.e., 7:00 a.m.), one-half of the M. vaccae-challenged mice and one-half of the saline-challenged mice were exposed to the FST, while the remaining mice were kept as home cage controls. For the FST, each mouse was individually placed into a 5 L Pyrex® glass beaker (height, 21 cm; diameter, 16 cm at the top) filled with water, at a temperature of 23 °C, to a depth of 14 cm for 6 min; adjacent beakers were separated by dividers to obstruct the view between them. A digital camera (Sony Handycam, DCR-SR68, Sony Corporation of America, New York, NY, USA) capturing two beakers at a time were positioned over the glass beakers for top-down video recording of the sessions. After the FST was completed, mice were gently dried with a towel and placed back into their cages. Behaviors were scored for the final 4-min period of the test during which investigators were absent from the room. All behavioral analysis was performed by a single observer blind with respect to the treatment of individual mice using Noldus The Observer XT, ver. 10.0 software. Inter-rater reliability analysis was conducted based on behavioral scoring by two investigators blind to treatment group. The analysis was conducted for individual behaviors, using Pearson correlation with all observations. Analysis revealed high inter-rater reliability for all behaviors in both Experiment 1 and Experiment 2: Experiment 1, (1) climbing (r = 0.998; p < 0.01); (2) swimming (r = 0.998; p < 0.01); (3) immobility (r = 0.998; p < 0.01); Experiment 2, (1) climbing (r = 0.998; p < 0.01); 2) swimming (r = 0.998; p < 0.01); (3) immobility (r = 0.998; p < 0.01). Based on the high inter-rater reliability of behavioral scoring, only behavioral scores conducted by a single investigator blind to treatment group are presented. Behavior was scored as the duration of time each mouse spent (1) swimming (proactive coping behavior; slow-paced front & hind leg movements, no breaking of water surface), (2) climbing or struggling (proactive coping behavior; high-paced front leg paddling, breaking water surface, strong hind leg strokes), and (3) immobile (reactive, despair-like behavior; minimal hind leg and tail movements only to stay afloat and to keep nose above water surface, stiff body posture). Antidepressant-like behavioral effects are related to a reduction in immobility time (Borsini, 1995).
Tissue Collection
In Experiments 1 and 2, ninety min following the onset of the FST, all mice were anesthetized with sodium pentobarbital (90 mg/kg; Fatal-Plus®, Vortech Pharmaceutical, Dearborn, MI, USA). Mice were then transcardially perfused with ice cold 0.05 M phosphate-buffered saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB) and brains were post-fixed in the same fixative for 16-20 h and then placed in 30% sucrose in 0.1 M PB with 1% NaN3 at room temperature until sunk. Brains were then blocked into forebrain and hindbrain pieces with a cut in the coronal plane at the caudal border of the mammillary bodies (approximately − 3.40 mm bregma) using a mouse brain matrix (RBM-2000C, ASI Instruments, Warren, MI, USA), to ensure a consistent coronal plane of sectioning. Thereafter, brains were rapidly frozen in isopentane cooled in dry ice, individually wrapped in aluminum foil, and stored at − 80 °C until sectioned. Brains were sectioned using a Leica CM1900 cryostat (Leica Biosystems Inc., Buffalo Grove, IL, USA; six alternate sets of 30 µm sections) and stored in a cryoprotectant solution consisting of 30% ethylene glycol/20% glycerol/0.05 M sodium phosphate buffer, pH 7.4, stored at − 20 °C until immunohistochemical staining.
Immunohistochemistry
Technical problems prevented quantification of c-Fos expression in Experiment 1. Briefly, the immunostaining of the tissue was of insufficient quality to reliably discern serotonergic neurons from non-serotonergic neurons, or c-Fos-immunoreactive cells from c-Fos immunonegative cells. In Experiment 2, to examine activation of serotonergic cell groups, brains were immunostained for the protein product of the immediate-early gene, c-fos, as a marker of neuronal activation (1:3000 rabbit anti-c-Fos; Cat. No. PC-38, Calbiochem, EMD Chemicals, Gibbstown, NJ, USA), and tryptophan hydroxylase (Tph), as a marker of serotonergic neurons (1:12,000 sheep anti-Tph, Cat. No. T8575, Sigma-Aldrich, St. Louis, MO, USA) in brainstem sections encompassing the rostral brainstem raphe complex. Brain sections were washed twice in 0.05 M PBS, then rinsed in 1% H2O2 in 0.05 M PBS, followed by washing in 0.05 M PBS and preincubation in 0.05 M PBS with 0.3% Triton X-100 (Cat No. T9284, Sigma-Aldrich; 0.3% PBST). Sections were then incubated overnight at room temperature (RT) with rabbit anti-c-Fos polyclonal antiserum (1:3000) in 0.05 M PBS with 0.1% Triton X-100 (0.1% PBST). After 16 h, tissue was washed twice in 0.05 M PBS followed by incubation with a biotinylated donkey anti-rabbit secondary antibody (1:200, Cat. No. E0353, Dako, Carpinteria, CA, USA) in 0.05 M PBS for 90 min. Tissue was washed twice in 0.05 M PBS followed by incubation with an avidin–biotin–peroxidase complex (Elite ABC reagent, Cat. No. PK-6100, 1:200; Vector Laboratories, Burlingame, CA, USA) in 0.05 M PBS for 90 min. Tissue was then washed twice in 0.05 M PBS, and incubated in a peroxidase chromogen substrate (Vector SG; Vector Laboratories; Cat. No. SK4700; diluted as recommended by the vendor) in 0.05 M PBS for 20 min. After the chromogen reaction, tissue was immediately washed in 0.05 M PBS, then in 1% H2O2 in 0.05 M PBS, and twice in 0.05 M PBS. The tissue was then incubated with sheep anti-Tph antiserum (1:12,000) in 0.1% PBST overnight at RT. All subsequent steps were identical to those described earlier for the immunoperoxidase localization of c-Fos immunoreactivity, except for the secondary antibody and chromogen reaction steps; these used a rabbit anti-sheep secondary antibody (1:200, Cat. No. PK-6106, Vector Laboratories), and a peroxidase chromogen substrate solution consisting of 0.01% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Cat. No. D9015, Sigma-Aldrich) and 0.0015% H2O2 in 0.05 M PBS (25 min). Finally, sections were washed twice in 0.05 M PBS to stop the reaction. Following the immunohistochemical procedure, sections were rinsed briefly in distilled water with 0.15% gelatin (gelatin from porcine skin, type A, Cat. No. G-2500, Sigma-Aldrich) and were then mounted onto microscope slides (VWR VistaVision microscope slides, Cat No. 16004-368, VWR, West Chester, PA, USA), followed by being dehydrated through an alcohol series and cleared with xylene. The slides were then mounted with cover slips using Entellan mounting medium (Electron Microscopy Science, Hatfield, PA, USA). The color reaction of the c-Fos immunostaining was blue–black and localized to the cell nucleus while that of the Tph immunostaining was orange–brown and localized to the cytoplasm.
Cell Counting
Cell counting for the c-Fos/Tph immunohistochemistry was conducted using brightfield microscopy. The numbers of c-Fos-immunoreactive (c-Fos-ir) serotonergic neurons (i.e., c-Fos-ir/Tph-ir neurons), the numbers of c-Fos-ir, non-serotonergic cells (i.e. c-Fos-ir/Tph-immunonegative cells), and the total numbers of Tph-ir neurons (i.e., both c-Fos-ir/Tph-ir and c-Fos-immunonegative/Tph-ir neurons) were counted in different regions of the dorsal raphe nucleus (DR) covering five rostrocaudal levels including the rostral (–4.42 mm bregma), mid-rostrocaudal (–4.60 mm bregma), and caudal (–4.78 mm, –4.96 mm, –5.14 mm bregma) parts of the DR. The subdivisions of the DR that were selected for final analysis include the dorsal raphe nucleus, caudal part (DRC) (at –4.96 mm, –5.14 mm bregma), the dorsal raphe nucleus, interfascicular part (DRI) (at –4.78 mm, –4.96 mm, –5.14 mm bregma), and the dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray (DRVL/VLPAG) (at –4.60 mm, –4.78 mm bregma). These DR subdivisions were chosen to replicate previous findings, in that the DRI is activated by acute M. vaccae challenge, whereas the DRC is not (Lowry et al., 2007); both the DRI and DRVL/VLPAG (but not the DRC) are activated by lipopolysaccharide (LPS) (Hollis et al., 2006), and, therefore, the DRVL/VLPAG was chosen for comparison to these previous findings. The DRVL and adjacent VLPAG contain a distinct cluster of multipolar serotonergic neurons (Johnson et al., 2004), together defined as the “lateral wings” of the DR (Steinbusch 1981, 1984). As serotonergic neurons in the DRVL and VLPAG appear to be functionally related (Johnson et al. 2004), cells in these structures were counted as a single population, using the designation DRVL/VLPAG. Cells were counted in both the left and the right DRVL/VLPAG and the cell counts were summed to give a total number of cells in the DRVL/VLPAG. Cell counts were conducted using a 10× objective lens (100× total magnification) by an investigator blind to the assignment of treatment groups. Potential double immunostained (i.e., c-Fos-ir/Tph-ir) neurons were confirmed using a 40× objective (400× total magnification).
Statistical Analysis
Statistical outliers were identified using Grubbs’ test for single outliers (Grubbs, 1969) and were excluded from the dataset. In Experiments 1 and 2, for the Tb data, outliers were removed from individual 1-min interval sampling data prior to averaging of 30 min interval values; furthermore, for Tb data, presumed technical artifacts for individual 1-min interval sampling data were removed prior to removal of Grubbs’ single outliers if temperature was > 39 °C or < 34 °C, and if change from the previous data point was > 0.35 °C or if change from second to previous data point was > 0.7 °C. Likewise, in Experiments 1 and 2, for LMA data, Grubbs’ single outliers were removed from the averaged 30 min interval values. Core body temperature data, LMA data, home cage behavior data, and c-Fos-ir/Tph-ir, c-Fos-ir/Tph-immunonegative, and total Tph-ir cell count data (i.e., both c-Fos-ir/Tph-ir and c-Fos-immunonegative/Tph-ir neurons) were analyzed using linear mixed model analysis, a statistical approach ideal for repeated measures data that efficiently accounts for multiple measures derived from the same unit of observation (i.e., mouse), while also accommodating missing data and complex covariance structures (Duricki et al. 2016). The primary analyses were followed, when appropriate, with planned pairwise post hoc analyses using Fisher’s least significant difference (LSD) tests. Forced swim test behavior was analyzed using Student’s t-test. All statistical procedures were performed using IBM SPSS Statistics (Version 22.0.0.0 for Windows, SPSS Inc., Chicago, IL, USA).
Results
Experiment 1
Telemetry Data
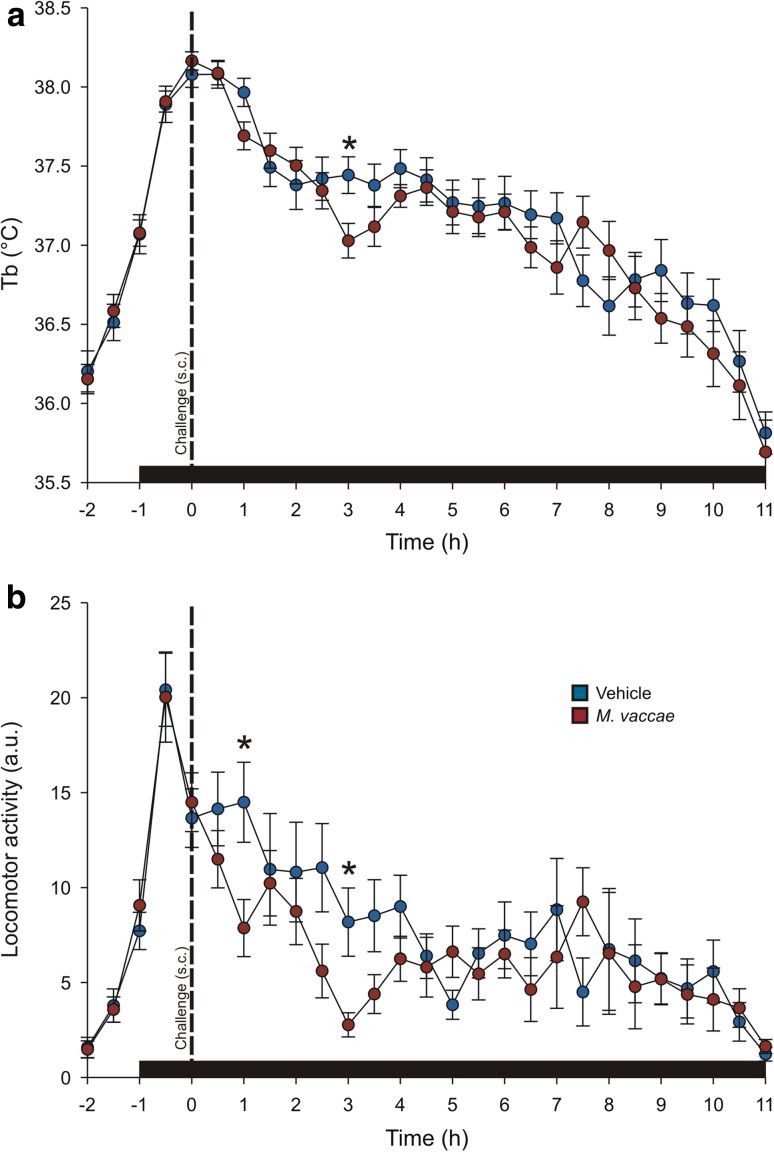
All telemetric recording data were analyzed using linear mixed model analysis using a first-order factor analytic (constant diagonal offset) covariance structure. In Experiment 1, using continuous telemetric recordings, linear mixed model analysis revealed an M. vaccae × time interaction on Tb (Fig. 2a; F (27,336.39) = 1.524, p < 0.05). Likewise, there was an M. vaccae × time interaction on LMA (Fig. 2b; F (27,111.25) = 2.12, p < 0.01). Mice that were challenged with M. vaccae, compared to vehicle-challenged controls, displayed a decrease in Tb at the 30-min interval beginning 3 h following injection (Fig. 2a). Mice that were challenged with M. vaccae, compared to vehicle-challenged controls, displayed a decrease in LMA at the 30-min intervals beginning 1 h and 3 h following injection (Fig. 2b). Neither Tb nor LMA was different between M. vaccae-and vehicle-immunized mice at any other time point studied.
Fig. 2.
Graphs illustrating effects of s.c. M. vaccae challenge on a core body temperature (Tb) and b locomotor activity (LMA) in Experiment 1. All mice were immunized (s.c) with heat-killed M. vaccae NCTC 11659 (0.1 mg/100 µl, s.c.) on Days − 28 and − 14 and were challenged (s.c.) with either M. vaccae (n = 17; 0.1 mg/100 µl s.c.) or borate-buffered saline (BBS) vehicle (n = 16) on Day 1. Core body temperature (Tb; °C) and locomotor activity (LMA; arbitrary units, a.u.) were monitored in the home cage environment; cages were placed on receivers 24 h before challenge injections for habituation. Telemetry data were sampled for a 5 s period at 128 Hz at 1 min intervals. The graphs display the mean ± S.E.M. for average values for each 30-min interval, illustrated at the start time of each averaged 30-min interval. Vertical dashed lines indicate the time at which the s.c. injections were given; horizontal black bars indicate the dark phase of the light/dark–light cycle. Statistical significance was assessed by linear mixed model analysis followed by Fisher’s least significant difference (LSD) tests at individual time points; *p < 0.05, relative to vehicle-treated controls at the same time point. a.u., arbitrary units; Tb, core body temperature
Forced Swim Test
In Experiment 1, using continuous telemetric recordings and undisturbed mice, M. vaccae-immunized mice that were challenged with M. vaccae, compared to M. vaccae-immunize mice challenged with vehicle, spent less time immobile in the FST (Fig. 3; t (16) = 2.34, p < 0.05). There were no differences between M. vaccae-challenged mice and vehicle-challenged mice in time spent climbing or swimming (Fig. 3).
Fig. 3.
Effects of s.c. M. vaccae challenge, in undisturbed M. vaccae-immunized mice, on forced swim test (FST) behavior in Experiment 1. All mice were immunized (s.c) with M. vaccae (0.1 mg/100 µl, s.c.) on Days − 28 and − 14 and were challenged (s.c.) with either M. vaccae (0.1 mg/100 µl, s.c.) or borate-buffered saline (BBS) vehicle on Day 1. Mice underwent a 6-min FST 12 h after challenge. Bars representing group means + SEM are shown for % time spent engaged in each behavior during the final 4 min of the test. Statistical significance was assessed by Student’s t-tests. *p < 0.05, compared to vehicle-challenged mice. Sample sizes: vehicle, n = 9; M. vaccae, n = 9
Experiment 2
Telemetry Data
In Experiment 2, using intermittent telemetric recordings during three 30-min periods, (1) a baseline period from –30 min to 0 min, immediately before acute challenge with M. vaccae or vehicle, (2) from 2 h to 2 h, 30 min following challenge, and (3) from 10 h to 10 h, 30 min following challenge, linear mixed model analysis revealed an effect of time on Tb (Fig. 4a; F (2,33.47) = 20.75, p < 0.01). In contrast, neither the main effect of M. vaccae (F (1,25.41) = 3.22, p = 0.09), nor the M. vaccae x time interaction (F (2,33.47) = 2.01, p = 0.15), was significant.
Fig. 4.
Graphs illustrating effects of s.c. M. vaccae challenge on a core body temperature (Tb) and b locomotor activity (LMA) in Experiment 2. All mice were preimmunized (s.c) with heat-killed M. vaccae NCTC 11659 (0.1 mg/100 µl, s.c.) on Days − 28 and − 14 and were challenged (s.c.) with either M. vaccae (0.1 mg/100 µl s.c.) or borate-buffered saline (BBS) vehicle on Day 1. Core body temperature (Tb; ºC) and locomotor activity (LMA; arbitrary units) were monitored in the home cage environment during three 30-min periods, (1) a baseline period from − 30 min to 0 min, immediately before acute challenge with M. vaccae or vehicle (HC1), (2) from 2 h to 2 h, 30 min following challenge (HC2), and (3) from 10 h to 10 h, 30 min following challenge (HC3). Statistical significance was assessed by linear mixed model analysis followed by Fisher’s least significant difference tests. Sample sizes vehicle, n = 15; M. vaccae, n = 16. a.u., arbitrary units; Tb, core body temperature
Likewise, linear mixed model analysis revealed an effect of time on LMA (Fig. 4b; F (2,44.94) = 56.03, p < 0.01). In contrast, neither the main effect of M. vaccae (F (1,26.82) = 2.23, p = 0.147), nor the M. vaccae x time interaction (F (2,44.94) = 1.34, p = 0.27), was significant.
Home Cage Behavior
In Experiment 2, linear mixed model analysis of specific home cage behaviors during the periods of intermittent telemetric recordings, using a first-order factor analytic (heterogeneous diagonal offset) covariance structure, revealed a main effect of M. vaccae on locomotion (Fig. 5a; F (1,26.62) = 5.085, p < 0.05). Mice challenged with M. vaccae had reduced locomotion, relative to vehicle-challenged controls, during the period from 2 h to 2 h, 25 min following challenge. Linear mixed model analysis also revealed an effect of time on rearing behavior (Fig. 5e; F (2,58.48) = 5.743, p < 0.01). In contrast, there were no effects of M. vaccae, time, or M. vaccae × time interactions, on other home cage behaviors, i.e. burrowing, non-ambulatory motor activity, grooming, sniffing, feeding-related behavior, and inactivity (Fig. 5b–d, f–h).
Fig. 5.
Effects of s.c. M. vaccae challenge, in M. vaccae-immunized mice, on home cage behavior in Experiment 2. All mice were immunized (s.c) with M. vaccae (0.1 mg/100 µl s.c.) on Days − 28 and − 14 and were challenged (s.c.) with either M. vaccae (0.1 mg/100 µl s.c.) or borate-buffered saline (BBS) vehicle on Day 1. Behaviors were monitored in the home cage environment during three 30-min periods, (1) a baseline period from − 30 to 0 min, immediately before acute challenge with M. vaccae or vehicle (HC1), 2) from 2 h to 2 h, 30 min following challenge (HC2), and 3) from 10 h to 10 h, 30 min following challenge (HC3). Bars representing group means + SEM are shown for time spent engaged in each behavior. Statistical significance was assessed by linear mixed model analysis followed by Fisher’s least significant difference tests. *p < 0.05, compared to M. vaccae-immunized, vehicle-challenged mice. + p < 0.01, main effect of time. Sample sizes: vehicle, n = 15; M. vaccae, n = 16
Forced Swim Test
In Experiment 2, using intermittent telemetric recordings, which resulted in intermittent disturbances of mice between challenge and behavioral testing, there were no effects of M. vaccae on climbing, swimming, or immobility behaviors when tested 12 h following challenge (Table 1).
Table 1.
Behavioral responses during the forced swim test in Experiment 2
| Vehiclea | M. vaccae | |
|---|---|---|
| Climbing | 26.79 ± 7.67 | 12.27 ± 2.47 |
| Swimming | 36.59 ± 13.36 | 47.02 ± 17.41 |
| Immobile | 180.26 ± 10.30 | 180.72 ± 16.22 |
aData represent mean ± SEM
c-Fos-ir/Tph-ir Cell Count Data in the Dorsal Raphe Nucleus
Linear mixed model analysis of numbers of c-Fos-ir/Tph-ir serotonergic neurons at different rostrocaudal levels of the DR, nested within subregions, revealed a main effect of M. vaccae challenge (Fig. 6; F (1,81.46) = 5.781, p < 0.05). An M. vaccae by FST interaction (Fig. 6; F (1,76.47) = 3.363, p = 0.071) and an M. vaccae × rostrocaudal level (subregion) interaction approached statistical significance (Fig. 6; F (2,63.14) = 2.794, p = < 0.069). Among mice exposed to the FST, mice that were challenged with M. vaccae, compared to vehicle-challenged controls, had a greater number of c-Fos-ir/Tph-ir serotonergic neurons in the DRI at –4.78 mm and –5.14 mm bregma and in the DRVL/VLPAG at –4.60 mm bregma. In contrast, there was no effect of M. vaccae on numbers of c-Fos-ir/Tph-ir serotonergic neurons in the DRC, which was chosen as a negative control.
Fig. 6.
Effects of s.c. M. vaccae challenge, in M. vaccae-immunized mice, on c-Fos expression in serotonergic neurons in subregions of the dorsal raphe nucleus (DR) in Experiment 2. a Photomicrographs illustrating subregions of the DR. b Cell count data for c-Fos-immunoreactive/tryptophan hydroxylase (Tph)-immunoreactive neurons (closed bars) and the total number of serotonergic neurons sampled (i.e. c-Fos-immunoreactive/Tph-immunoreactive neurons and c-Fos-immunonegative/Tph-immunoreactive neurons; open bars) in selected subdivisions of the DR, measured 13.5 h following immunization with either vehicle or M. vaccae, and 1.5 h following exposure to home cage control conditions or a 6-min forced swim test. All mice were immunized (s.c.) with M. vaccae (0.1 mg/100 µl, s.c.) on Days − 28 and − 14 and were challenged (s.c.) with either M. vaccae (0.1 mg/100 µl, s.c.) or borate-buffered saline (BBS) vehicle on Day 1. Mean cell counts + S.E.M. are shown. Statistical significance was assessed by linear mixed model analysis followed, when appropriate, by Fisher’s least significant difference tests; *p < 0.05, compared to vehicle-challenged mice within the same forced swim test condition. Sample sizes: vehicle/home cage, n = 7; vehicle/forced swim test (FST), n = 8; M. vaccae/home cage, n = 8; M. vaccae/FST), n = 8. Abbreviations: Aq, cerebral aqueduct; CLi, caudal linear nucleus; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; ir, immunoreactive; mlf, medial longitudinal fasciculus; M.v., M. vaccae; Tph, tryptophan hydroxylase; Veh, borate-buffered saline vehicle. Rostrocaudal coordinates are indicated in mm with reference to bregma. Scale bar = 200 µm
c-Fos-ir/Tph-Immunonegative Cell Count Data in the Dorsal Raphe Nucleus
Linear mixed model analysis of numbers of c-Fos-ir/Tph-immunonegative non-serotonergic cells within different rostrocaudal levels of the DR, nested within subregions, revealed a main effect of FST exposure (Fig. 7; F (1,55.23) = 5.720, p < 0.05), and a FST exposure × rostrocaudal level interaction (F (2,36.97) = 3.425, p < 0.05). A main effect of M. vaccae (F (1,55.23) = 2.699, p = 0.11) and an M. vaccae by FST interaction (F (1,52.848) = 2.204, p = 0.14) did not reach statistical significance.
Fig. 7.
Effects of s.c. M. vaccae challenge, in M. vaccae-immunized mice, on c-Fos expression in non-serotonergic cells in subregions of the dorsal raphe nucleus (DR) in Experiment 2. a Photomicrographs illustrating subregions of the DR. b Cell count data for c-Fos-immunoreactive/tryptophan hydroxylase (Tph)-immunonegative neurons in selected subdivisions of the DR, measured 13.5 h following immunization with either vehicle or M. vaccae, and 1.5 h following exposure to home cage control conditions or a 6-min forced swim test (FST). All mice were immunized (s.c.) with M. vaccae (0.1 mg/100 µl, s.c.) on Days − 28 and − 14 and were challenged (s.c.) with either M. vaccae (0.1 mg/100 µl, s.c.) or borate-buffered saline (BBS) vehicle on Day 1. Mean cell counts + S.E.M. are shown. Statistical significance was assessed by linear mixed model analysis followed, when appropriate, by Fisher’s least significant difference tests; +p < 0.05, compared to home cage controls within the same M. vaccae challenge condition. Sample sizes: vehicle/home cage, n = 7; vehicle/FST, n = 8; M. vaccae/home cage, n = 8; M. vaccae/FST, n = 8. Aq, cerebral aqueduct; CLi, caudal linear nucleus; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray (Note: serotonergic neurons in the DRVL and VLPAG appear to be functionally related (Johnson et al. 2004) and were counted as a single population); ir, immunoreactive; mlf, medial longitudinal fasciculus; M.v., M. vaccae; Tph, tryptophan hydroxylase; Veh, borate-buffered saline vehicle. Rostrocaudal coordinates are indicated in mm with reference to bregma. Scale bar = 200 µm
Among mice challenged with M. vaccae, mice that were exposed to the FST, compared to home cage controls, had a greater number of c-Fos-ir/Tph-immunonegative non-serotonergic cells in the DRI and DRC at –5.14 mm bregma.
Total Tph-ir Serotonergic Neurons
Linear mixed model analysis of the total number of serotonergic neurons sampled (i.e., c-Fos-ir/Tph-ir and c-Fos-immunonegative/Tph-ir cells) revealed a FST × rostrocaudal level interaction (Fig. 6; F (2,55.63) = 5.151, p < 0.01). However, post hoc pairwise comparisons detected no differences between mice exposed to the FST and home cage controls.
Discussion
Acute challenge with a heat-killed preparation of M. vaccae, in M. vaccae-immunized mice, transiently decreased core body temperature and locomotor activity 3 h following challenge, as measured by telemetric recordings, and decreased ambulatory locomotion assessed 2 h following challenge, as measured by manual scoring of home cage behavior. These effects were associated with a selective activation of serotonergic neurons in the DRI and in the lateral wings of the DR (DRVL/VLPAG), and, in mice that were left undisturbed between challenge with M. vaccae and behavioral testing, antidepressant-like behavioral responses, as measured in the FST. Together, these data suggest that an acute peripheral immune activation with a heat-killed preparation of M. vaccae activates DRI and DRVL/VLPAG serotonergic neurons and induces antidepressant-like behavioral responses.
Acute challenge with a heat-killed preparation of M. vaccae, in M. vaccae-immunized mice, transiently decreased core body temperature 3 h following challenge. A typical response to activation of the immune system in mice, particularly following administration of Gram-negative bacteria containing lipopolysaccharide (LPS), or LPS itself, is a pronounced hyperthermia. Indeed, in carefully controlled studies, mice maintained in a thermoneutral ambient temperature respond to LPS with fever in a dose-dependent fashion (Rudaya et al. 2005). However, thermoregulatory responses to LPS in a cool environment, as used in our study, is different, and consists of an early hyperthermia response (absent in our study), followed by a late hypothermia (beginning ~ 3 h post-injection, coinciding with the onset of hypothermia in our study) (Rudaya et al. 2005). Other studies also have documented a delayed (> 120 min) hypothermia response to LPS in mice that may last up to 48 h (Nautiyal et al. 2009; Oka et al. 2003; Kozak et al. 1998; Blanqué et al. 1996; Ochalski et al. 1993; Wardlaw et al. 1971). This delayed response appears to be dependent on ongoing inflammation as it is sensitive to the synthetic immunosuppressive glucocorticoid, dexamethasone, as well as inhibitors of eicosanoid synthesis (Zhang et al. 2003; Ochalski et al. 1993). Furthermore, this delayed hypothermia response to LPS is absent in mast cell-deficient mice, suggesting that mast cells mediate the delayed hypothermia response following LPS-induced immune activation (Nautiyal et al. 2009). Of further interest, bacterial cell wall components including peptidoglycan (PGN) from the Gram-positive bacterium, Staphylococcus aureus, LPS from Escherichia coli, and lipoarabinomannan (LAM) from Mycobacterium smegmatis induce synthesis and release of leukotrienes from mast cells, while other studies implicate leukotrienes in LPS-induced hypothermia in mice (Paul et al. 1999). Thus, in mice, following injection of M. vaccae at a subneutral ambient temperature (23 °C in the current study), the response is similar to the response to LPS, but without the early hyperthermia response, which is dependent on direct activation of toll-like receptor 4 (TLR4) (Hoshino et al. 1999; Steiner et al. 2006). M. vaccae is a Gram-positive organism and, therefore, does not express LPS, does not increase TLR4 signaling (Le Bert et al. 2011), and does not induce an early TLR4-dependent hyperthermia. Previous studies have confirmed different metabolic responses in mice following infection with Gram-negative and Gram-positive bacterial strains (Hoerr et al. 2012), and that Gram-negative strains induce a stronger inflammatory response, relative to Gram-positive strains, in humans (Abe et al. 2010). M. vaccae does induce a delayed inflammatory response (measured 12 h after injection), as we have described in previous studies (Lowry et al. 2007), and signals in part through activation of TLR2 (Le Bert et al. 2011). Determination of mechanisms underlying M. vaccae-induced hypothermia 3–4 h following injection will require further study. Although mechanisms underlying the antidepressant-like behavioral effects of M. vaccae, measured 12 h following injection, are not known, treatment with some inflammatory agents, such as interferon-alpha, and even LPS, have been found to induce antidepressant-like behavioral responses when given peripherally in mice (Wang et al. 2009; Renault and Aubert 2006); determination of mechanisms underlying the antidepressant-like behavioral effects of M. vaccae will require further study.
Among mice exposed to the FST, acute challenge with M. vaccae, in M. vaccae-immunized mice, increased c-Fos expression in DRI serotonergic neurons. Mice were euthanized in Experiments 1 and 2 90 min following the onset of the FST; as the FST is a major stressor by itself, it may have affected the excitability of 5-HT neurons. Indeed, previous studies in adult male Sprague–Dawley rats found that exposure to forced swimming for 15 min at 25 °C increased c-Fos expression in the DRI (identified as the caudal ventral region of the dorsal raphe nucleus in that study) (Commons 2008). In addition, we have documented similar findings in adult male Wistar rats, exposed to forced swimming for 15 min at 25 °C (Kelly et al., 2011). Consistent with the present study, a 5-min forced swim at 25 °C in adult male Wistar rats was not sufficient, by itself, to increase c-Fos expression in DRI serotonergic neurons (Drugan et al. 2013). Nevertheless, the 6-min forced swim at 23 °C, although it did not increase c-Fos expression by itself, may have altered the excitability of DRI serotonergic neurons, leading to M. vaccae-induced increases in c-Fos expression in serotonergic neurons, but only in mice that were exposed to forced swimming. Both acute challenge with M. vaccae, in M. vaccae-immunized mice, and acute challenge with LPS, activate DRI serotonergic neurons (Hollis et al., 2006;Lowry et al., 2007). The effects of LPS on DRI serotonergic neurons can be prevented by prior treatment with the anti-inflammatory drug, indomethacin (Hollis et al. 2006), suggesting that inflammatory mediators may play an important role. Meanwhile, whole-body heating and exposure to cold swimming have been shown to activate DRI serotonergic neurons in rats, suggesting that different peripheral stimuli (immune signals, thermal signals) converge on DRI serotonergic neurons (Hale et al. 2011; Kelly et al. 2011), possibly through activation of afferent spinoparabrachial or spinothalamic pathways (Hale and Lowry 2011; Hale et al. 2013; Raison et al. 2015). Although c-Fos induction has been widely used for neuronal activity mapping, it is best described as a response that involves both transcriptional changes and alterations in intra- and intercellular signal transduction (Morgan and Curran 1995). Verification that M. vaccae treatment in fact increases neuronal firing of DRI serotonergic neurons will require in vivo electrophysiological techniques.
Acute challenge with a heat-killed preparation of M. vaccae in Experiment 1, in M. vaccae-immunized mice that were subsequently left undisturbed in their home cages, induced antidepressant-like behavioral effects, as measured by decreases in immobility. This effect was not due to an overall increase in behavioral activity, as multiple measures in the home cage environment indicated that M. vaccae challenge decreased locomotor activity 1–3 h following challenge, while no effects of M. vaccae challenge on home cage behaviors were observed after the 3 h time point. Although M. vaccae challenge decreased immobility in the FST, it had no effect on either climbing or swimming behavior alone. These data are in line with previous findings using M. vaccae (Lowry et al. 2007). However, acute challenge with a heat-killed preparation of M. vaccae in Experiment 2, in M. vaccae-immunized mice that were repeatedly transferred from the housing room to an experimental room fitted with biotelemetry equipment, had no antidepressant-like behavioral effects. Each cage transfer was associated with a transient stress-induced hyperthermia (data not shown), which may have interfered with subsequent antidepressant-like behavioral responses.
Although previous studies have shown that challenge with a heat-killed preparation of M. vaccae, in M. vaccae-immunized mice, activates DRI serotonergic neurons, this study expands that finding to show that M. vaccae activates both DRI serotonergic neurons and DRVL/VLPAG serotonergic neurons. Again, this pattern of activation resembles the pattern observed previously using i.p. administration of LPS, consisting of selective activation of the DRI and DRVL/VLPAG serotonergic neurons, but not DRC serotonergic neurons (Hollis et al. 2006). In contrast, whole-body heating activates DRC serotonergic neurons in rats, suggesting that whole-body heating, either through activation of afferent spinoparabrachial or spinothalamic pathways (Hale and Lowry 2011; Hale et al. 2013; Raison et al. 2015), or through direct actions on thermosensitive serotonergic neurons (Cronin and Baker, 1977), impacts a more diverse population of DR serotonergic neurons, relative to peripheral immune signals. This could happen if peripheral immune signals, i.e., though activation of cytokine receptors on peripheral sensory fibers (Goehler et al. 1997), activate a unique subset of afferent sensory fibers, relative to thermal signals. The mechanism through which peripheral immune activation with M. vaccae and LPS selectively activates DRI and DRVL/VLPAG serotonergic neurons is not known, but may involve activation of spinoparabrachial pathways, leading to activation of the lateral parabrachial nucleus, which gives rise to glutamatergic projections to the DR (for review, see Raison et al. 2015).
Serotonergic neurons within the DRI in turn project to forebrain limbic structures that are dysregulated in patients with major depressive disorder (for review, see Raison et al. 2015). Data from the present study suggest that immune signaling molecules, or hypothermia per se, may be important for signaling peripheral immune signals to serotonergic neurons projecting to forebrain circuits involved in control of cognitive and affective function. Specific identification of mechanisms involved remains to be determined.
We did not detect effects of exposure to the FST on c-Fos expression in serotonergic neurons, when assessed 90 min following the onset of the test. This is in contrast to our previous studies in rats, showing that forced swimming resulted in moderate activation of serotonergic neurons in multiple subregions of the DR (Kelly et al. 2011). However, there were multiple methodological differences between the two studies. For example, the previous study used different water temperatures (19, 25, and 35 °C), longer duration swim (15 min instead of 6 min), and a longer post-swim interval (2 h instead of 90 min), in addition to the species differences. The lack of c-Fos expression in serotonergic neurons in the present study is more in line with a previous study by Commons (Commons 2008), in which a 15-min swim at 25 °C had no effect on c-Fos expression in the majority of subregions of the DR (with the exception of modest, but statistically significant, increases in the DRC and DRI) in rats. Of interest, however, prior treatment with the 5-HT1A receptor antagonist, WAY-100635, was permissive for swim stress-induced increases in c-Fos expression in serotonergic neurons in the rostral dorsal and ventral DR, DRVL/VLPAG, and median raphe nucleus, suggesting that tonic activation of inhibitory 5-HT1A autoreceptors in these regions contributes to control of serotonergic neuronal firing rates during forced swimming, and thus attenuates or prevents increases in c-Fos expression in these subregions.
Acute challenge with a heat-killed preparation of M. vaccae, in M. vaccae-immunized mice, decreased core body temperature and locomotor activity, as measured using telemetric recordings 3 h following challenge, and also by direct observation of behaviors in the home cage 2 h following M. vaccae challenge. Although effects of M. vaccae on core body temperature and locomotor activity occurred within a similar time frame, these responses may involve distinct mechanisms. For example, previous studies have shown that indomethacin inhibits the febrile response to LPS, but has no effect on LPS-induced reductions in locomotor activity (Kozak et al. 1994). In contrast, LPS-induced reductions in locomotor activity can be attenuated by minocycline, a microglia inhibitor that suppresses brain indoleamine 2,3-dioxygenase (IDO) activity, and can be prevented by treatment with 1-methyltryptophan, an IDO competitive inhibitor (O’Connor et al. 2009a, b, c; Wang et al., 2010). These data raise the interesting possibility that acute challenge with M. vaccae, in M. vaccae-immunized mice, may affect brain microglia function and IDO activity. Consistent with this hypothesis, previous studies in mice have shown that depressive-like behavior following administration of the mycobacterium, Bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is dependent on induction of IDO, and that IDO-deficient mice, although they continued to respond to proinflammatory cytokines, failed to respond to BCG (O’Connor et al. 2009b).
Conclusions
These data demonstrate that acute challenge with a heat-killed preparation of the saprophytic, immunoregulatory bacterium, M. vaccae, in M. vaccae-immunized mice, activates DRI and DRVL/VLPAG serotonergic neurons in association with transient hypothermia, decreased locomotor activity, and antidepressant-like behavioral responses. These findings support a role for DRI and DRVL/VLPAG serotonergic neurons, in particular, regarding antidepressant-like behavioral responses. Future studies will be required to determine the mechanisms underlying this selective activation of brainstem serotonergic systems by peripheral immune activation and the roles of these serotonergic systems in antidepressant-like behavioral responses.
Acknowledgements
This work is dedicated to Dr. Richard Kvetnansky and his tireless dedication to understanding the neurochemical basis of physiologic and behavioral responses to stress.
Abbreviations
- BBS
Borate-buffered saline
- DR
Dorsal raphe nucleus
- DRC
Dorsal raphe nucleus, caudal part
- DRI
Dorsal raphe nucleus, interfascicular part
- DRVL/VLPAG
Dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray
- FST
Forced swim test
- LMA
Locomotor activity
- LPS
Lipopolysaccharide
- PB
Phosphate buffer
- PBS
Phosphate-buffered saline
- RT
Room temperature
- Tb
Core body temperature
- Tph
Tryptophan hydroxylase
Author Contributions
PHS and CAL designed research; PHS, MWH, JLL, NCD, JMK, OAR, and JDH performed research; PHS, JDH, DMK, and CAL analyzed data; and PHS and CAL wrote the paper.
Funding
This material is based upon work supported by the National Science Foundation under Grant No. 0845550. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. Dr. Christopher A. Lowry is supported by the Department of the Navy, Office of Naval Research Multidisciplinary University Research Initiative (MURI) Award (Grant Number N00014-15-1-2809), Department of Veterans Affairs Office of Research and Development (VA-ORD) RR&D Small Projects in Rehabilitation Research (SPiRE) (I21) (Grant Number 1 I21 RX002232-01), Colorado Clinical & Translational Sciences Institute (CCTSI) Center for Neuroscience (Grant Number CNSTT-15-145), the Colorado Department of Public Health and Environment (CDPHE; Grant Number DCEED-3510), and the Alfred P. Sloan Foundation (Grant Numbers G-2015-14165, G-2016-7077).
Compliance with Ethical Standards
Conflict of interest
Christopher A. Lowry serves on the Scientific Advisory Board of Immodulon Therapeutics Ltd. The remaining authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All studies were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, Eighth Edition (The National Academies Press, 2011), and the Institutional Animal Care and Use Committee at the University of Colorado Boulder approved all procedures. All possible efforts were made to minimize the number of animals used and their suffering.
References
- Abe R, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Tateishi Y, Shinozaki K, Hirasawa H (2010) Gram-negative bacteremia induces greater magnitude of inflammatory response than Gram-positive bacteremia. Crit Care 14:R27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt SS, Laarakker MC, van Lith HA, van der Staay FJ, Gieling E, Salomons AR, van’t Klooster J, Ohl F (2009) Individual housing of mice—impact on behaviour and stress responses. Physiol Behav 97:385–393 [DOI] [PubMed] [Google Scholar]
- Blanqué R, Meakin C, Millet S, Gardner CR (1996) Hypothermia as an indicator of the acute effects of lipopolysaccharides: comparison with serum levels of IL1β, IL6, and TNFα. Gen Pharmacol 27:973–977 [DOI] [PubMed] [Google Scholar]
- Borsini F (1995) Role of the serotonergic system in the forced swimming test. Neurosci Biobehav Rev 19:377–395 [DOI] [PubMed] [Google Scholar]
- Busch M, Chourbaji S, Dammann P, Haemisch A, Jirkof P, Oehlert P, Osterkamp A, Ott S, Peters S, Spekl K, Tsai PP (2014) Tiergerechte Haltung von Labormäusen: Ausschuss für Tiergerechte Laborierhaltung. GV-SOLAS, Gesellschaft für Versuchstierkunde
- Commons KG (2008) Evidence for topographically organized endogenous 5-HT-1A receptor-dependent feedback inhibition of the ascending serotonin system. Eur J Neurosci 27:2611–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin MJ, Baker MA (1977) Thermosensitive midbrain neurons in the cat. Brain Res 128:461–472 [DOI] [PubMed] [Google Scholar]
- Drugan RC, Hibl PT, Kelly KJ, Dady KF, Hale MW, Lowry CA (2013) Prior cold water swim stress alters immobility in the forced swim test and associated activation of serotonergic neurons in the dorsal raphe nucleus. Neuroscience 253:221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duricki DA, Soleman S, Moon LD (2016) Analysis of longitudinal data from animals with missing values using SPSS. Nat Protoc 11:1112–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JH, Hassell JE Jr, Siebler PH, Arnold MR, Lamb AK, Smith DG, Day HEW, Smith TM, Simmerman EM, Outzen AA, Holmes KS, Brazell CJ, Lowry CA (2017) Preimmunization with a heat-killed preparation of Mycobacterium vaccae enhances fear extinction in the fear-potentiated startle paradigm. Brain Behav Immun 66:70–84 [DOI] [PubMed] [Google Scholar]
- Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, Watkins LR (1997) Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull 43:357–364 [DOI] [PubMed] [Google Scholar]
- Grubbs FE (1969) Procedures for detecting outlying observations in samples. Technometrics 11:1–21 [Google Scholar]
- Hale MW, Lowry CA (2011) Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology 213:243–264 [DOI] [PubMed] [Google Scholar]
- Hale MW, Dady KF, Evans AK, Lowry CA (2011) Evidence for in vivo thermosensitivity of serotonergic neurons in the rat dorsal raphe nucleus and raphe pallidus nucleus implicated in thermoregulatory cooling. Exp Neurol 227:264–278 [DOI] [PubMed] [Google Scholar]
- Hale MW, Raison CL, Lowry CA (2013) Integrative physiology of depression and antidepressant drug action: implications for serotonergic mechanisms of action and novel therapeutic strategies for treatment of depression. Pharmacol Ther 137:108–118 [DOI] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, de Malmanche MT, Attia J (2012) Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: a meta-analysis. Psychol Med 42:2015–2026 [DOI] [PubMed] [Google Scholar]
- Hodes GE et al (2014) Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci USA 111:16136–16141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerr V, Zbytnuik L, Leger C, Tam PP, Kubes P, Vogel HJ (2012) Gram-negative and Gram-positive bacterial infections give rise to a different metabolic response in a mouse model. J Proteome Res 11:3231–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis JH, Evans AK, Bruce KP, Lightman SL, Lowry CA (2006) Lipopolysaccharide has indomethacin-sensitive actions on Fos expression in topographically organized subpopulations of serotonergic neurons. Brain Behav Immun 20:569–577 [DOI] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S (1999) Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162:3749–3752 [PubMed] [Google Scholar]
- Johnson PL, Lightman SL, Lowry CA (2004) A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann N Y Acad Sci 1018:58–64 [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Donner NC, Hale MW, Lowry CA (2011) Swim stress activates serotonergic and nonserotonergic neurons in specific subdivisions of the rat dorsal raphe nucleus in a temperature-dependent manner. Neuroscience 197:251–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB (2014) Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry 71:1121–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimaki M, Shipley MJ, Batty GD, Hamer M, Akbaraly TN, Kumari M, Jokela M, Virtanen M, Lowe GD, Ebmeier KP, Brunner EJ, Singh-Manoux A (2014) Long-term inflammation increases risk of common mental disorder: a cohort study. Mol Psychiatry 19:149–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak W, Conn CA, Kluger MJ (1994) Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice. Am J Physiol 266:R125–R135 [DOI] [PubMed] [Google Scholar]
- Kozak W, Kluger MJ, Soszynski D, Conn CA, Rudolph K, Leon LR, Zheng H (1998) IL6 and IL1β in fever. Studies using cytokine-deficient (knockout) mice. Ann N Y Acad Sci 856:33–47 [DOI] [PubMed] [Google Scholar]
- Langgartner D, Füchsl AM, Uschold-Schmidt N, Slattery DA, Reber SO (2015) Chronic subordinate colony housing paradigm: a mouse model to characterize the consequences of insufficient glucocorticoid signaling. Front Psychiatry 6:18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bert N, Chain BM, Rook G, Noursadeghi M (2011) DC priming by M. vaccae inhibits Th2 responses in contrast to specific TLR2 priming and is associated with selective activation of the CREB pathway. PLoS ONE 6:e18346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xiao B, Qiu W, Yang L, Hu B, Tian X, Yang H (2010) Altered expression of CD4(+)CD25(+) regulatory T cells and its 5-HT(1a) receptor in patients with major depression disorder. J Affect Disord 124:68–75 [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hollis JH, De Vries A, Pan B, Brunet LR, Hunt JR, Paton JF, van Kampen E, Knight DM, Evans AK, Rook GA (2007) Identification of an immune-responsive mesolimbocortical serotonergic system: potential role in regulation of emotional behavior. Neuroscience 146(2):756–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Evans AK, Gasser PJ, Hale MW, Staub DR, Shekhar A (2008) Topographical organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In: Monti JM, Pandi-Perumal BL, Jacobs BL, Nutt DL (eds) Serotonin and sleep: molecular, functional and clinical aspects. Birkhauser, Basel, pp 25–68 [Google Scholar]
- Miller AH, Raison CL (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16:22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T (1995) Immediate-early genes: ten years on. TINS 18:66–67 [PubMed] [Google Scholar]
- Nautiyal KM, McKellar H, Silverman AJ, Silver R (2009) Mast cells are necessary for the hypothermic response to LPS-induced sepsis. Am J Physiol Regul Integr Comp Physiol 296:R595–R602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochalski SJ, Hartman DA, Belfast MT, Walter TL, Glaser KB, Carlson RP (1993) Inhibition of endotoxin-induced hypothermia and serum TNF-a levels in CD-1 mice by various pharmacological agents. Agents Actions 39:C52–C54 [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R (2009a) Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci 29:4200–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW (2009b) Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol 182:3202–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R (2009c) Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 14:511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Oka K, Kobayashi T, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S, Saper CB (2003) Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J Physiol 551:945–954 (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul L, Fraifeld V, Kaplanski J (1999) Evidence supporting involvement of leukotrienes in LPS-induced hypothermia in mice. Am J Physiol 276:R52–R58 [DOI] [PubMed] [Google Scholar]
- Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C, Lazaropoulou C, Papassotiriou I, Tsiantis J, Chrousos GP (2007) Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology 32:991–999 [DOI] [PubMed] [Google Scholar]
- Raison CL, Hale MW, Williams LE, Wager TD, Lowry CA (2015) Somatic influences on subjective well-being and affective disorders: the convergence of thermosensory and central serotonergic systems. Front Psychol 5:1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber SO et al (2016) Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc Natl Acad Sci USA 113:E3130–E3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault J, Aubert A (2006) Immunity and emotions: lipopolysaccharide increases defensive behaviours and potentiates despair in mice. Brain Behav Immun 20:517–526 [DOI] [PubMed] [Google Scholar]
- Rohleder N (2014) Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med 76:181–189 [DOI] [PubMed] [Google Scholar]
- Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA (2005) Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol 289:R1244–R1252 [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM (1981) Distribution of serotonin-immunoreactivity in the central nervous system of the rat. Cell bodies and terminals. Neuroscience 6:557–618 [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM (1984) Serotonin-immunoreactive neurons and their projections in the CNS. In: Björklund A, Hökfelt T (eds) Classical transmitters and transmitter receptors in the CNS. Elsevier Science Publishers B.V, Amsterdam, pp 68–125 [Google Scholar]
- Steiner AA, Chakravarty S, Rudaya AY, Herkenham M, Romanovsky AA (2006) Bacterial lipopolysaccharide fever is initiated via Toll-like receptor 4 on hematopoietic cells. Blood 107:4000–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dunn AJ, Roberts AJ, Zhang H (2009) Decreased immobility in swimming test by homologous interferon-alpha in mice accompanied with increased cerebral tryptophan level and serotonin turnover. Neurosci Lett 452:96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lawson MA, Dantzer R, Kelley KW (2010) LPS-induced indoleamine 2,3-dioxygenase is regulated in an interferon-gamma-independent manner by a JNK signaling pathway in primary murine microglia. Brain Behav Immun 24:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw AC, Boorman L, Reid R (1971) Assay of endotoxin by the hypothermic response of mice. Br J Exp Pathol 52:198–208 [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Lu J, Elmquist JK, Saper CB (2003) Specific roles of cyclooxygenase-1 and cyclooxygenase-2 in lipopolysaccharide-induced fever and Fos expression in rat brain. J Comp Neurol 463:3–12 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhen H, Yao W, Bian F, Mao X, Yang X, Jin S (2013) Antidepressant drug, desipramine, alleviates allergic rhinitis by regulating Treg and Th17 cells. Int J Immunopathol Pharmacol 26:107–115 [DOI] [PubMed] [Google Scholar]