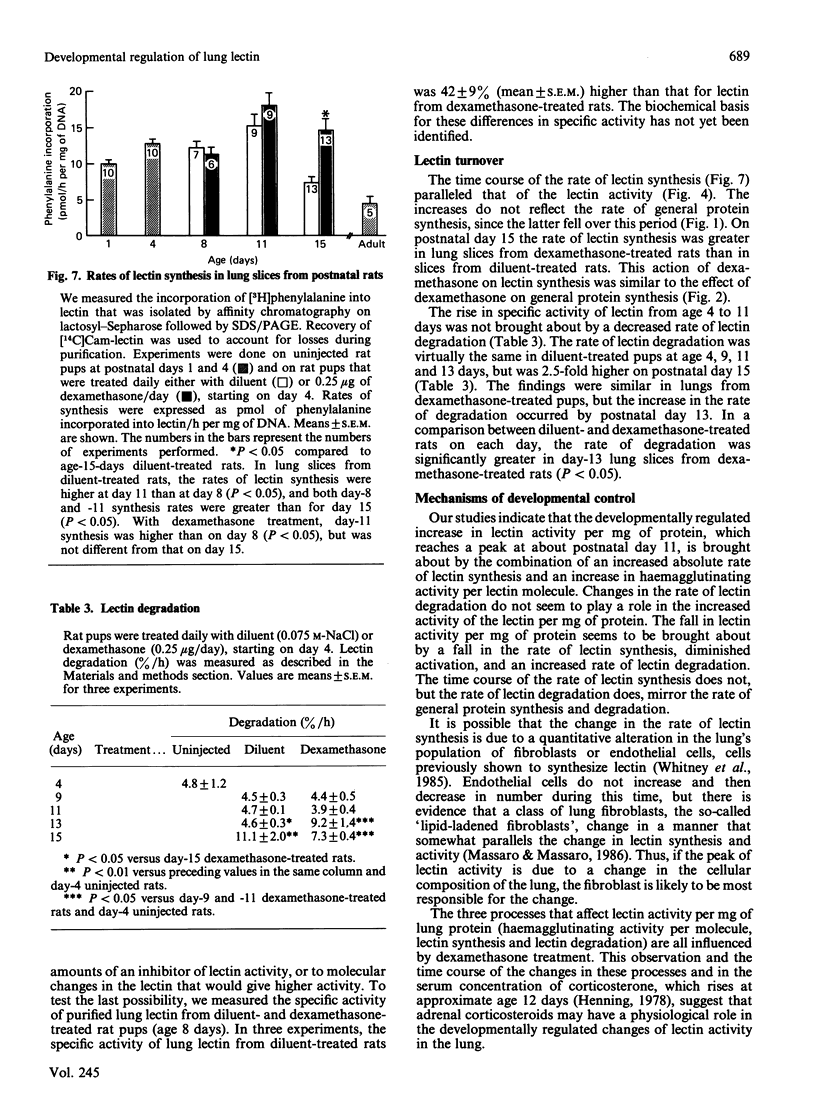
Abstract
Soluble lectins are widely distributed cell-agglutinating proteins. Their activity is developmentally regulated in several tissues, including the lung, but virtually nothing is known about the mechanisms of the developmental regulation or the turnover of these proteins. We studied mechanisms that might be responsible for the developmentally regulated changes in the activity of a lectin (beta-galactoside-binding protein) found in the lung, and determined if its activity or turnover could be modulated by treatment of rat pups with a glucocorticosteroid hormone (dexamethasone). Our studies on the activity and turnover of the lectin indicated that the peak of lectin activity (units/mg of protein) that occurred at age 12 days appeared to be brought about by two means: an increase in the activity of the lectin molecule itself (units/micrograms of lectin) that occurred at age 8 days, and 1.5-fold increase in the absolute rate of lectin synthesis at age 11 days. The decline in lectin activity was associated with a decrease in its rate of synthesis, return to the baseline extent of activation, and an increased rate of degradation. Treatment of rat pups with dexamethasone diminished the peak of lectin activity (units/mg of protein) by about 25%. This effect of dexamethasone was due, at least in part, to the complete prevention of activation of the lectin molecule (units/micrograms of lectin) and a premature increase in the rate of lectin degradation. Perhaps the normal fall in lectin activity after age 11 days is caused by mechanisms induced by the increase in serum corticosteroid that occurs at that age.
Full text
PDF


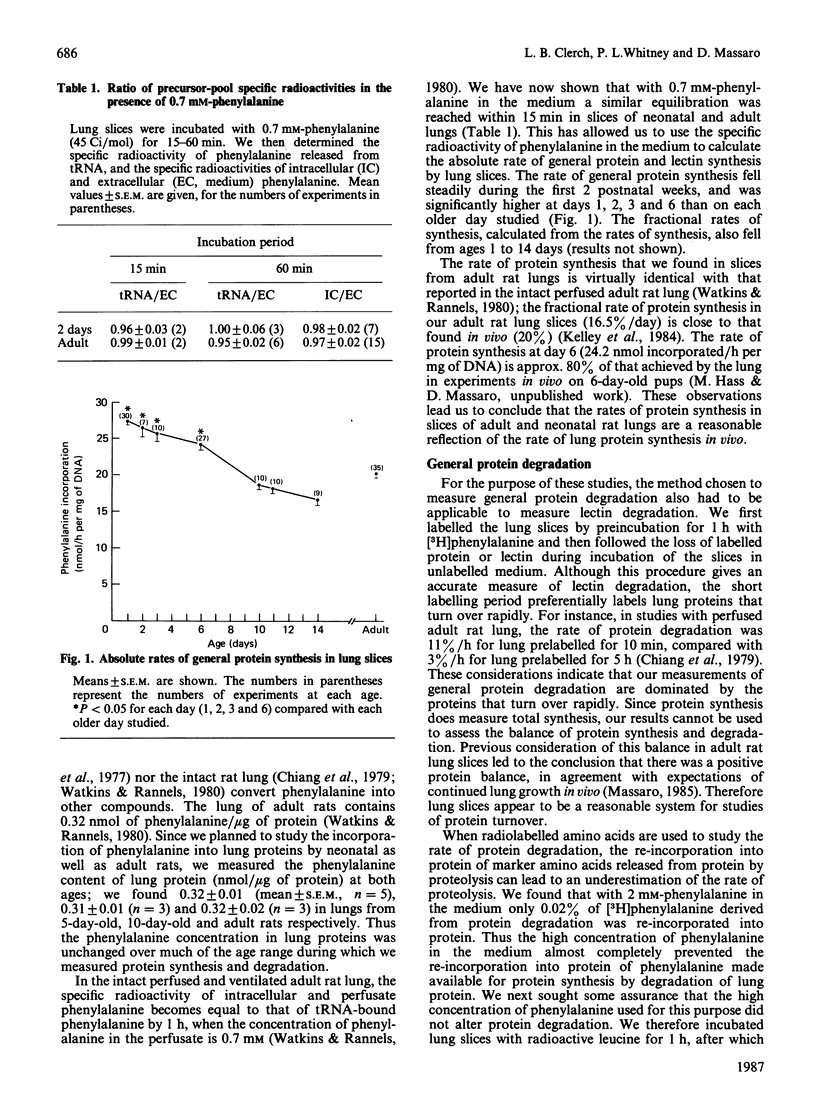
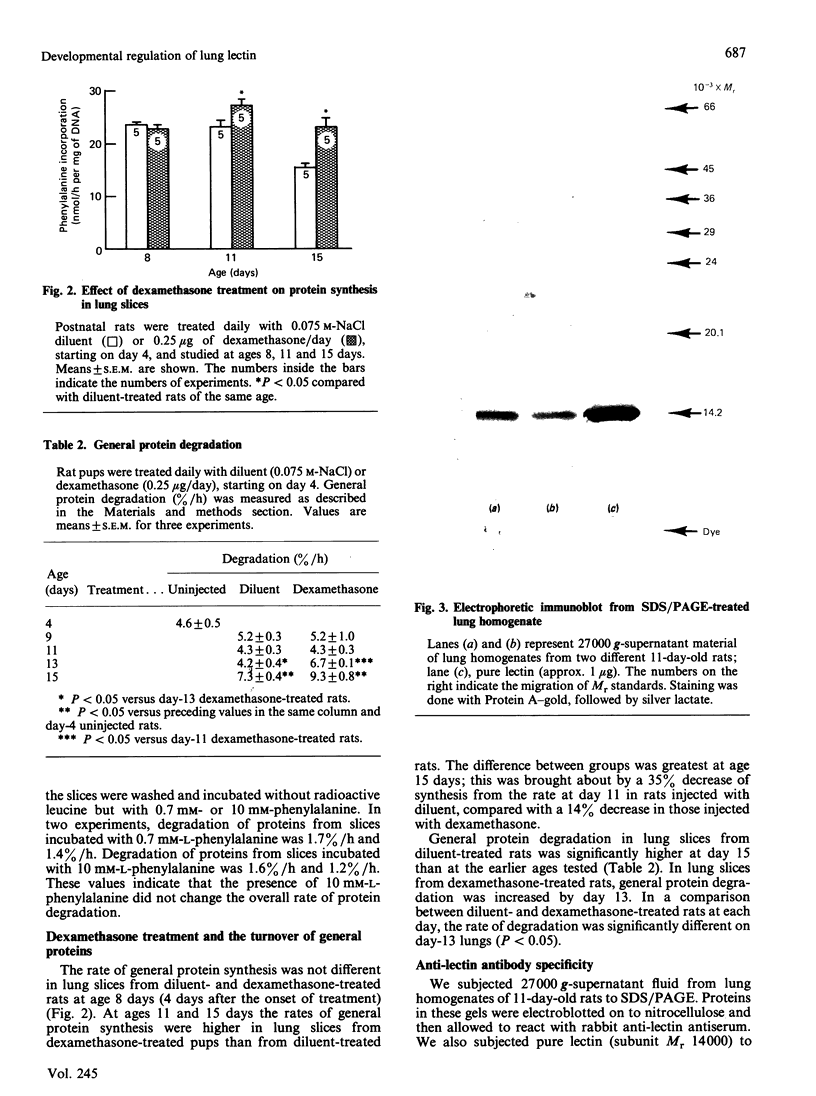
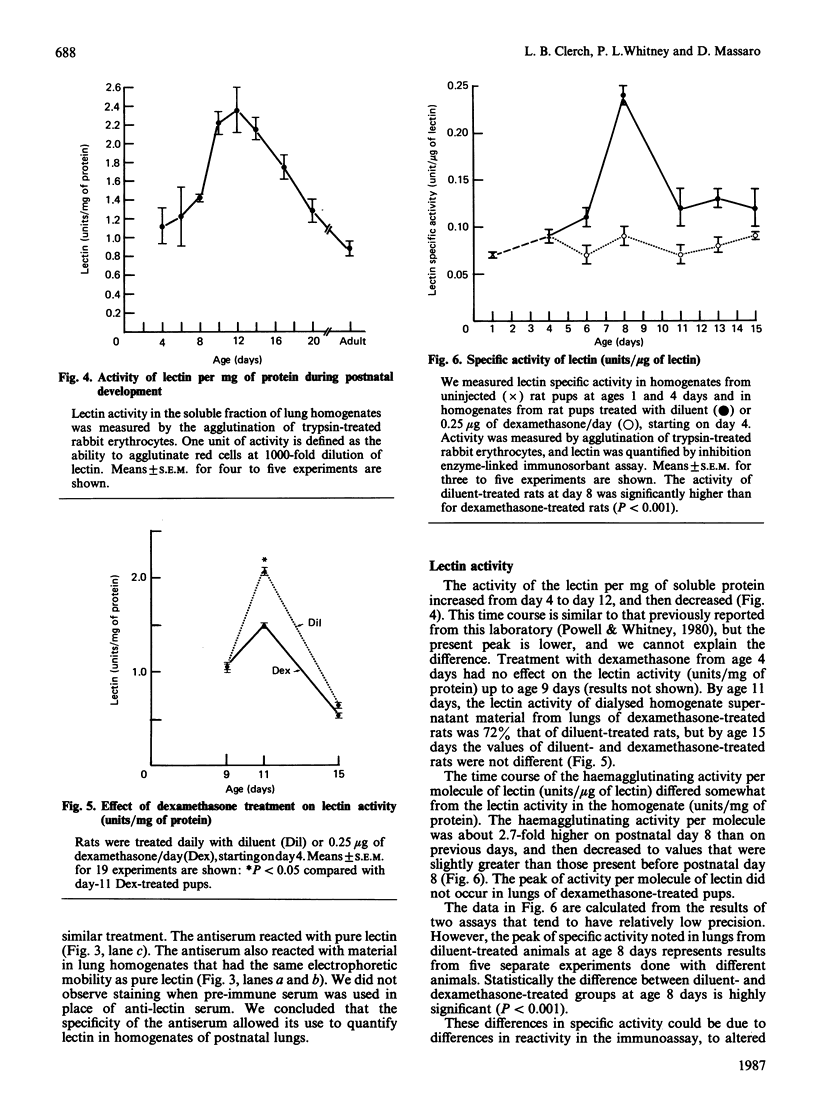

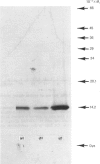
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airhart J., Kelley J., Brayden J. E., Low R. B., Stirewalt W. S. An ultramicro method of amino acid analysis: application to studies of protein metabolism in cultured cells. Anal Biochem. 1979 Jul 1;96(1):45–55. doi: 10.1016/0003-2697(79)90552-9. [DOI] [PubMed] [Google Scholar]
- Barondes S. H. Lectins: their multiple endogenous cellular functions. Annu Rev Biochem. 1981;50:207–231. doi: 10.1146/annurev.bi.50.070181.001231. [DOI] [PubMed] [Google Scholar]
- Barondes S. H. Soluble lectins: a new class of extracellular proteins. Science. 1984 Mar 23;223(4642):1259–1264. doi: 10.1126/science.6367039. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Barondes S. H. Quantitation of two endogenous lactose-inhibitable lectins in embryonic and adult chicken tissues. J Cell Biol. 1982 Jan;92(1):23–27. doi: 10.1083/jcb.92.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chiang M. J., Whitney P., Jr, Massaro D. Protein metabolism in lung: use of isolated perfused lung to study protein degradation. J Appl Physiol Respir Environ Exerc Physiol. 1979 Jul;47(1):72–78. doi: 10.1152/jappl.1979.47.1.72. [DOI] [PubMed] [Google Scholar]
- Dalle M., Delost P. Plasma and adrenal cortisol concentrations in foetal, newborn and mother guinea-pigs during the perinatal period. J Endocrinol. 1976 Aug;70(2):207–214. doi: 10.1677/joe.0.0700207. [DOI] [PubMed] [Google Scholar]
- Danscher G., Nörgaard J. O. Light microscopic visualization of colloidal gold on resin-embedded tissue. J Histochem Cytochem. 1983 Dec;31(12):1394–1398. doi: 10.1177/31.12.6631001. [DOI] [PubMed] [Google Scholar]
- Girard J. R., Guillet I., Marty J., Marliss E. B. Plasma amino acid levels and development of hepatic gluconeogenesis in the newborn rat. Am J Physiol. 1975 Aug;229(2):466–473. doi: 10.1152/ajplegacy.1975.229.2.466. [DOI] [PubMed] [Google Scholar]
- Henning S. J. Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol. 1978 Nov;235(5):E451–E456. doi: 10.1152/ajpendo.1978.235.5.E451. [DOI] [PubMed] [Google Scholar]
- Kelley J., Stirewalt W. S., Chrin L. Protein synthesis in rat lung. Measurements in vivo based on leucyl-tRNA and rapidly turning-over procollagen I. Biochem J. 1984 Aug 15;222(1):77–83. doi: 10.1042/bj2220077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G., Teichberg V. I. Isolation and physicochemical characterization of electrolectin, a beta-D-galactoside binding lectin from the electric organ of Electrophorus electricus. J Biol Chem. 1981 Jun 10;256(11):5735–5740. [PubMed] [Google Scholar]
- Massaro D., Massaro G. D. Dexamethasone accelerates postnatal alveolar wall thinning and alters wall composition. Am J Physiol. 1986 Aug;251(2 Pt 2):R218–R224. doi: 10.1152/ajpregu.1986.251.2.R218. [DOI] [PubMed] [Google Scholar]
- Massaro D., Teich N., Maxwell S., Massaro G. D., Whitney P. Postnatal development of alveoli. Regulation and evidence for a critical period in rats. J Clin Invest. 1985 Oct;76(4):1297–1305. doi: 10.1172/JCI112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan H. E., Earl D. C., Broadus A., Wolpert E. B., Giger K. E., Jefferson L. S. Regulation of protein synthesis in heart muscle. I. Effect of amino acid levels on protein synthesis. J Biol Chem. 1971 Apr 10;246(7):2152–2162. [PubMed] [Google Scholar]
- Powell J. T., Whitney P. L. Postnatal development of rat lung. Changes in lung lectin, elastin, acetylcholinesterase and other enzymes. Biochem J. 1980 Apr 15;188(1):1–8. doi: 10.1042/bj1880001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thet L. A., Delaney M. D., Gregorio C. A., Massaro D. Protein metabolism by rat lung: influence of fasting, glucose, and insulin. J Appl Physiol Respir Environ Exerc Physiol. 1977 Sep;43(3):463–467. doi: 10.1152/jappl.1977.43.3.463. [DOI] [PubMed] [Google Scholar]
- Watkins C. A., Rannels D. E. Measurement of protein synthesis in rat lungs perfused in situ. Biochem J. 1980 Apr 15;188(1):269–278. doi: 10.1042/bj1880269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P. L., Powell J. T., Sanford G. L. Oxidation and chemical modification of lung beta-galactoside-specific lectin. Biochem J. 1986 Sep 15;238(3):683–689. doi: 10.1042/bj2380683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P., Maxwell S., Ryan U., Massaro D. Synthesis and binding of lactose-specific lectin by isolated lung cells. Am J Physiol. 1985 Mar;248(3 Pt 1):C258–C264. doi: 10.1152/ajpcell.1985.248.3.C258. [DOI] [PubMed] [Google Scholar]