ABSTRACT
Sooty mangabeys (SMs) are natural hosts of simian immunodeficiency virus (SIV) and do not progress to AIDS despite high viral replication. The main factors involved in the benign nature of this infection are (i) low level of immune activation, (ii) relative preservation of specific CD4+ T-cell subsets from direct virus infection, and (iii) absence of microbial translocation from the gut to the systemic circulation. To determine the impact of SIV infection on underlying cause of death, we retrospectively analyzed data from 307 SMs (219 SIV infected and 88 uninfected) housed at the Emory Primate Center that have died between 1986 and 2022. Interestingly, we found that SIV-infected SMs live ~4 years longer than SIV-uninfected SMs, although this result is hard to interpret due to differences in how animals were housed and assigned to specific experimental studies. While the causes of death were not different between SIV-infected and uninfected SMs that died before age 15 (i.e., adult), we found significant differences in the relative frequency of specific causes of death in the elderly population (≥15 years old). Specifically, we observed that SIV-infected SMs were more likely to die from infections but less likely to die from cardiovascular disease (and diabetes in female animals) as compared to uninfected SMs. While confirming the non-pathogenic nature of SIV infection in SMs, these data reveal, for the first time, a qualitative impact of SIV infection on the host physiology that induces a significant change in the mortality pattern in these natural SIV hosts.
IMPORTANCE
In this study, we demonstrate, for the first time, that the natural, non-pathogenic SIV infection of the African monkey SM has a clinical impact which is revealed in terms of main causes of mortality, which are significantly different in the infected animals as compared to the uninfected ones. Indeed, SIV-infected SMs are at higher risk of dying of infectious diseases but appear to be somewhat protected from cardiovascular causes of death. The identification of a specific pattern of mortality associated with the infection suggests that the host-pathogen interaction between SIV and the SM immune system, while non-pathogenic in nature, has a detectable impact on the overall health status of the animals.
KEYWORDS: SIV, sooty mangabeys, SIV infection, cause of death
INTRODUCTION
Naturally occurring simian immunodeficiency virus (SIV) infections have been identified in over 40 different African non-human primate species. Interestingly, natural SIV infections of sooty mangabeys (SMs; Cercocebus atys) and African green monkeys (Chlorocebus spp.) do not lead to progressive CD4+ T-cell loss and development of simian AIDS, even though the virus is equally cytopathic in productively infected CD4+ T cells and plasma viral loads are comparable to those observed during pathogenic HIV infection of humans and SIV infection of rhesus macaques (RMs) (reviewed in references 1–5). In SIV-infected SMs, the resistance to disease progression has been attributed to several non-mutually exclusive specific aspects of the host-pathogen interaction, including (i) the absence of chronic immune activation, (ii) the low levels of microbial translocation from the gut, and (iii) the relative preservation from direct virus infection of specific CD4+ T-cell subsets including central memory cells, stem-cell memory cells, and follicular helper cells (6–15). The fact that natural SIV infection of SMs is non-pathogenic has emerged over many years of clinical and pathological observation of the large colony of infected and uninfected animals housed at the Emory National Primate Research Center (ENPRC), which culminated in a report by Keele et al. indicating that there is no significant difference in the average lifespan of SIV-infected vs uninfected SMs (shown in Fig. S5) in contrast to pathogenic nature of SIVcpz, the immediate precursor of HIV-1, in chimpanzees (16).
Of note, a few reports have also suggested that, with aging, SIV-infected SMs may develop signs of immune decline, including decrease in CD4+ T cells and increased immune activation, which could be partially restored after anti-retroviral treatment, with at least one case of “classic” AIDS reported in a 24-year-old captive SM that was naturally SIV infected (7, 17–20). To better assess the long-term impact of SIV infection on the overall clinical conditions of SMs, we have conducted a systematic analysis of the causes of death in 307 SMs, of which 219 were SIV infected and 88 were uninfected, that were housed at ENPRC and have died of natural causes or elected clinical euthanasia between 1986 and 2022. To the best of our knowledge, this is the first time that such a comprehensive and detailed analysis has been conducted through full and updated access to the ENPRC record of clinical and pathological observations, as well as necropsy reports. While this study clearly confirmed that SIV infection does not reduce the average lifespan of captive SMs, it also revealed that, in elderly animals (defined as older than 15 years), the pattern of causes of death is significantly different between SIV-infected and uninfected animals. In particular, we observed that in SIV, infection is associated with an increased risk of death by infectious diseases and a decreased risk of death by cardiovascular diseases, with no differences between infected and uninfected animals in the risk of death by cancer, diabetes, trauma, and miscellaneous other causes. We believe that the results of this study provide valuable and unprecedented insights into the potential long-term, large-scale impact of SIV infection on the overall health of SMs and, in fact, of any SIV infection in a natural non-human primate host species.
RESULTS
Average lifespan of SIV-infected and uninfected SMs
Between January 1986 and December 2022, 307 necropsies were conducted on adult SMs (defined as older than 3 years), who had died either due to natural death or because of euthanasia performed due to the presence of naturally acquired illness. As shown in Table 1, this retrospective analysis included 219 SIV-infected SMs (71.3% of the total), of which 205 were naturally infected (66.8%) and 14 were experimentally infected (4.5%), and 88 were uninfected animals (28.7% of the total). We also found that a total of 116 deaths (37.8% of the total) occurred in adult SMs aged between 3 and 14 years old, including 74 SIV-infected and 42 uninfected animals, while 191 deaths (62.2%) occurred in elderly animals, defined as >15 years old (Fig. S1). As also shown in Table 1, we found a similar distribution of infected and uninfected animals across sex and age groups.
TABLE 1.
Characteristics of studied sooty mangabeys
| Group | Total (%) | SIV+ (%) | SIV− (%) |
|---|---|---|---|
| Overall | |||
| Total | 307 | 219 (71.3) | 88 (28.7) |
| Females | 175 (57.0) | 126 (41.0) | 49 (16.0) |
| Males | 132 (43.0) | 93 (30.3) | 39 (12.7) |
| Adult (3–14 years old) | |||
| Total | 116 (37.8) | 74 (63.8) | 42 (36.2) |
| Females | 62 (53.4) | 38 (32.8) | 24 (20.7) |
| Males | 54 (46.6) | 36 (31.0) | 18 (15.5) |
| Elderly (≥15 years old) | |||
| Total | 191 (62.2) | 145 (75.9) | 46 (24.1) |
| Females | 113 (59.2) | 88 (46.1) | 25 (13.1) |
| Males | 78 (40.8) | 57 (29.8) | 21 (11.0) |
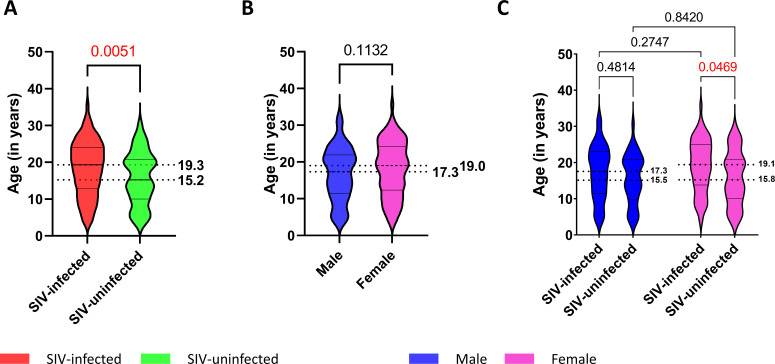
We next compared the overall lifespan in SIV-infected vs uninfected SMs and found that, on average, SIV-infected animals lived approximately 4 years longer than SIV-uninfected SMs (median 19.3 and 15.2 years, respectively; P = 0.0051; Fig. 1A). As females have a higher predicted survival in several primate species (21) and females have a higher, although not significant, longevity than males in our cohort (median 19.0 and 17.3 years, respectively; P = 0.1132; Fig. 1B), we further investigated the impact of SIV infection on life expectancy within sex groups. As shown in Fig. 1C, within the female group, we confirmed a significantly higher life expectancy in SIV-infected animals as compared to uninfected ones (median 19.1 and 15.8 years, respectively; P = 0.0469). A similar trend was also observed in male SIV-infected SMs as compared to uninfected males (median 17.3 and 15.5 years, respectively; P = 0.4814), although the observed difference did not reach statistical significance. Taken together, these results might suggest that SIV infection could increase the overall life expectancy in SMs. However, it is important to note that the presence of this relatively small difference is hard to interpret because the colonies of SIV-infected and uninfected SMs have been housed separately, undergoing distinct breeding, birth control, and hormone treatment regimens over the course of this 30-year retrospective study. For this reason, we prefer to conclude conservatively that the main message of this data set resides in clearly confirming the non-pathogenic nature of SIV infection of SMs.
Fig 1.
Life expectancy of SMs. (A) Age at death in SIV infected (red) and SIV uninfected (green). (B) Age at death in males (blue) and females (pink). (C) Age at death in male (blue) and female (pink) SIV infected and SIV uninfected. Mann-Whitney test was performed in panels A and B. One-way analysis of variance test was performed in panel C. P value indicated and highlighted in red font when <0.05.
Frequency of causes of death in SMs, all animals, and divided based on age (i.e., adult vs geriatric) and sex
In this study, we defined the cause of death based on the primary pathology diagnosis from the necropsy report of each individual deceased animal as established by the team of veterinarian pathologists at the ENPRC of Emory University. Based on these reports, we have elected to classify causes of death into six major categories: cardiovascular, infectious diseases, neoplasia, diabetes, trauma, and “others/unknown.” While we recognize that there is an unavoidable element of uncertainty in establishing the exact cause of death in the event of complex clinical pictures, we reasoned that by strictly adhering to the definition of cause of death as made by the veterinarian pathologists at the time of necropsy and over a long period of time (1986–2022), we would minimize the risk of biased interpretation of the existing pathology records.
As shown in Table 2, we found that, in terms of relative frequency, the most common cause of death in all SMs (i.e., SIV infected and uninfected) was infectious diseases (24.1%), followed by diabetes (19.5%), neoplasia (12.7%), cardiovascular diseases (9.82%), and trauma (5.2%). In 88 cases (28.7%), the primary cause of death was due to one of a series of relatively rare conditions (i.e., unclassified intestinal diseases, endometriosis, osteoarthritis, and many other conditions, grouped as “others”) or was unable to be determined (i.e., “unknown”), either due to insufficient information in the necropsy report or due to complex clinical pictures in which more than one disease played an important role, thus precluding a unique classification of the causes of death. Of note, throughout the following analyses, others and unknown causes of deaths were grouped together.
TABLE 2.
Cause of death in the studied sooty mangabeys
| Group | Total (%) | Females | Males | ||
|---|---|---|---|---|---|
| SIV+ (%) | SIV− (%) | SIV+ (%) | SIV− (%) | ||
| Overall | |||||
| Total | 307 | 126 | 49 | 93 | 39 |
| Infectious diseases | 74 (24.1) | 30 (23.8) | 10 (20.4) | 27 (29.0) | 7 (17.9) |
| Diabetes | 60 (19.5) | 26 (20.6) | 14 (28.6) | 17 (18.3) | 3 (7.7) |
| Neoplasia | 39 (12.7) | 21 (16.7) | 5 (10.2) | 8 (8.6) | 5 (12.8) |
| Cardiovascular | 30 (9.8) | 2 (1.6) | 5 (10.2) | 15 (16.1) | 8 (20.5) |
| Trauma | 16 (5.2) | 8 (6.3) | 3 (6.1) | 4 (4.3) | 1 (2.6) |
| Other/unknown | 88 (28.7) | 39 (31.0) | 12 (24.5) | 22 (23.7) | 15 (38.5) |
| Adult (3–14 years old) | |||||
| Total | 116 | 38 | 24 | 36 | 18 |
| Infectious diseases | 48 (41.4) | 16 (42.1) | 9 (37.5) | 16 (44.4) | 7 (38.9) |
| Diabetes | 7 (6.0) | 0 (0.0) | 5 (20.8) | 2 (5.6) | 0 (0.0) |
| Neoplasia | 9 (7.8) | 3 (7.9) | 2 (8.3) | 2 (5.6) | 2 (11.1) |
| Cardiovascular | 3 (2.6) | 0 (0.0) | 0 (0.0) | 2 (5.6) | 1 (5.6) |
| Trauma | 10 (8.6) | 4 (10.5) | 2 (8.3) | 4 (11.1) | 0 (0.0) |
| Unknown/other | 39 (33.6) | 15 (39.5) | 6 (25.0) | 10 (27.8) | 8 (44.4) |
| Elderly (≥15 years old) | |||||
| Total | 191 | 88 | 25 | 57 | 21 |
| Infectious diseases | 26 (13.6) | 14 (15.9) | 1 (4.0) | 11 (19.3) | 0 (0.0) |
| Diabetes | 54 (28.3) | 26 (29.5) | 9 (36.0) | 15 (26.3) | 4 (19.0) |
| Neoplasia | 30 (15.7) | 18 (20.5) | 3 (12.0) | 6 (10.5) | 3 (14.3) |
| Cardiovascular | 27 (14.1) | 2 (2.3) | 5 (20.0) | 13 (22.8) | 7 (33.3) |
| Trauma | 6 (3.1) | 4 (4.5) | 1 (4.0) | 0 (0.0) | 1 (4.8) |
| Unknown/other | 48 (25.1) | 24 (27.3) | 6 (24.0) | 12 (21.1) | 6 (28.6) |
We next examined the relative frequencies of causes of death in SMs who died as “adult” (defined as between 3 and 14 years of age) or “elderly” (over 15 years of age), and then in female vs male animals. The analysis of these data is shown in Table 2 and reveal, as expected, a significant difference among these two age groups, with a clear increase of mortality caused by cardiovascular diseases and diabetes in the older animals.
Lower frequency of death by cardiovascular diseases and diabetes, and higher frequency of death by infectious diseases in SIV-infected SMs as compared to uninfected
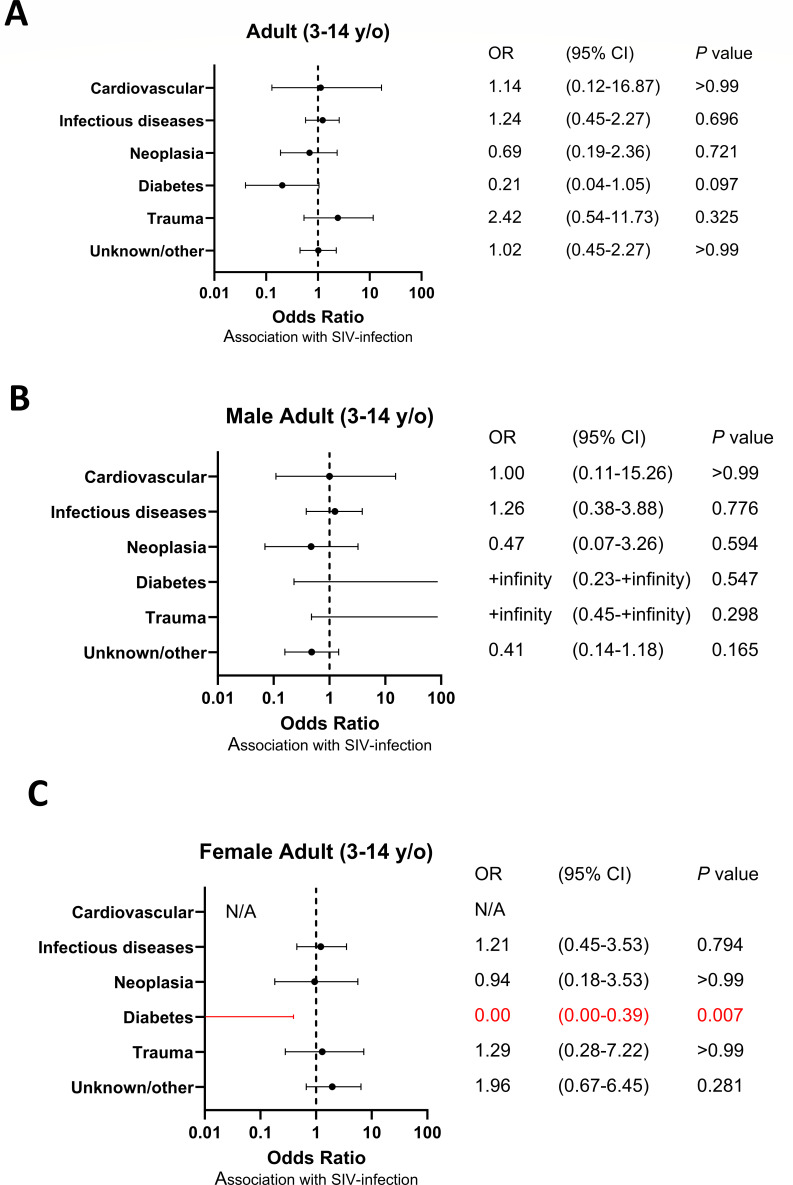
We next compared the relative frequency of the main categories of causes of death in the SIV-infected SMs (n = 219) as compared to the uninfected ones (n = 88). We found that, as a whole group, SIV-infected SMs showed a trend toward a lower frequency of death due to cardiovascular disease as compared to uninfected animals (odds ratio [OR] 0.48, 95% confidence interval [CI] 0.23–1.02, P = 0.087; Fig. S2A), with this trend reaching statistical significance within the female group (OR 0.14, 95% CI 0.03–0.71, P = 0.019; Fig. S2C). We then divided the deaths observed in SIV-infected SMs based on the age of the deceased animals and defined death as “adults,” those occurring between 3 and 14 years of age, and geriatric deaths, those occurring in animals that were 15 years old or older. We first observed that, within the adult group, there was no statistically significant association between SIV infection and any category of cause of death (Fig. 2A). However, we observed, in the same animals, a trend toward decreased risk of death from diabetes (OR 0.21, 95% CI 0.04–1.05, P = 0.097; Fig. 2A), which reached statistical significance only in the female group (OR 0.00, 95% CI 0.00–0.39, P = 0.007; Fig. 2C).
Fig 2.
Odds ratio of cause of death in adult (3- to 14-year-old) SIV-infected SMs. (A) Odds ratio of causes of death of adult (3- to 14-year-old) SIV-infected SMs not divided by sex, (B) in the male group and (C) in the female group. Ninety-five percent confidence intervals (CIs) for the odds ratio are shown as error bars on the graph. P value less than 0.05 is highlighted in red font.
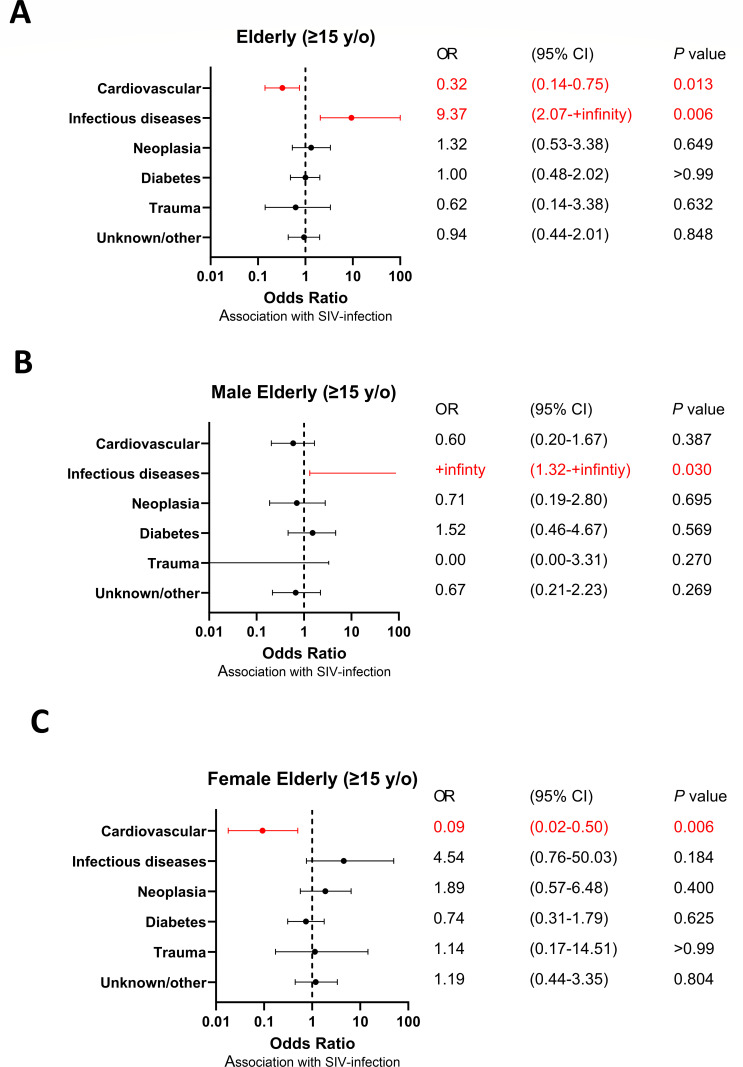
We next investigated the effect of age of death on the presence of specific changes in the relative frequency of specific causes of death between SIV-infected SMs and uninfected animals. In particular, we focused on the group of SMs that experienced an elderly death (i.e., ≥15 years old). As shown in Fig. 3, we observed that SIV infection was associated with a statistically significant reduction in the frequency of death caused by cardiovascular diseases in both the total group of SMs (OR 0.32, 95% CI 0.14–0.75, P = 0.013; Fig. 3A) and when the analysis was conducted within the female animals (OR 0.09, 95% CI 0.02–0.50, P = 0.006; Fig. 3C), thus suggesting that sex distribution and female sex’s protective role from cardiovascular disease were not confounding factors for this observation. A similar trend was also observed within the male group but did not reach statistical significance (OR 0.60, 95% CI 0.120–1.67, P = 0.387; Fig. 3B).
Fig 3.
Odds ratio of cause of death in elderly (≥15-year-old) SIV-infected SMs. (A) Odds ratio of causes of death in adult (≥15-year-old) SIV-infected SMs not divided by sex, (B) in the male group, and (C) in the female group. NInety-five percent confidence intervals (CIs) for the odds ratio are shown as error bars on the graph. P values less than 0.05 are highlighted in red font.
Interestingly, the comparative analysis of the causes of death between SIV-infected and uninfected SMs as conducted in the group of animals deceased in elderly age also revealed that SIV-infected SMs were significantly more likely to die from infectious diseases (OR +infinity, 95% CI 2.0 to +infinity, P = 0.006; Fig. 3A), with the statistical significance confirmed for males (OR +infinity, 95% CI 1.32 to +infinity, P = 0.030; Fig. 3B), while a non-significant trend was observed in the female group (OR 4.54, 95% CI 0.76–50.03, P = 0.184; Fig. 3C). Table 3 describes in greater detail the deaths caused by infectious diseases in elderly SIV-infected SMs, including, whenever available, the involved pathogen, the time of death, and the tissue and/or organ that was the main site of infection. In this regard, it is important to note that, overall, these analyses do not provide any evidence in favor of the possibility that the increased frequency of death by infectious diseases observed in SIV-infected SMs, which were housed separately from the uninfected animals, was due to specific epidemic events, defined as caused by a common pathogen within a restricted period of time.
TABLE 3.
Cause of death for infectious diseases in elderly (≥15-year-old) SMs
| ID | Sex | SIV | Necropsy data (mo-yr) | Age at death (years) | Pathogen | Type of infection |
|---|---|---|---|---|---|---|
| 1 | F | + | Sep-92 | 21 | Peritonitis | |
| 2 | F | + | 1999 | 23 | Escherichia coli | Septicemia |
| 3 | F | + | Jan-00 | 17 | Gastrointestinal | |
| 4 | M | + | Mar-01 | 16 | Gastrointestinal | |
| 5 | F | + | Apr-02 | 16 | Streptococcus pneumoniae | Septicemia |
| 6 | F | + | May-02 | 17 | Streptococcus pneumoniae | Meningitis |
| 7 | F | + | Aug-02 | 17 | Mycobacterium fortuitum | Peritonitis |
| 8 | F | + | 2002 | 25 | Streptococcus pneumoniae | Meningitis |
| 9 | F | − | Oct-03 | 15 | Peritonitis | |
| 10 | F | + | Feb-04 | 19 | Post-trauma | |
| 11 | F | + | 2005 | 22 | Urinary tract | |
| 12 | M | + | 2006 | 21 | Campylobacter fetus | Peritonitis |
| 13 | M | + | July-09 | 21 | Meningitis | |
| 14 | M | + | 2009 | 24 | Zygomycosis | |
| 15 | F | + | May-13 | 24 | Staphylococcus aureus | Endocarditis |
| 16 | M | + | Aug-14 | 19 | Septicemia | |
| 17 | M | + | Dec-15 | 24 | Escherichia coli and Staphylococcus aureus | Septicemia |
| 18 | M | + | Jan-16 | 25 | Pneumonia | |
| 19 | F | + | Jan-18 | 25 | Gastrointestinal | |
| 20 | F | + | Jan-18 | 36 | Pneumonia | |
| 21 | M | + | June-19 | 24 | Staphylococcus warneri | Endocarditis |
| 22 | M | + | Nov-19 | 22 | Staphylococcus warneri | Endocarditis |
| 23 | F | + | Oct-20 | 26 | Pneumonia | |
| 24 | M | + | Dec-20 | 24 | Pneumonia | |
| 25 | F | + | Jan-22 | 29 | Campylobacter fetus | Gastrointestinal |
| 26 | M | + | Aug-22 | 33 | Other |
DISCUSSION
SIV infection in natural hosts, such as SMs, is typically non-pathogenic and does not progress to AIDS. This outcome is in sharp contrast with HIV infection in humans as well as pathogenic SIVmac or SHIV infection of Asian macaques, which represent the most widely used experimental animal model used in studies of HIV/AIDS pathogenesis, prevention, and treatment (1–5). The main factors contributing to the non-pathogenic nature of SIV infection in SMs are the absence of chronic immune activation, the observed low levels of microbial translocation, and a relative preservation of a specific CD4+ T-cell subset from direct virus infection (6–15). While both previous studies (16) and the current comprehensive analysis have clearly demonstrated that, in SMs, SIV infection is not associated with any detectable reduction of the observed lifespan, it is reasonable to posit that some of the observed immunological adaptations to chronic SIV infection have substantially modified the overall “immunological landscape” of the animals. Given the well-established but complex role that the immune system plays in the onset and severity of numerous pathological conditions, we sought to investigate whether SIV infection has a discernible impact on the relative distribution of specific causes of morbidity and mortality in this colony of captive SMs.
In this study, we compared and contrasted the pattern of causes of death among SIV-infected and uninfected SMs and added a sub-analysis of the groups of adults (i.e., ages 3–14) and elderly (i.e., ages 15 and older) deaths, as well as in the groups of female and male animals. This study revealed a clear and previously unrecognized pattern that includes (i) increased frequency of death by infectious diseases in SIV-infected SMs and (ii) increased frequency of death by cardiovascular disease and diabetes in uninfected animals. We wish to emphasize that these observations reached statistical significance despite a relatively small number of events (307 deaths in total), thus indicating a relatively strong biological effect of the studied variable (i.e., SIV infection) on the observed causes of death in SMs. Please also note that we elected not to include in this analysis any pediatric causes deaths (defined as occurring before 3 years of age), as SIV infection of SMs is very rarely transmitted from mother to infant, with the vast majority of naturally SIV-infected SMs becoming seropositive around 2–3 years of age (21, 22).
While the current study is clearly descriptive and observational in nature, with no investigation of specific mechanisms of pathogenesis and their relationship with the immune-pathogenesis of SIV infection, it is tempting to speculate on the reasons why SIV-infected SMs experience a higher frequency of deaths by infectious diseases and a lower frequency of deaths by cardiovascular disease and diabetes. For instance, previous reports suggest a slow decline of CD4+ count with age in SIV-infected SMs compared to uninfected animals (9, 17), which may contribute to the increased frequency of deaths caused by infectious diseases. Similarly, the ability of SIV-infected SMs to rapidly suppress the immune activation caused by the SIV infection (12) may somewhat interfere with the immune responses to specific pathogens and thus predispose elderly animals to succumb to certain infectious diseases. Of note, our study did not reveal any increased frequency of death due to neoplasia in SIV-infected SMs.
In the context of cardiovascular disease, the role of the immune system is well established in humans, and it appears to involve a disrupted balance between pro- and anti-inflammatory mechanisms. In keeping with this notion, chronic immune activation has been linked to onset of cardiovascular diseases in both HIV-infected humans (23–25) and SIV-infected macaques (26). The observed lower frequency of deaths by cardiovascular diseases in SIV-infected SMs emphasizes the striking different outcomes of lentiviral infections between natural and non-natural hosts. Moreover, it is not unreasonable to speculate that the early establishment of an anti-inflammatory and/or immune-regulatory environment in SMs soon after SIV infection might play a role in reducing the overall risk of cardiovascular diseases, which would then translate into a reduced frequency of these diseases as the main cause of death in the SIV-infected animals. The fact that the differences observed between SIV-infected and uninfected SMs reached statistical significance only in the female group may be related to the small sample size of the male SMs populations and/or the possibility of a more pronounced impact of this putative SIV-associated anti-inflammatory effect in female than male SMs. Of note, we also observed a decreased risk of diabetes-related death in the adult female group, and given the well-established link between cardiovascular diseases and diabetes (24), it is possible to hypothesize a common underlying mechanism by which SIV infection is associated with lower frequency of death as caused by these diseases in SIV-infected SMs.
Due to the retrospective study design, samples near time of death were not available, precluding any evaluation of specific comorbidities markers. Future studies in both male and female SMs, with specific focus on inflammatory balance, sex differences, and longitudinal analyses, may help in understanding the mechanism behind the potential protective role of SIV infections against cardiovascular disease and diabetes.
In conclusion, this comprehensive analysis of the causes of death in SMs confirms the non-pathogenic nature of SIV infection in these animals and introduces—for the first time and in a statistically significant way—the concept that the causes of death are different in SIV-infected vs uninfected animals. This observation suggests that specific features of the host-pathogen interaction that emerge during chronic SIV infection may change the overall in vivo immunological landscape of SMs with a long-term impact on the risk of developing specific diseases. Additional studies in other SIV natural host species would be valuable to confirm and expand on the findings presented here, while further investigation of the underlying mechanisms may have important implications for our understanding of the immune pathogenesis of HIV infection in humans.
MATERIALS AND METHODS
Study population
All animals involved were housed at the ENPRC and maintained in accordance with United States Department of Agriculture and National Institutes of Health guidelines at an Association for Assessment and Accreditation of Laboratory Animal Care accredited facility. The study population consisted of animals living under normal conditions until their natural death or euthanasia based on their clinical status. Necropsies were performed at the ENPRC between January 1986 and December 2022 to reveal the causes of death. The SIV-infected SMs utilized in this study were either naturally infected in the ENPRC colony or experimentally infected before 2007 (n = 14). SIV infectious status was determined by SIV PCR of plasma, and negative HIV-2 serology confirmed the absence of SIV infection.
Pathology
Post-mortem evaluations were performed according to standard necropsy protocol used at the ENPRC between 1986 and 2022. Necropsy involved an external body inspection and evaluation of organ morphology. Organs were evaluated in situ, systematically removed, sampled, and placed in 10% neutral buffered formalin for preservation and eventual histological evaluation. Based on anamnestic information, macroscopic and histopathological findings, the final diagnosis of death was determined.
Statistical analysis
Data were analyzed using GraphPad Prism (version 9.5.1). Mann-Whitney and one-way analysis of variance tests were used as appropriate. The significance level was set at P < 0.05.
To evaluate the association between variables, ORs were calculated. The ORs were calculated as the odds of cause of death outcome in SIV-infected SMs divided by the odds of the outcome in the SIV uninfected SMs group. The 95% CI and exact P value were calculated.
ACKNOWLEDGMENTS
We thank all animal care and veterinary staff at the Emory National Primate Research Center (ENPRC).
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under award number P51 OD011132 (to the ENPRC), by HHS/National Institutes of Health (NIH) (R37 AI66998 to G.S., R01 AI050529 and R37 AI150590 to B.H.H.).
C.C. and S.G. conceptualized and designed the study; C.C., B.B., M.C., J.C., and I.N.M. acquired the data. C.C., D.A.K, B.H.H., and G.S. wrote the manuscript and provided critical revision of the manuscript.
Footnotes
This article is a direct contribution from Beatrice H. Hahn, a Fellow of the American Academy of Microbiology, who arranged for and secured reviews by Jason Brenchley, National Institute of Allergy and Infectious Diseases, Division of Intramural Research, and Michaela Müller-Trutwin, Institut Pasteur.
Contributor Information
Guido Silvestri, Email: gsilves@emory.edu.
Satya Dandekar, University of California, Davis, Davis, California, USA.
ETHICS APPROVAL
As this study involved only post-mortem sampling, ethical approval was not required.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.01639-24.
Figures S1 and S2.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Pandrea I, Silvestri G, Onanga R, Veazey RS, Marx PA, Hirsch V, Apetrei C. 2006. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: common patterns and species-specific differences. J Med Primatol 35:194–201. doi: 10.1111/j.1600-0684.2006.00168.x [DOI] [PubMed] [Google Scholar]
- 2. Klatt NR, Villinger F, Bostik P, Gordon SN, Pereira L, Engram JC, Mayne A, Dunham RM, Lawson B, Ratcliffe SJ, Sodora DL, Else J, Reimann K, Staprans SI, Haase AT, Estes JD, Silvestri G, Ansari AA. 2008. Availability of activated CD4+ T cells dictates the level of viremia in naturally SIV-infected sooty mangabeys. J Clin Invest 118:2039–2049. doi: 10.1172/JCI33814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pandrea I, Silvestri G, Apetrei C. 2009. AIDS in african nonhuman primate hosts of SIVs: a new paradigm of SIV infection. Curr HIV Res 7:57–72. doi: 10.2174/157016209787048456 [DOI] [PubMed] [Google Scholar]
- 4. Bosinger SE, Sodora DL, Silvestri G. 2011. Generalized immune activation and innate immune responses in simian immunodeficiency virus infection. Curr Opin HIV AIDS 6:411–418. doi: 10.1097/COH.0b013e3283499cf6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. 2012. Natural SIV hosts: showing AIDS the door. Science 335:1188–1193. doi: 10.1126/science.1217550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silvestri G, Sodora DL, Koup RA, Paiardini M, O’Neil SP, McClure HM, Staprans SI, Feinberg MB. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441–452. doi: 10.1016/s1074-7613(03)00060-8 [DOI] [PubMed] [Google Scholar]
- 7. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12:1365–1371. doi: 10.1038/nm1511 [DOI] [PubMed] [Google Scholar]
- 8. Dunham R, Pagliardini P, Gordon S, Sumpter B, Engram J, Moanna A, Paiardini M, Mandl JN, Lawson B, Garg S, McClure HM, Xu YX, Ibegbu C, Easley K, Katz N, Pandrea I, Apetrei C, Sodora DL, Staprans SI, Feinberg MB, Silvestri G. 2006. The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood 108:209–217. doi: 10.1182/blood-2005-12-4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sumpter B, Dunham R, Gordon S, Engram J, Hennessy M, Kinter A, Paiardini M, Cervasi B, Klatt N, McClure H, Milush JM, Staprans S, Sodora DL, Silvestri G. 2007. Correlates of preserved CD4+ T cell homeostasis during natural, nonpathogenic simian immunodeficiency virus infection of sooty mangabeys: implications for AIDS pathogenesis. J Immunol 178:1680–1691. doi: 10.4049/jimmunol.178.3.1680 [DOI] [PubMed] [Google Scholar]
- 10. Gordon SN, Dunham RM, Engram JC, Estes J, Wang Z, Klatt NR, Paiardini M, Pandrea IV, Apetrei C, Sodora DL, Lee HY, Haase AT, Miller MD, Kaur A, Staprans SI, Perelson AS, Feinberg MB, Silvestri G. 2008. Short-lived infected cells support virus replication in sooty mangabeys naturally infected with simian immunodeficiency virus: implications for AIDS pathogenesis. J Virol 82:3725–3735. doi: 10.1128/JVI.02408-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, Khoruts A, Frank I, Else J, Schacker T, Silvestri G, Douek DC. 2008. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112:2826–2835. doi: 10.1182/blood-2008-05-159301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, Zeng M, Masopust D, Carlis JV, Ran L, Vanderford TH, Paiardini M, Isett RB, Baldwin DA, Else JG, Staprans SI, Silvestri G, Haase AT, Kelvin DJ. 2009. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest 119:3556–3572. doi: 10.1172/JCI40115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paiardini M, Cervasi B, Reyes-Aviles E, Micci L, Ortiz AM, Chahroudi A, Vinton C, Gordon SN, Bosinger SE, Francella N, Hallberg PL, Cramer E, Schlub T, Chan ML, Riddick NE, Collman RG, Apetrei C, Pandrea I, Else J, Munch J, Kirchhoff F, Davenport MP, Brenchley JM, Silvestri G. 2011. Low levels of SIV infection in sooty mangabey central memory CD4+ T cells are associated with limited CCR5 expression. Nat Med 17:830–836. doi: 10.1038/nm.2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brenchley JM, Vinton C, Tabb B, Hao XP, Connick E, Paiardini M, Lifson JD, Silvestri G, Estes JD. 2012. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood 120:4172–4181. doi: 10.1182/blood-2012-06-437608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cartwright EK, McGary CS, Cervasi B, Micci L, Lawson B, Elliott STC, Collman RG, Bosinger SE, Paiardini M, Vanderford TH, Chahroudi A, Silvestri G. 2014. Divergent CD4+ T memory stem cell dynamics in pathogenic and nonpathogenic simian immunodeficiency virus infections. J Immunol 192:4666–4673. doi: 10.4049/jimmunol.1303193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keele BF, Jones JH, Terio KA, Estes JD, Rudicell RS, Wilson ML, Li Y, Learn GH, Beasley TM, Schumacher-Stankey J, Wroblewski E, Mosser A, Raphael J, Kamenya S, Lonsdorf EV, Travis DA, Mlengeya T, Kinsel MJ, Else JG, Silvestri G, Goodall J, Sharp PM, Shaw GM, Pusey AE, Hahn BH. 2009. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature 460:515–519. doi: 10.1038/nature08200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taaffe J, Chahroudi A, Engram J, Sumpter B, Meeker T, Ratcliffe S, Paiardini M, Else J, Silvestri G. 2010. A five-year longitudinal analysis of sooty mangabeys naturally infected with simian immunodeficiency virus reveals a slow but progressive decline in CD4+ T-cell count whose magnitude is not predicted by viral load or immune activation. J Virol 84:5476–5484. doi: 10.1128/JVI.00039-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Milush JM, Reeves JD, Gordon SN, Zhou D, Muthukumar A, Kosub DA, Chacko E, Giavedoni LD, Ibegbu CC, Cole KS, Miamidian JL, Paiardini M, Barry AP, Staprans SI, Silvestri G, Sodora DL. 2007. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J Immunol 179:3047–3056. doi: 10.4049/jimmunol.179.5.3047 [DOI] [PubMed] [Google Scholar]
- 19. Calascibetta F, Micci L, Carnathan D, Lawson B, Vanderford TH, Bosinger SE, Easley K, Chahroudi A, Mackel J, Keele BF, Long S, Lifson J, Paiardini M, Silvestri G. 2016. Antiretroviral therapy in simian immunodeficiency virus-infected sooty mangabeys: implications for AIDS pathogenesis. J Virol 90:7541–7551. doi: 10.1128/JVI.00598-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ling B, Apetrei C, Pandrea I, Veazey RS, Lackner AA, Gormus B, Marx PA. 2004. Classic AIDS in a sooty mangabey after an 18-year natural infection. J Virol 78:8902–8908. doi: 10.1128/JVI.78.16.8902-8908.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chahroudi A, Cartwright E, Lee ST, Mavigner M, Carnathan DG, Lawson B, Carnathan PM, Hashempoor T, Murphy MK, Meeker T, Ehnert S, Souder C, Else JG, Cohen J, Collman RG, Vanderford TH, Permar SR, Derdeyn CA, Villinger F, Silvestri G. 2014. Target cell availability, rather than breast milk factors, dictates mother-to-infant transmission of SIV in sooty mangabeys and rhesus macaques. PLoS Pathog 10:e1003958. doi: 10.1371/journal.ppat.1003958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chahroudi A, Meeker T, Lawson B, Ratcliffe S, Else J, Silvestri G. 2011. Mother-to-infant transmission of simian immunodeficiency virus is rare in sooty mangabeys and is associated with low viremia. J Virol 85:5757–5763. doi: 10.1128/JVI.02690-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, Martin JN, Deeks SG. 2009. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 23:1059–1067. doi: 10.1097/QAD.0b013e32832b514b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ke C, Lipscombe LL, Weisman A, Zhou L, Austin PC, Shah BR, Booth GL. 2022. Trends in the association between diabetes and cardiovascular events, 1994-2019. JAMA 328:1866–1869. doi: 10.1001/jama.2022.14914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palella Jr FJ, Phair JP. 2011. Cardiovascular disease in HIV infection. Curr Opin HIV AIDS 6:266–271. doi: 10.1097/COH.0b013e328347876c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pandrea I, Cornell E, Wilson C, Ribeiro RM, Ma D, Kristoff J, Xu C, Haret-Richter GS, Trichel A, Apetrei C, Landay A, Tracy R. 2012. Coagulation biomarkers predict disease progression in SIV-infected nonhuman primates. Blood 120:1357–1366. doi: 10.1182/blood-2012-03-414706 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 and S2.