Abstract
Continuous exposure to cold leads to activation of adaptive thermogenesis in brown adipose tissue but also to induction of brown/beige cell phenotype in white adipose tissue. The aim of this work was to investigate whether prior exposure to immobilization (IMO) stress may affect immune response associated with adipocyte “browning” in mesenteric adipose tissue (mWAT). In the first experiment, Sprague–Dawley rats were exposed to acute (3 h) or prolonged (7 days) cold exposure (4 ± 1 °C). 7-day cold stimulated gene expression of uncoupling protein 1 and other “browning”-associated factors. In the second experiment, rats were immobilized for 7 days (2 h daily) followed by exposure to continuous cold for 1 or 7 days. Prior IMO exaggerated cold-induced sympathetic response manifested by elevated tyrosine hydroxylase (TH) protein and norepinephrine in mWAT. Induction of non-sympathetic catecholamine production demonstrated by elevated TH and PNMT (phenylethanolamine N-methyltransferase) mRNAs was observed after 7-day cold; however, prior IMO attenuated this response. 7-day cold-induced gene expression of anti-inflammatory mediators (IL-4, IL-13, IL-10, adiponectin), markers of M2 macrophages (Arg1, Retnlα), and eosinophil-associated molecules (eotaxin, IL-5), while inhibited expression of pro-inflammatory cytokines (IFNγ, IL-1b, IL-6, IL-17) and monocytes (MCP-1, Ly6C). This immune response was accompanied by elevated expression of uncoupling protein-1 and other thermogenic factors. Rats exposed to prior IMO exhibited inhibition of cold-induced immune and “browning”-related expression pattern. Overall, we demonstrated that 7-day cold-induced browning”-associated changes in rat mWAT, while prior history of repeated stress prevented this response.
Keywords: Adipose tissue, Immobilization, Cold exposure, Uncoupling protein, Heterotypic stressor, Inflammatory mediators, Macrophages
Introduction
Repeated exposure to various stressors has adverse effects on human health and represents a significant risk factor in the development of cardiovascular, metabolic, autoimmune, neurologic, and other diseases. The mechanisms of stress-induced pathological responses involve sustained or maladaptive reactivity of the sympathetic nervous system, hypothalamic-pituitary-adrenocortical (HPA) axis and other endocrine and immune factors. Activation of stress systems in the periphery results in a rapid energy mobilization mediated primarily by catecholamines (CAs) released locally from sympathetic nerves or by uptake from the circulation which originate from both, sympathetic nerves and adrenal medulla. CA interaction with β-adrenergic receptors activates lipolysis in the white adipose tissue (WAT), while inducing the uncoupling activity leading to thermogenesis in brown adipose tissue (BAT) and WAT. Some of WAT depots in rodents and humans contain cells that can express functional uncoupling protein (UCP1) and exhibit BAT-like multilocular lipid droplet morphology. These cells are called brown-in-white (brite) cells, beige cells, or adaptive or recruitable brown adipocytes (Seale et al. 2008). Beige adipocytes are distinct from classical brown adipocytes as they are not derived from Myf5-positive progenitor cells (Petrovic et al. 2010). Depending on their location, the beige adipocytes may differentiate from various cell-types or progenitors including: white adipocytes (Barbatelli et al. 2010), Sca-1+ progenitor cells (Schulz et al. 2011), smooth muscle cells Myh11+ progenitors (Long et al. 2014), or PDGFRα+ cells (Lee et al. 2012). Beige phenotype of adipose cells was usually observed in subcutaneous depots but very low expression of UCP1 has been found also in visceral WATs. The special type of WAT is perivascular adipose tissue (PVAT) which surrounds most of the vasculature and is very alike rodent BAT and expresses high level of thermogenic genes such as UCP1 or peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α). The process of WAT “browning” is induced primarily by exposure to cold environment or by stimulation with β-adrenergic agonists (Cousin et al. 1992; Loncar 1992; Young et al. 1984). Mediators or factors that can induce beige adipogenesis and functional uncoupling include norepinephrine (Qiu et al. 2014), thyroid hormone T3 (Bianco and McAninch 2013; Alvarez-Crespo et al. 2016), lactate (Carrière et al. 2014), γ-amino butyric acid, irisine, and other factors (Wang et al. 2016). PGC-1α, a transcriptional coactivator of UCP1 protein appears to be a significant regulator of uncoupling respiration in BAT or WAT (Lin et al. 2004; Kleiner et al. 2012). Recently, innate immune system is recognized as a central player in the regulation of beige and brown adipose tissue function (Alexaki and Chavakis 2016; van den Berg et al. 2017; Lee et al. 2015). Nguyen et al. (2011) described the role of alternatively activated macrophages (M2 class) producing and secreting catecholamines during cold exposure to induce thermogenic changes in the brown while enhancing the lipolysis in the white adipose tissue. A balance between classically activated macrophages exhibiting pro-inflammatory response (M1 class) and anti-inflammatory M2 macrophage populations appears to be a key regulator of thermogenesis in BAT or WAT. Type 2 cytokines (IL-4, IL-5 and IL-13) are the major stimuli for M2 macrophage polarization and subsequent “browning” process. IL-5 production by type 2 innate lymphoid cells (ILC2 s) promotes survival and proliferation of eosinophils, which on the other hand produce IL-4 and alongside the ILC2 s-derived IL-13 supports alternative activation of macrophages and proliferation of PDGFRα stromal cells (Wu et al. 2011; Lee et al. 2015; Molofsky et al. 2013; Nussbaum et al. 2013). Furthermore, IL-4 triggers tyrosine hydroxylase (TH) activation and thus norepinephrine secretion by M2 macrophages, which in turn interacts with β3-adrenergic receptors (ARs) activating cAMP-PKA-p38 MAPK pathway which stimulates thermogenic response in brown/beige adipose cells. Sympatho-adrenomedullary and thyroid systems act synergistically during adaptation to cold. Endogenous catecholamines during adaptation to cold raise iodothyronine deiodinase (DIO2) activity in brown fat due to α1-adrenergic receptor stimulation. Saturation of thyroid-hormone receptors with DIO2-generated T3 in BAT stimulates expression of UCP1, a T3-dependent gene (Bianco et al. 1988; Bianco and McAninch 2013). Mesenteric adipose tissue (mWAT) contains abundant perivascular-originating adipocytes and exhibits high immunoendocrine activity affecting metabolic, as well as inflammatory bowel diseases (Bertin et al. 2010). mWAT inflammation is mostly related to both, insulin resistance and metabolic syndrome in contrast to other visceral fat depots (Kranendonk et al. 2015). We previously showed that mWAT of rats continuously exposed to cold temperature (4 ± 1 °C) for 7 days exhibited adipocyte “browning”-specific gene expression profile suggesting a typical innate immune response associated with browning and up-regulation of thermogenic genes, UCP1 and PGC1α, as described by group of Chawla (Nguyen et al. 2011; Qiu et al. 2014). Since we previously focused on mWAT and its stress-induced catecholamine production (Kvetnansky et al. 2012; Vargovic et al. 2013) as well as stress-induced inflammatory response in mWAT (Vargovic et al. 2016a, 2016b), both involved in adipocyte “browning”, the aim of this study was to evaluate the effect of prior exposure to repeated psychological stressor—immobilization on cold-induced changes associated with brown/beige phenotype of adipocytes in rat mWAT.
Materials and Methods
Animals
In the all experiments, we used male Sprague–Dawley rats, 4 months old (300–350 g, Charles River, Suzfeld, Germany). Animals were housed two per cage in a controlled environment (23 ± 1 °C, 12 h light–dark cycle, lights on at 6 AM) 1 week before the experiments. The animals received care in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. All experimental procedures with animals used in this study were approved by the State Veterinary and Food Administration of the Slovak Republic (Approval No. Ro 2804/07-221/3).
Stress Procedures
To find out whether an acute or a chronic cold exposure can induce browning-associated changes in mWAT, the rats were initially exposed to either acute cold (4 ± 1 °C) for 3 h or chronic cold for 7 days. These experimental groups were sacrificed by decapitation after termination of cold exposure directly in the cold chamber at the same day as the control group housed at 23 °C. To investigate adipocyte-specific changes associated with “browning”, the adipocyte fraction was obtained from mWAT by collagenase digestion, as described previously (Vargovic et al. 2011). The markers of beige adipocytes or of thermogenic response which were markedly altered by cold exposure were further analyzed in the next experiment combining prior repeated IMO with the cold exposure. However, since 3-h cold was not sufficient to activate many factors evaluated, we decided to use longer exposures (1 or 7 days). For all the other analyses, we used whole pads of mWAT, which in addition to adipocytes also contain other cells (e.g., macrophages) that play an essential role in adipocyte browning.
Thus, in the next batch of experiments the rats were initially exposed to 2-h immobilization (IMO) for 7 consecutive days and then challenged with 1 or 7-day cold exposure. IMO, which represents an intensive emotional stressor, was performed as described previously (Kvetnansky and Mikulaj 1970). Repeated (2 h for 7 consecutive days) rather than a single IMO (2 h) was selected based on our previous observations showing a pronounced stimulation of sympathoadrenal and hypothalamus-pituitary-adrenocortical systems.
Accordingly, the cohort of animals consisted of six groups (7–8 rats per group):
Untreated control
Cold exposure for 1 day (24 h)
Cold exposure for 7 days
7xIMO + 1 day at room temperature (RT)
7xIMO + 1 day cold
7xIMO + 7 days cold
Catecholamine Determination
Catecholamine concentration was measured in mWAT samples homogenized in 0.01 M HCl. Supernatants were used for catecholamine determination using 2-CAT ELISA kits (Labor Diagnostica Nord, Nordhorn, Germany) as described previously (Vargovic et al. 2011). Values were normalized to the cytosolic protein in homogenate determined by bicinchoninic acid (BCA) Protein Assay (Thermo Scientific, Rockford, IL).
RNA Isolation and Relative Quantification of mRNA Levels by Real-Time RT-PCR
Total RNA from mWAT samples as well as the reverse transcription and the real-time PCR were performed as described previously (Vargovic et al. 2011, 2013). For PCR amplifications, aliquots containing 20 ng of total RNA were used. Tyrosine hydroxylase (TH) and phenylethanolamine N-methyltransferase (PNMT) were amplified using specific TaqMan probe (Applied Biosystems) (Rn00562500_m1 for TH and Rn01495588_m1for PNMT) and Maxima Probe/ROX qPCR Master Mix (Applied Biosystems) according to manufacturer´s protocol. All other transcripts were measured using SYBR Green Mastermix (Bioline) with specific primers (5 pmol) in 10 μl of final volume. The primer sequences are displayed in Table 1. Data were analyzed with SDS software version 2.3 (Applied Biosystems) according to the ΔΔCT method described by Livak and Schmittgen (2001). Expressions of analyzed transcripts were normalized to average values of three internal controls, i.e., tata-binding protein (TBP), glyceraldehyde-3-phospate dehydrogenase (GAPDH), and ribosomal protein S29 (RPS29).
Table 1.
Primers used in real-time RT-PCR
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| Adiponectin | GGGAGACGCAGGTGTTCTTG | CTGAATGCTGAGTGATACATGTAAGC |
| Arginase1 | CCCGTGGTCTCTCACATTG | CCGCAGCATTAAGGAAAGC |
| β3-AR | GCAACCTGCTGGTAATCACA | GGATTGGAGTGACACTCTTG |
| Cox8β | GTGCGACCCCGAGAATCAT | TGGCTTGGAAGAGATACGGG |
| DIO2 | CTTCCTGGCGCTCTATGACT | CCCAATTTAACCTGTTTGTAGGC |
| Eotaxin | CACCCAGGTTCCATCCCAAC | GCTTTCAGCGTGCATCTGTT |
| F4/80 | ATAATCGCTGCTGGCTGAAT | AGACTGGCCCCAAGAAACTC |
| Fgf21 | CCTTGAAGCCAGGGGTCATT | GGATCAAAGTGAGGCGATCCA |
| IL1b | GACCTGTTCTTTGAGGCTGACA | CTCATCTGGACAGCCCAAGTC |
| IL4 | GGCTTCCAGGGTGCTTCGCAAAT | TGTGAGCGTGGACTCATTCACGG |
| IL4r | TGAACACACAGGTGCTGGAGAG | AGGTGCCTGGGCTATACAGG |
| IL5 | TGTTGACGAGCAATGAGACGA | CCCCCTCGGACAGTTTGATT |
| IL6 | ATACCACCCACAACAGACCAGT | GATGAGTTGGATGGTCTTGGT |
| IL10 | TGGATGTGGTAGCCGTTTCTCAGG | AACTGCACCCACTTCCCAGT |
| IL13 | GATCCACATCTCCCCCTGTG | GGGAAGTCTTCTGGTCTTGTGT |
| IL17 | CTCTTGCTGGATGAGAACAGAAT | TACTCATCCCTCAAAGTTCAGTGT |
| LY6C | TGCAGAAAGAGCTCAGGGC | TGCTCTTTGCTGTCCGTCTT |
| MCP1 | TCCACCACTATGCAGGTCTC | CATTAACTGCATCTGGCTGAG |
| Metrnl | AGGACCACAGGCTTCCAGTA | AGCACGTGCAGGTCCAGT |
| PGC1α | TGCAGCGGTCTTAGCACTCA | CATGAATTCTCGGTCTTAACAATGG |
| Retnlα | GGCACGAGGGGACACTGA | AGGGATAGTTATCTTGATGGGCT |
| Tmem26 | AGTATTGCAGCACGCAGTCT | AAAGCCTCTGGCCAGTTCAA |
| UCP1 | TGCCTAGCAGACATCATCACC | TGGCCTTCACCTTGGATCTGAA |
| GAPDH | AGATCCACAACGGATACATT | TCCCTCAAGATTGTCAGCAA |
| RPS29 | GGTCGCTTAGTCCAACTTAATGAA | GCTGAACATGTGCCGACAGT |
| TBP | TTCGTGCCAGAAATGCTGAA | GTTCGTGGCTCTCTTATTCTCATG |
Western Blot Analysis
Samples of mWAT (100–200 mg) were homogenized in 2 ml of 20 mM Tris pH 7.0; 5 mM PMSF (Pefabloc SC + Compete, Roche Diagnostic), incubated on ice for 1 h and centrifuged for 20 min (16,100×g, 4 °C) in order to extract the cytosolic protein fraction. Western blot was performed as described previously (Laukova et al. 2010). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control. We used the following primary antibodies: monoclonal anti-TH (1:3000; MAB5280, Chemicon International, Temecula, CA) and monoclonal anti-GAPDH (1:5000, MAB374, Chemicon International, Temecula, CA). Membranes were incubated with anti-mouse horseradish peroxidase-linked secondary antibodies (ECL, Amersham Biosciences) diluted 1:5000 for 1 h at room temperature. The signals were detected and evaluated as described by Laukova et al. (2010).
Statistical Analysis
Statistical differences were determined by one-way or two-way analysis of variance (ANOVA) followed by Bonferonni post hoc test [SigmaStat, version 3.1, Systat Software, Inc., USA]. Results are presented as mean ± SEM. and each value represents 6–8 rats. Values of * p < 0.05, ** p < 0.01, *** p < 0.001 defined the statistical significance versus control group and values of + p < 0.05, ++ p < 0.01, +++ p < 0.001 for non-immobilized and immobilized groups.
Results
Cold Exposure Induces Gene Expression of “Browning” Factors in Mesenteric Adipose Tissue of Rats
In the first experiment, we analyzed the gene expression of several factors related to production of brown/beige fat phenotype in adipocyte fraction of mWAT. This analysis was performed using freshly isolated adipocyte fractions obtained from rat mWAT. 7-day long exposure to the cold (4 ± 1 °C) elevated gene expression of the following beige adipocyte markers: UCP1 (p < 0.01, Fig. 1a), PPAR-c coactivator 1α (PGC1α, p < 0.01), cytochrome c oxidase subunit 8β (COX8b, p < 0.01), transmembrane protein 26 (TMEM26, p < 0.05). 3-h cold exposure increased only expression of COX8β (p < 0.05). Based on the current literature, we also evaluated other factors proposed to induce brown/beige adipocyte phenotype, such as iodothyronine deiodinase type 2 (DIO2), which converts T4 into biologically active T3 (Nedergaard et al. 1997; Martínez-Sánchez et al. 2017), meteorine-like (Metrnl) which is produced by muscle or adipose cells during exercise and induces brown/beige phenotype in WAT (Rao et al. 2014) and eotaxin, the chemokine attracting eosinophils which contribute to induction of adipocyte “beiging” (Qiu et al. 2014). Acute 3-h cold stimulated expression of eotaxin (p < 0.05), while 7-day cold up-regulated expression of DIO2 (p < 0.01), Metrnl (p < 0.05), and eotaxin (p < 0.01).
Fig. 1.

Expression of “browning”-associated factors in mesenteric adipose tissue of rats a Effect of acute (3 h) or prolonged (7 days) cold (4 °C) on gene expression of UCP1, PGC1α, Cox8b, Tmem26, Fgf21, DIO2, Metrnl, eotaxin in adipocyte fraction. b Effect of cold exposure (4 °C; 1 or 7-day) on gene expression of UCP1, PGC1α, Cox8b, Tmem26, DIO2, and Metrnl in unstressed rats (rest) or previously immobilized rats (prior IMO). Values of relative expression are evaluated by ΔΔCt method. Each column is displayed as mean ± SEM. and represents an average of 6–8 animals. Values of * p < 0.05, ** p < 0.01 or *** p < 0.01 defined the statistical significance versus control groups (housed at 23 °C) and values of + p < 0.05 immobilized versus non-stressed group at same cold interval (1- or 7- days)
In the next experiment, the whole pads of mWAT were analyzed after exposing the rats to repeated IMO followed by 1- or 7-day cold. The key thermogenic factor UCP1 showed 4-times higher expression after 7-day cold exposure (p < 0.05) but prior repeated IMO completely blocked this response in the mWAT (Fig. 1b, p < 0.05). PGC1α transcript showed augmented expression in the rats exposed to repeated IMO and kept at room temperature (p < 0.05) as well as in non-stressed animals exposed to 7-day cold (p < 0.05). Combination of IMO and cold exposure caused a decrease (p < 0.05) of IMO-induced PGC1α mRNA level to levels similar to those in non-stressed rats exposed to cold only. Similarly, the beige adipocyte marker, Tmem26, showed up-regulated mRNA levels (p < 0.05) in IMO group without cold exposure as well as in the rats exposed to 1-day cold only. Combination of cold and IMO did not produce any significant changes. Another beige adipocyte marker, Cox8b, displayed attenuated expression in rats exposed to prior IMO and not exposed to cold (p < 0.05) or rats exposed to 7-day cold (p < 0.05). DIO2 mRNA also showed reduced level in IMO group housed at room temperature (p < 0.05) or exposed to 7-day cold (p < 0.05). The levels of mRNA for Metrnl did not show any alterations after the cold exposure but rats exposed to both, repeated IMO and 7-day cold exhibited lower (p < 0.05) Metrnl mRNA levels as compared with non-immobilized rats exposed to 7-day cold.
Effect of Cold Exposure on Sympathetic and Non-sympathetic Catecholamine Production in Mesenteric Adipose Tissue
To evaluate the effect of cold exposure on catecholaminergic system, we analyzed tissue levels of norepinephrine (NE) and epinephrine (EPI), protein level of tyrosine hydroxylase (TH), gene expressions of TH and PNMT as well as β3- adrenergic receptor (Fig. 2).
Fig. 2.
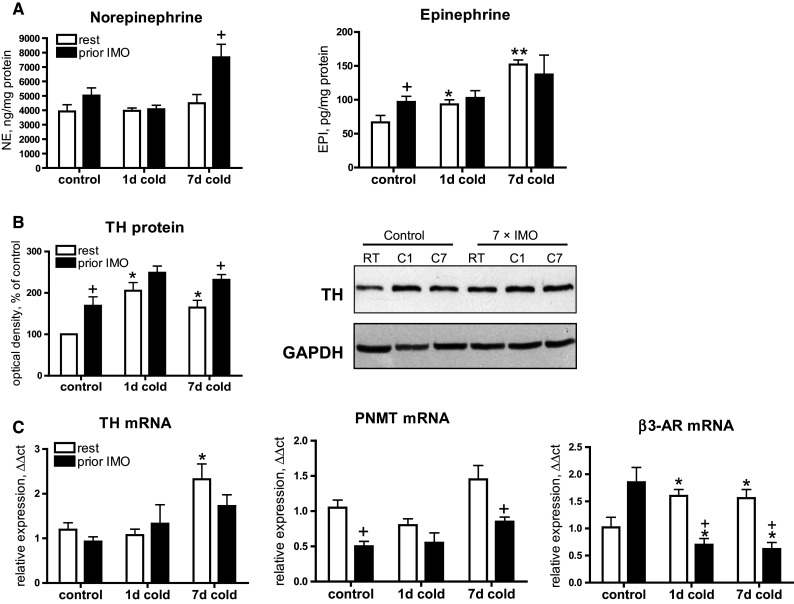
Effect of cold exposure (1 or 7-day) on sympathetic and non-sympathetic catecholamine system in mesenteric adipose tissue of non-stressed rats (rest) or previously immobilized rats (prior IMO). a Norepinephrine (NE) and epinephrine (EPI) concentrations in mWAT. b Western blot of tyrosine hydroxylase (TH) in protein homogenates of mWAT (10 µg of soluble cytosolic fraction). Loading control: glyceraldehyde-3-phosphate dehydrogenase (GAPDH). c Gene expression of TH and PNMT reflecting non-sympathetical catecholamine production in mWAT. d Gene expression of β3-adrenergic receptors. Each column is displayed as mean ± SEM. and represents an average of 6–8 animals. Values of * p < 0.05 or ** p < 0.01 defined the statistical significance versus control group (housed at 23 °C) and values of + p<0.05, immobilized versus non-stressed group at same cold interval (1- or 7- days)
Cold exposure of non-stressed rats did not affect norepinephrine (NE) concentrations but elevated epinephrine levels (EPI) after 1-day (p < 0.05) or 7-day cold (p < 0.01, Fig. 2a). Repeated IMO elevated EPI (p < 0.05) but in combination with the cold it did not show any changes. NE levels were not affected by IMO or cold while combination of IMO and cold produced a significant up-regulation (p < 0.05). TH which is the first and rate–limiting enzyme involved in catecholamine biosynthesis showed elevated protein levels in response to cold exposure as well as to IMO (Fig. 2b). Cold exposure elevated TH level after 1-day (p < 0.05) or 7-day exposure (p < 0.05). Repeated IMO alone also elevated TH protein (p < 0.05). Moreover, prior IMO significantly exaggerated TH level (p < 0.05) induced by 7-day cold.
In order to assess de novo synthesis of catecholamines in mWAT, we also analyzed TH and PNMT expressions which reflect a non-sympathetic source of catecholamine production, such as by macrophages, adipocytes, and other cells, as reported previously (Nguyen et al. 2011; Vargovic et al. 2011; Ayala-Lopez et al. 2014). Real-time RT-PCR analysis (Fig. 2c) showed that 7-day cold induces rise (p < 0.05) of TH mRNA in the rats not exposed to prior IMO, while IMO rats did not show significant response to subsequent 1- or 7-day cold exposure. PNMT expression was not affected by cold exposure but prior IMO caused decreased PNMT mRNA in control rats and in rats exposed to 7-day cold (both p < 0.05).
Next, we examined the expression of β3-ARs, activation of which is essential for stimulation of thermogenic response mediated by PGC1α and UCP1 (Cannon and Nedergaard 2004). Exposure to 1 or 7-day cold induced a rise of β3-AR mRNA (p < 0.05), while down-regulation of these receptors was observed after IMO followed by 1- or 7-day cold challenge (both p < 0.05).
Immune Cells and Inflammatory Factors Controlling Adipocyte “Browning”
To investigate the effect of stress on cold-induced immune response associated with adipocyte browning, we evaluated gene expression of pro- and anti-inflammatory factors, as well as chemokines and markers of immune cells potentially participating in immune response associated with “browning” mechanism. Cold exposure significantly affected gene expression of pro- and anti-inflammatory factors in mWAT (Fig. 3). Exposure to 1-day cold only produced a mild immune response represented by down-regulation of pro-inflammatory cytokine interferon gamma (IFNγ, p < 0.05) and up-regulation of anti-inflammatory M2-macrophage markers, arginase 1 (Arg1, p < 0.05), and resistin-like alpha protein (Retnlα, p < 0.05). A substantial immunomodulation was induced by 7-day cold exposure manifested by elevated expression of following anti-inflammatory factors: Arg1 (p < 0.05), Retnlα (p < 0.05), interleukin 4 (IL-4, p < 0.05), interleukin 5 (IL-5, p < 0.05), interleukin 13 (IL-13 p < 0.05) and adiponectin (p < 0.05), and reduced expression of pro-inflammatory cytokines: IFNγ (p < 0.05), interleukin 1β (p < 0.05) interleukin 6 (p < 0.05) and interleukin 17 (p < 0.05). Previous exposure to repeated IMO significantly modulated immune response induced by cold exposure. Cold-induced inhibition of IFNγ expression was not observed in IMO rats exposed to cold. Most of the anti-inflammatory effects observed after 7-day cold exposure were not present in the rats exposed to both prior IMO and subsequent 7-day cold.
Fig. 3.
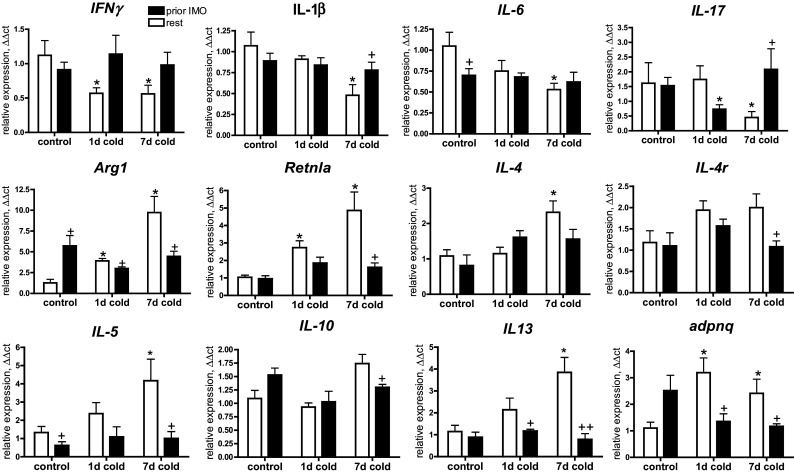
Effect of cold exposure combined with prior IMO experience on the gene expression of anti-inflammatory (Arg1, Retnla, IL4, IL4R, IL13, IL5, IL10) and pro-inflammatory (IFNγ, IL1β, IL6, IL17) factors in mWAT. Each column is displayed as mean ± SEM. and represents an average of 6–8 animals. Values of * p < 0.05 defined the statistical significance versus control groups and values of + p < 0.05, ++ p < 0.01 resting versus immobilized groups
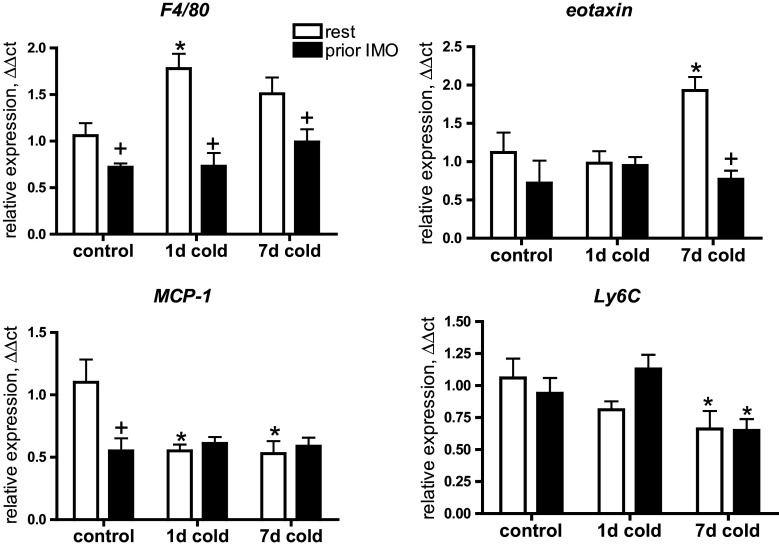
Next, we evaluated changes in chemokines and markers of immune cells potentially involved in immune regulation of “browning” (Fig. 4). Expression of F4/80 reflecting resident macrophages in adipose tissue (Khazen et al. 2005) was up-regulated by 1-day cold exposure in non-IMO rats, while significantly lower (p < 0.05) F4/80 mRNA levels were observed in all groups exposed to IMO. Eotaxin, a chemokine for eosinophils showed elevated (p < 0.05) expression in the rats exposed to 7-day cold but prior IMO completely attenuated (p < 0.05) this response. Macrophage chemoattractant protein (MCP-1) showed down-regulation in all IMO and cold-exposed rats (p < 0.05 for all). Peripheral monocytes analyzed using Ly6C expression showed a decreased mRNA levels in both IMO and unstressed rats exposed to 7-day cold (both p < 0.05).
Fig. 4.
Effect of cold exposure combined with prior IMO experience on immune cells markers and chemokines in mWAT. Gene expression of resident macrophages marker (F4/80), MCP1—macrophage chemoatractant protein, Ly6C—a marker of circulating monocytes. Each column is displayed as mean ± SEM. and represents an average of 6–8 animals. Values of * p < 0.05 defined the statistical significance versus control groups and values of + p < 0.05 resting versus immobilized groups
A link between immune response and thermogenesis-associated changes (UCP1 expression) was confirmed by positive correlations between the expression of UCP1 and IL4/eotaxin (Fig. 5).
Fig. 5.
Correlations between gene expressions of UCP1 and IL-4 or eotaxin
Changes in Body Weight and Mesenteric Adipose Tissue Mass
Exposure to both stressors produced alterations of the body weight as well as of the mWAT mass (Fig. 6). Repeated IMO significantly reduced body weight (p < 0.01). Cold exposure also decreased the body weight (p < 0.05 for both 1 and 7-day cold). Moreover, rats which were subjected to both IMO and cold exposure exhibited the lowest body weights. On the other hand, almost all the rats gained their body weight during the experiment (14 days in total). The weight gain was negatively proportional to duration of stress (cold or/and IMO) exposure (Fig. 6 middle). IMO alone decreased the weight gain (p < 0.05), but prior IMO-induced even smaller weight gain following 1-day (p < 0.01) or 7-day (p < 0.05) cold comparing with cold exposure only.
Fig. 6.

Effect of cold exposure combined with prior IMO experience on the body and mesenteric adipose tissue weights. (Left) Body weight determined at the end of experiment. (Middle) Relative body weight gain normalized to initial body weight. (Right) Relative weight (g/100 g of body weight) of mesenteric adipose tissue pads (mWAT) after dissection. (Down) Correlations between TH protein levels determined by Western blot and body weight gain (left) or mWAT weight (right). Each column is displayed as mean ± SEM. and represents an average of 6–8 animals. Values of * p < 0.05, ** p < 0.01 defined the statistical significance versus control groups and values of + p < 0.05, ++ p < 0.01 resting versus immobilized groups
Both stressors (IMO and cold) significantly affected mWAT mass. 7-day cold exposure as well as repeated IMO alone reduced mWAT weight (both p < 0.01). Marked loss of mWAT mass was observed in the rats exposed to both prior IMO and subsequent 1 or 7-day cold exposure (both p < 0.01 compared to unstressed rats exposed to cold). Body weight gain or mWAT weight negatively correlated with TH protein level in mWAT (Fig. 6) suggesting that energy metabolism in mesenteric fat is primarily regulated by sympathetic activity.
Discussion
Prolonged or repeated exposure to psychological stressors has been associated with either weight gain or weight loss (Razzoli and Bartolomucci 2016). Uncoupling activity found in BAT but also in WAT is an effective regulatory factor determining fat mass and therefore metabolic health. Sympathetic system activity is crucial in the regulation of energy metabolism in WAT including uncoupling-associated energy dissipation utilized in heat production during adaptive thermogenesis. Chronic psychological stress exposure can result in abnormal or maladaptive response of sympathoadrenal and hypothalamus-pituitary-adrenocortical systems suggesting pathological consequences in the energy metabolism and particularly in the regulation of overall neuro-immuno-endocrine homeostasis. We have previously shown that mesenteric adipose tissue (mWAT) shares the key features of brown-fat phenotype such as UCP1 and PGC1α expressions that are up-regulated during long-term cold exposure (Vargovic et al. 2016b). Activation of UCP1 expression by 7-day cold exposure was accompanied by up-regulated expression of anti-inflammatory factors potentially linked to alternatively activated macrophages, similarly as described previously (Nguyen et al. 2011; Qiu et al. 2014). In the present study, we have addressed the role of psychological stress in the cold-induced “browning”-associated changes found in mWAT (Vargovic et al. 2016b). Since cold exposure is a significant activator of sympathetic system producing norepinephrine which induces UCP1 expression and thermogenesis in BAT/WAT, we assumed that combined effect of psychological stress and cold exposure would affect “browning”-associated program in mWAT. It is known that prior stress experience alters the response to subsequent homotypic or heterotypic stressor (Sabban and Serova 2007). Repeated exposure to homotypic stressor usually results in adaptation of stress systems, but exposure to heterotypic stressors may result in exaggerated or maladaptive response. (Dronjak et al. 2004; Spiga et al. 2009). For example, when cold-acclimated rats are exposed to a new acute stressor such as immobilization stress, the increase in plasma EPI and NE concentrations in response to the novel stressor is substantially greater than that in naive animals exposed to the same stressor (Dronjak et al. 2004). Exaggerated or maladapted response of the main stress-responding systems (sympathoadrenal system and HPA axis) very likely results in changes in immunomodulation, particularly, in the equilibrium between pro-and anti-inflammatory responses (e.g., M1/M2 macrophages or Th1/Th2 responses). We previously showed that prior exposure to a single or repeated IMO significantly affects inflammatory response in mWAT elicited by lipopolysaccharide challenge (Vargovic et al. 2016a). In the present study, we showed that cold-induced anti-inflammatory modulation in mWAT (Fig. 3) manifested by augmented expression of markers specific for M2-polarized macrophages (Arg1, Retnlα) and anti-inflammatory cytokines (IL-4, IL-13, IL-5), while inhibiting expression of pro-inflammatory factors (IFNγ, IL-1β, IL-6, IL-17). This observation is in agreement with previous studies describing the mechanism of WAT “browning” (Nguyen et al. 2011; Qiu et al. 2014). Additionally, since we observed a cold-induced expression of chemokine attracting eosinophils (eotaxin) as well as IL-5 which facilitates recruitment of eosinophils into adipose tissue (Molofsky et al. 2013), eosinophils are very likely the main source of IL-4 induced by a cold challenge. This is very similar to the previous studies describing eosinophil-derived IL-4 which was required for activation of M2 macrophages in adipose tissue (Wu et al. 2011; Qiu et al. 2014). Furthermore, we observed cold-induced expression of tyrosine hydroxylase (Fig. 2c) suggesting potential activation of cold-induced catecholamine production in M2 macrophages as described by Nguyen et al. (2011). These cold-regulated mechanisms were accompanied by up-regulation of thermogenic genes UCP1 and PGC1α, similarly as described in aforementioned studies.
In addition to confirming the cold-induced mechanisms of adipocyte browning in mWAT we observed a significant effect of prior repeated stress in modulation of immune and browning response induced by cold exposure. We found out that prior IMO completely attenuated cold-induced up-regulation of anti-inflammatory factors and M2 macrophage markers while stimulating expression of pro-inflammatory cytokines (Fig. 3). We also showed that prior IMO inhibited cold-induced expression of tyrosine hydroxylase, an rate-limiting catecholamine biosynthetic enzyme which is linked to thermogenic response in adipose tissues (Fig. 2c). Epinephrine-synthesizing enzyme PNMT did not show cold-induced expression, but its expression in the rats exposed to both prior IMO and cold was significantly lower comparing rats exposed to cold only. Also, epinephrine concentration in mWAT increased after cold exposure, but in IMO rats, epinephrine did not respond to subsequent cold challenge. Despite all the known facts about norepinephrine role in UCP1—mediated thermogenesis, a specific role of epinephrine has not been described yet. This IMO/cold-associated expression pattern was associated with co-expression of thermogenesis/browning-associated genes UCP1, PGC1α, Cox8b, DIO2. Thus, these data suggest that repeated intensive stress can disrupt normal immune response to the cold in mWAT. We can propose a few explanations of these stress-induced effects on cold-associated immunomodulation observed here. IMO exposure is an intensive psychological stressor altering main components of stress systems such as sympathetic nervous system and HPA axis. It is known that repeated or chronic activation or a malfunctioning stress system characterized by sustained hyper- or hypoactivity may contribute to various pathological abnormalities across a wide range of organ systems, potentially resulting in endocrine, inflammatory, and psychiatric disorders (Vaccarino and Bremner 2005; Chrousos and Gold 1992). Based on our data, it is important to consider stress-induced abnormalities in the immune system and thus balance between pro- and anti-inflammatory local or systemic environment. It is known that balance between pro- and anti-inflammatory polarizations of macrophages (M1/M2) may determine thermogenic response in adipose tissue (Qiu et al. 2016). Macrophages colonize adipose tissues in two distinct ways: recruitment of monocyte precursors and proliferation of resident cells. Several studies suggested that the M1–M2 switch in adipose tissues is caused by different recruitment of various monocyte subtypes (Lumeng et al. 2008). In the lean body, resident macrophages expressing F4/80 are represented mostly by M2 macrophages contributing to WAT homeostasis. They produce anti-inflammatory cytokines such as IL-10, which down-regulates MCP-1 production. Cold exposure reduces M1 macrophages but also expands alternatively polarized, anti-inflammatory M2 macrophages, triggering white adipose tissue browning, fat burning, and restoration of insulin sensitivity (Qiu et al. 2016). Our data showed cold-induced elevation of F4/80 expression suggesting increased proliferation of resident macrophages in mWAT which most likely participate in the anti-inflammatory M2 response in rats exposed to 7-day cold.
However, in animals exposed to both, prior IMO and subsequent cold challenge, the polarization of macrophages rather points to a shift towards M1 state as evidenced by decreased expression of anti-inflammatory and M2 markers, marker of resident macrophages (F4/80) and also by elevated expression of pro-inflammatory cytokines. This shift is hardly a result of higher recruitment of pro-inflammatory polarized monocytes into WAT, as we found decreased expression of MCP-1 or Ly6C in all IMO or cold-exposed rats.
Although SAS and HPA functions are linked rather to anti-inflammatory modulation or immunosuppression, chronic or repeated exposures to intensive stressors or heterotypic stressors usually lead to alterations of normal stress-responses. Generally, normal stress responses are protective mechanisms to maintain homeostasis during exposure to internal or external stimuli (stressors). The system (organism, tissues, cells) can be maintained within acceptable range of variable values characterizing homeostatic state by appropriate stress-response reactions. If intensity, character or durability of stressors overwhelms a defending capacity of stress systems, a maladaptive response or disruption of normal stress response may occur with pathophysiological consequences. These mechanisms usually trigger inflammatory response that can result in further alterations of system homeostasis causing diseases (Chovatiya and Medzhitov 2014). According to our data, we can consider a cold challenge a natural stressor inducing adequate response of sympathetic system and other systems leading to gene expression pattern typical for anti-inflammatory response and recruitment of brown/beige cell phenotype in mWAT, while prior IMO appears to be a significant disruptor of these responses. We previously reported (Vargovic et al. 2013) that IMO is an intensive psychological stressor that activates sympathetic system in mWAT. Thus, repeated IMO prior to the cold exposure likely increases the sympathetic activity as demonstrated by elevated TH protein in mWAT found here, and also after the cold exposure (Fig. 2b). High sympathetic activity in mWAT was primarily associated with high metabolic activity in mWAT as evidenced by reduced body weights as well as mWAT mass, which were proportional to the duration of stress (IMO/cold) exposure (Fig. 6). Although combined effect of IMO and cold causes exaggerated response of sympathetic system resulting to the acceleration of fat/weight loss, an unknown mechanism probably induced inhibition of browning–related processes. To explain this paradigm, we can also consider changes in the expression of adrenergic receptors β3-ARs, which are essential for activation of thermogenic adipose tissue activity (Fig. 2c). Since IMO or cold alone elevated β3-ARs mRNA, combined effect of both stressors led to down-regulation of β3-ARs. Therefore, lower β3-AR levels due IMO-elicited desensitization could adequately affect activation of UCP1 transcription. On the other hand, we also showed that IMO substantially down-regulated expression of DIO2 (Fig. 1b), an enzyme producing thyroid hormone T3, which also enhances UCP1 transcription. This suggests another stress-induced mechanism of UCP1 inhibition. Overall, it appears that intensity of stress-induced activation of sympathetic nerves innervating adipose tissues and neuro-immunoendocrine axes could be crucial in BAT or brown-like phenotype recruitment and function. Different stressors trigger the HPA axis, SAS or other neuroendocrine axes with various intensity (Koolhaas et al. 2011), but direct comparison of how different stress models activate BAT sympathetic nerves or other neuroendocrine mechanisms associated with UCP1-thermogenesis is not yet available (Razzoli and Bartolomucci 2016).
However, since the innate immune system regulation is essential in the “browning” process, it is most plausible to assume that combination of IMO and cold can inhibit “browning” rather by promoting pro-inflammatory phenotype. A number of studies reported alterations in the immune functions and increased inflammation due to psychological stress (Vaccarino and Bremner 2005; Ippoliti et al. 2013; Dhabhar 2014). mWAT mostly consists of perivascular fat (PVAT) which is modestly anti-inflammatory and enhances endothelial dependent and independent vasorelaxation (Fitzgibbons and Czech 2014). Catecholaminergic system in PVAT may be an integral link connecting obesity with the vascular dysfunction observed in obesity-associated hypertension (Ayala Lopez and Watts 2016). Furthermore, it is known that UCP1 expression in adipose tissues contributes to blood pressure in humans (Li et al. 2015). The PVAT depot expand in obesity and in diabetes, in conjunction with the accumulation of inflammatory cells and production of various inflammatory mediators leading to altered PVAT functions or pathophysiological consequences associated with atherosclerosis and hypertension (Chang et al. 2013). Since PVAT functions are in close relationship with brown-like characteristics, chronic stress-elicited alterations of inflammatory regulation or other physiological mechanisms controlling browning-associated responses could contribute to PVAT dysfunction associated with health disorders and leading to metabolic, cardiovascular, and other diseases. On the other hand, recent studies indicated that beige adipocyte activity affects systemic metabolism and contributes significantly to whole body insulin sensitivity (Qiu et al. 2014; Cohen et al. 2014).
In summary, present data confirmed our previous observation that 7-day cold exposure induces anti-inflammatory response as well as expression of BAT thermogenesis- associated genes and beige adipocyte markers in rat mWAT. Furthermore, we found out that prior exposure to repeated IMO completely attenuates both anti-inflammatory and thermogenic gene expression pattern suggesting stress-induced mechanisms preventing the cold-induced immunoendocrine response in mWAT and formation of beige phenotype. Our findings suggest a novel mechanism of stress-regulated metabolic activity with implications in stress-associated obesity or other stress-related metabolic diseases. However, a more detailed investigation of the key mechanisms confirming our observations is needed in the future studies.
Acknowledgements
This research was supported by Slovak Research and Development Agency (No. APVV-0088-10); and VEGA Grant (2/0067/14).
Author’s Contribution
The authors’ responsibilities were as follow: R.K., P.V., and J.U. conceived and designed the study; P.V., G.M., and J.U. contributed to optimization of methods, sample and data collection and data analysis; P.V. and M.L. wrote the manuscript; P.V., M.L., J.U., R.K. researched data and contributed to the discussion. All authors reviewed and edited the manuscript.
Compliance with Ethical Standards
Conflict of interest
No potential conflicts of interest were disclosed.
Research Involving Human and Animal Participants
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the Institute of Experimental Endocrinology at which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
References
- Alexaki VI, Chavakis T (2016) The role of innate immunity in the regulation of brown and beige adipogenesis. Rev Endocr Metab Disord 17(1):41–49 [DOI] [PubMed] [Google Scholar]
- Alvarez-Crespo M, Csikasz RI, Martínez-Sánchez N, Diéguez C, Cannon B, Nedergaard J, López M (2016) Essential role of UCP1 modulating the central effects of thyroid hormones on energy balance. Mol Metab 5(4):271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala-Lopez N, Watts SW (2016) New actions of an old friend: perivascular adipose tissue’s adrenergic mechanisms. Br J Pharmacol. doi:10.1111/bph.13663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala-Lopez N, Martini M, Jackson WF, Darios E, Burnett R, Seitz B, Fink GD, Watts SW (2014) Perivascular adipose tissue contains functional catecholamines. Pharmacol Res Perspect 2(3):e00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S (2010) The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298(6):E1244–E1253 [DOI] [PubMed] [Google Scholar]
- Bertin B, Desreumaux P, Dubuquoy L (2010) Obesity, visceral fat and Crohn’s disease. Curr Opin Clin Nutr Metab Care 13:574–580 [DOI] [PubMed] [Google Scholar]
- Bianco AC, McAninch EA (2013) The role of thyroid hormone and brown adipose tissue in energy homoeostasis. Lancet Diabetes Endocrinol 1(3):250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco AC, Sheng XY, Silva JE (1988) Triiodothyronine amplifies norepinephrine stimulation of uncoupling protein gene transcription by a mechanism not requiring protein synthesis. J Biol Chem 263:18168–18175 [PubMed] [Google Scholar]
- Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84(1):277–359 [DOI] [PubMed] [Google Scholar]
- Carrière A, Jeanson Y, Berger-Müller S, André M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, Villageois P, Louche K, Collas P, Moro C, Dani C, Villarroya F, Casteilla L (2014) Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63:3253–3265 [DOI] [PubMed] [Google Scholar]
- Chang L, Milton H, Eitzman DT, Chen YE (2013) Paradoxical roles of perivascular adipose tissue in atherosclerosis and hypertension. Circ J 77(1):11–18 [DOI] [PubMed] [Google Scholar]
- Chovatiya R, Medzhitov R (2014) Stress, inflammation, and defense of homeostasis. Mol Cell 54(2):281–288 [DOI] [PMC free article] [PubMed]
- Chrousos GP, Gold PW (1992) The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267(9):1244–1252 Erratum in: JAMA 268(2):200 [PubMed] [Google Scholar]
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ (2014) Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156:304–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Casteilla L (1992) Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 103(4):931–942 [DOI] [PubMed] [Google Scholar]
- Dhabhar FS (2014) Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res 58(2–3):193–210 [DOI] [PubMed] [Google Scholar]
- Dronjak S, Jezova D, Kvetnansky R (2004) Different effects of novel stressors on sympathoadrenal system activation in rats exposed to long-term immobilization. Ann N Y Acad Sci 1018:113–123 [DOI] [PubMed] [Google Scholar]
- Fitzgibbons TP, Czech MP (2014) Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc 3(2):e000582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippoliti F, Canitano N, Businaro R (2013) Stress and obesity as risk factors in cardiovascular diseases: a neuroimmune perspective. J Neuroimmune Pharmacol 8(1):212–226 [DOI] [PubMed] [Google Scholar]
- Khazen W, M’Bika JP, Tomkiewicz C, Benelli C, Chany C, Achour A, Forest C (2005) Expression of macrophage-selective markers in human and rodent adipocytes. FEBS Lett 579(25):5631–5634 [DOI] [PubMed] [Google Scholar]
- Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR (2012) Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc Natl Acad Sci USA 109:9635–9640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wöhr M, Fuchs E (2011) Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev 35(5):1291–1301 [DOI] [PubMed] [Google Scholar]
- Kranendonk ME, van Herwaarden JA, Stupkova T, de Jager W, Vink A, Moll FL, Kalkhoven E, Visseren FL (2015) Inflammatory characteristics of distinct abdominal adipose tissue depots relate differently to metabolic risk factors for cardiovascular disease: distinct fat depots and vascular risk factors. Atherosclerosis 239(2):419–427 [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Mikulaj L (1970) Adrenal and urinary catecholamines in rats during adaptation to repeated immobilization stress. Endocrinology 87:738–743 [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Ukropec J, Laukova M, Manz B, Pacak K, Vargovic P (2012) Stress stimulates production of catecholamines in rat adipocytes. Cell Mol Neurobiol 32(5):801–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukova M, Vargovic P, Krizanova O, Kvetnansky R (2010) Repeated stress down-regulates β(2)- and α (2C)-adrenergic receptors and up-regulates gene expression of IL-6 in the rat spleen. Cell Mol Neurobiol 30(7):1077–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova Anelia P, Mottillo Emilio P, Granneman JG (2012) In vivo identification of bipotential adipocyte progenitors recruited by beta 3-adrenoceptor activation and high-fat feeding. Cell Metab 15:480–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC et al (2015) Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160(1–2):74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu J, Wang G, Yu J, Sheng Y, Wang C, Lv Y, Lv S, Qi H, Di W, Yin C (2015) Determination of UCP1 expression in subcutaneous and perirenal adipose tissues of patients with hypertension. Endocrine 50(2):413–423 [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY (2004) Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119:121–135 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, Castellot JJ Jr, Rosen ED, Spiegelman BM (2014) A smooth muscle-like origin for beige adipocytes. Cell Metab 19(5):810–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncar D (1992) Brown adipose tissue as a derivative of mesoderm grafted below the kidney capsule. A model for differentiation of isolated rat mesoderm. Int J Dev Biol 36(2):265–274 [PubMed]
- Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR (2008) Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57:3239–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Sánchez N, Moreno-Navarrete JM, Contreras C, Rial-Pensado E, Fernø J, Nogueiras R, Diéguez C, Fernández-Real JM, López M (2017) Thyroid hormones induce browning of white fat. J Endocrinol 232(2):351–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM (2013) Innate lymphoid type 2 cells sustain visceral adiposetissue eosinophils and alternatively activated macrophages. J Exp Med 210(3):535–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Dicker A, Cannon B (1997) The interaction between thyroid and brown-fat thermogenesis. Central or peripheral effects? Ann N Y Acad Sci 813:712–717 [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A (2011) Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480(7375):104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM (2013) Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502(7470):245–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J (2010) Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285(10):7153–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Nguyen Khoa D, Odegaard Justin I, Cui X, Tian X, Locksley Richard M, Palmiter Richard D, Chawla A (2014) Eosinophils and Type 2 Cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157:1292–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Shan B, Yang L, Liu Y (2016) Adipose tissue macrophage in immune regulation of metabolism. Sci China Life Sci 59(12):1232–1240 [DOI] [PubMed] [Google Scholar]
- Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM (2014) Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157:1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M, Bartolomucci A (2016) The dichotomous effect of chronic stress on obesity. Trends Endocrinol Metab 27(7):504–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabban EL, Serova LI (2007) Influence of prior experience with homotypic or heterotypic stressor on stress reactivity in catecholaminergic systems. Stress 10(2):137–143 [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, Schrier D, Falb D, Kirkland JL, Wagers AJ, Tseng YH (2011) Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A 108(1):143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga F, Harrison LR, MacSweeney CP, Thomson FJ, Craighead M, Lightman SL (2009) Effect of vasopressin 1b receptor blockade on the hypothalamic-pituitary-adrenal response of chronically stressed rats to a heterotypic stressor. J Endocrinol 200(3):285–291 [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Bremner JD (2005) Stress response and the metabolic syndrome. Hosp Physician 11:1–11
- van den Berg SM, van Dam AD, Rensen PC, de Winther MP, Lutgens E (2017) Immune modulation of brown(ing) adipose tissue in obesity. Endocr Rev 38:46–68 [DOI] [PubMed] [Google Scholar]
- Vargovic P, Ukropec J, Laukova M, Cleary S, Manz B, Pacak K, Kvetnansky R (2011) Adipocytes as a new source of catecholamine production. FEBS Lett 585(14):2279–2284 [DOI] [PubMed] [Google Scholar]
- Vargovic P, Ukropec J, Laukova M, Kurdiova T, Balaz M, Manz B, Ukropcova B, Kvetnansky R (2013) Repeated immobilization stress induces catecholamine production in rat mesenteric adipocytes. Stress 16(3):340–352 [DOI] [PubMed] [Google Scholar]
- Vargovic P, Laukova M, Ukropec J, Manz G, Kvetnansky R (2016a) Lipopolysaccharide induces catecholamine production in mesenteric adipose tissue of rats previously exposed to immobilization stress. Stress 19(4):439–447 [DOI] [PubMed] [Google Scholar]
- Vargovic P, Manz G, Kvetnansky R (2016b) Continuous cold exposure induces an anti-inflammatory response in mesenteric adipose tissue associated with catecholamine production and thermogenin expression in rats. Endocr Regul 50(3):137–144 [DOI] [PubMed] [Google Scholar]
- Wang S, Yang X (2016) Inter-organ regulation of adipose tissue browning. Cell Mol Life Sci. doi:10.1007/s00018-016-2420-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK et al (2011) Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332(6026):243–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Wilson S, Arch JR (1984) Prolonged beta-adrenoceptor stimulation increases the amount of GDP-binding protein in brown adipose tissue mitochondria. Life Sci 34(12):1111–1117 [DOI] [PubMed] [Google Scholar]