Abstract
Purpose
Race-based correction is widely utilized in clinical practice, but may contribute to overestimation of lung function, underdiagnoses in minority groups, and exclusion of minority groups from research trials. The aim of this systematic review is to examine the usage of race-based correction in pulmonary function testing (PFT) within chronic obstructive lung disease (COPD) research and its impact on the exclusion of minority groups from research trials.
Methods
We systematically searched Medline from 2010 to 2022 to identify randomized controlled trials (RCTs) that examine inhaler therapy for COPD. Article screening, critical appraisal, and data extraction were completed in duplicate by independent reviewers. Data regarding study design, inclusion criteria, demographics, and race-based correction were extracted and synthesized narratively.
Results
Of the 774 screened articles, we included 21 RCTs in the review, which were multinational trials involving 70696 study participants. All studies had an inclusion criteria of an FEV1 cutoff of 50% to 80%. Racial minorities remained underrepresented in the trials, with the proportion of black participants ranging from <1% to 4.7%. Four studies directly mentioned race-based correction, while the remainder of the studies did not provide any explicit details. After obtaining additional information by contacting authors and reviewing the citations, 15 were estimated to utilize race-based correction.
Conclusion
Race-based correction may be frequently utilized in major COPD RCTs, but there remains inconsistent reporting regarding the usage of race-based correction. This may contribute to the exclusion of racialized populations from research trials as there remains significant underrepresentation of racialized populations from research.
Keywords: chronic obstructive lung disease, pulmonary function testing, race-based correction, racial disparities, ethnic representation in research
Introduction
Chronic obstructive lung disease (COPD) is a lung condition characterized by progressive obstructive airflow limitation, affecting around 400 million people worldwide and associated with significant morbidity and mortality.1,2 Among patients with COPD, there are substantial disparities in health outcomes across racial groups.3 Black COPD patients are at higher risk of COPD exacerbations, lower quality of life, and even mortality.4–6 Many socioeconomic factors contribute to these disparities and there remains significant gaps in clinical care provided for racialized populations, including higher rates of undiagnosed COPD in African Americans.7 Despite the gaps and the need for additional research in this area, racial minority groups remain underrepresented not only in COPD research trials but across all areas of clinical research.8,9
Race-based correction has been widely used in pulmonary function testing for COPD, encompassing both fixed correction factors for race as well as race-specific equations for percent predicted values. While previously recommended by the Joint Working Group of the American Thoracic Society (ATS) and European Thoracic Society (ETS), a statement was released in 2023 currently recommending the usage of race-neutral equations in pulmonary function testing.10–12 The diagnosis of COPD is based upon pulmonary function testing, according to criteria defined by the Global Initiative for Chronic Obstructive Lung Disease.1 The assessment of the severity of COPD is graded by the percent predicted values of forced expiratory volume in 1 second (FEV1) through spirometry.1 With the usage of race-specific equations, predicted values of lung function, such as FEV1 and forced vital capacity (FVC) in milliliters, vary by 4–6% for Asian individuals and 10–15% for Black individuals compared to White individuals.10 Race-specific equations have been increasingly called into question within the literature for representing implicit bias and potentially resulting in the underdiagnosis of lung disease in racial groups.13
Using race-based correction to estimate lung function has significant implications for the diagnosis, management, and monitoring of COPD.14 With race-based corrections, racial minorities require lower absolute lung function values for their lung disease to be considered equally severe, which may result in underdiagnosis, undertreatment, and underrepresentation of racial populations in research. Recent studies have shown that race-based correction in spirometry did not improve the prediction of clinical events versus race-neutral equations.15 Race is a social construct, with a diverse range of genetic differences and phenotypes within each racial population; as such, identifying an individual by appearances or skin colour does not serve as an adequate proxy for biologic differences.16 When early differences in lung function were identified between white and non-white groups, it was noted that socioeconomic factors, such as air pollution, exercise, respiratory illnesses, may have resulted in these measured differences.17 The majority of studies used to develop modern race-based correction equations did not adjust for these social factors.18 Despite historical analyses suggesting that race-based correction had origins and underpinnings in colonialism and racism, the narrative of innate differences between racial groups persists.17,19,20 Currently, there is an emerging body of literature surrounding race-based correction, however there remains limited evidence-based data on the impact of race-based correction in clinical research trials.
In clinical research, race-based correction may contribute to the exclusion of certain racial groups from participating in research studies, given the FEV1 inclusion criteria required to participate in COPD trials. Racialized populations remain underrepresented in clinical trials in pulmonary medicine, with current COPD treatments predominantly evaluated in white and male populations.21,22 It is currently unknown the degree to which race-based corrections are utilized in clinical research and whether they are contributing to the exclusion of racialized populations in clinical trials. Therefore, the purpose of this study was to examine the usage of race-based correction within major COPD randomized control trials (RCT) and determine whether its usage led to the exclusion of racialized participants from clinical trials.
Methods
This study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see Additional File 1 for the PRISMA checklist). A protocol was developed a priori and published on the Open Science Framework registration.23
Study Eligibility
This systematic review included randomized controlled trials evaluating inhaler therapy in patients with COPD. The randomized controlled trials were included if their inclusion criteria for study participants involved pulmonary function testing criteria (which included a formal diagnosis of COPD) and the use of FEV1 as an outcome measure. The study was limited to RCTs with a sample size of 1500 or more to focus the analysis on large inhaler trials within COPD research, given the large number of COPD trials. Secondary or pooled analyses of RCTs, cost-effectiveness trials, protocols, or abstracts were excluded.
Search Strategy and Study Screening
Medline was searched from Jan 1, 2010, to June 23, 2022, for articles pertaining to COPD and inhaler therapy. Details regarding the search strategy can be found in the registered protocol.23 All the studies were screened in duplicate both at the title and abstract as well as the full-text screening phases. Disagreements were resolved by discussion between the two reviewers, and remaining conflicts after discussion were resolved by a third independent reviewer.
Data Extraction and Critical Appraisal
All RCTs included in the review were analyzed using a standardized data extraction form, which included key details such as study bibliographic details, study design and methods, outcomes, study population demographics, pulmonary function testing values, usage of race-based correction, and funding sources. Data was collected from the full text, supplementary files, and protocols of the articles. Corresponding authors for the studies were contacted for additional information regarding race-based correction if insufficient details were published. Critical appraisal was performed using the Cochrane Risk of Bias Tool for Randomized Trials, a tool validated for use in randomized controlled trials.24 Studies were reported as unknown, low, medium, or high risk of bias according to the Cochrane critical appraisal tool. Data extraction and critical appraisal were both performed independently in duplicate, with conflicts resolved via discussion between reviewers.
Data Analysis and Synthesis
Cohen’s kappa statistic was applied to assess interrater reliability during the full text and article screening phases. The proportion of studies that reported details regarding race-based spirometry were analyzed, including the applied race correction factor. If the study did not explicitly provide any details regarding race-based correction, data was gathered regarding whether the study cited that they performed spirometry according to ATS or ERS guidelines that recommend race-based pulmonary function testing. Patient demographics including ethnicity and pulmonary function testing data were also assessed. The extracted data was analyzed using narrative synthesis and descriptive analysis. There was insufficient data to perform statistical analysis given the heterogeneity between studies and limited publicly reported data regarding race-based correction.
Results
Characteristics of Included Studies
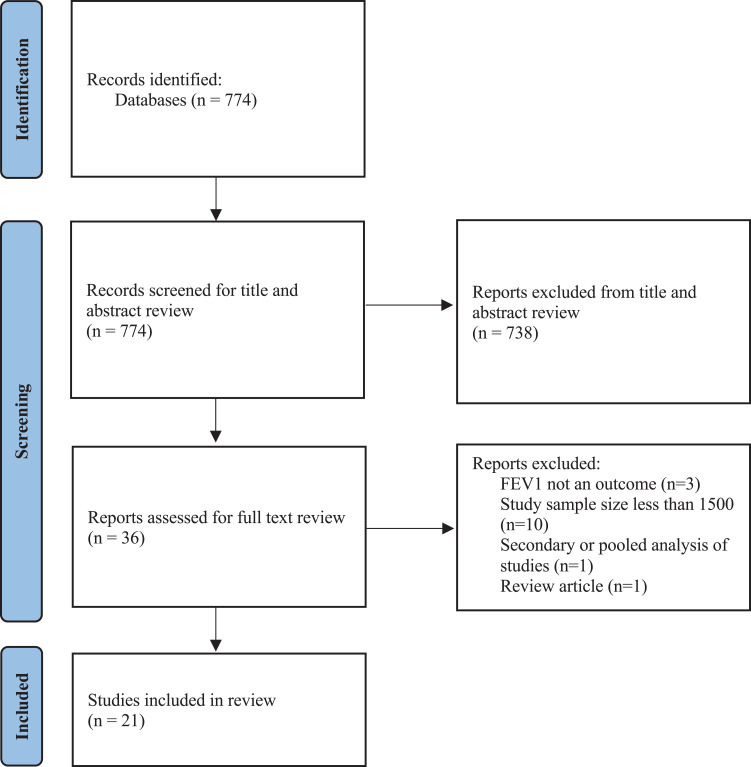
The search yielded 774 records, of which 738 articles were excluded at the title and abstract review stage with 36 records reviewed at the full-text review stage (see Figure 1). After full-text review, 21 articles met the study eligibility criteria and were included in the systematic review.25–45 There was a total of 70,626 study participants across all included studies. The flow diagram and reasons for exclusion of articles were reported in Figure 1. All studies reported an inclusion criterion of an FEV1 of less than 50% to less than 80% (see Table 1). The full characteristics of the studies are listed in Additional File 2.
Figure 1.
CONSORT diagram of article screening and inclusion.
Table 1.
Inclusion/Exclusion Criteria and Pulmonary Function Testing Values of the Randomized Control Trials
| Author Year | Inclusion Criteria for FEV1% Predicted | Other Major Inclusion Criteria | FEV1 (mL) | FEV1% Predicted | FVC (mL) | FEV1/FVC ratio (%) | Funding Source |
|---|---|---|---|---|---|---|---|
| Abrahams 201325 | <80% | Adults ≥40 years, FEV1/FVC <70%, smoking history of ≥10 pack years | 1180 | 43 | Pharma | ||
| Bateman 201026 | <60% | Adults ≥40 years, FEV1/FVC <70%, smoking history of ≥10 pack years, | 1110 | 40 | 2354 | 47.0 | Pharma |
| Buhl 201137 | ≥30% and <80% | Adults ≥40 years, FEV1/FVC <70%, smoking history of ≥10 pack years | 1520–1530† | 54.3–54.6† | 51.0–51.2† | Pharma | |
| D’Urzo 201439 | ≥30% and <80% | Adults ≥40 years, FEV1/FVC <70%, smoking history of ≥10 pack years | 1370 | 53.5 | Pharma | ||
| Dahl 201040 | ≥30% and <80% | Adults ≥40 years, FEV1/FVC <70%, smoking history of ≥20 pack years | 1288 | 51.7 | 51.2 | Pharma | |
| Decramer 201341 | ≥30% and <50% | Adults ≥40 years, FEV1/FVC <70%, smoking history of ≥10 pack years, documented history of one or more moderate or severe exacerbations in the previous 12 months | 1136 | 40.5 | 46.3 | Pharma | |
| Donohue 201342 | ≤70% | Adults ≥40 years, FEV1/FVC <70%, smoking history of ≥10 pack-years, MRC of ≥2 | 47.1 | 47.0 | Pharma | ||
| Donohue 201043 | ≥30% and <80% | Adults ≥40 years, FEV1/FVC <70%, smoking history of ≥ 20 pack-years | 3620 | 55.6 | 52.9 | Pharma | |
| Ferguson 201844 | ≥25% and <80% | Aged 40–80 years, FEV1/FVC <70%, smoking history of ≥10 pack years; with CAT>10 despite receiving two or more inhaled maintenance therapies for at least 6 weeks before screening | 50.3 | Pharma | |||
| Ferguson 201845 | ≥30% and <80% | Aged 40–80 years, FEV1/FVC <70%, smoking history of ≥10 pack years, (CAT score ≥10) despite treatment with one or more inhaled bronchodilator as COPD maintenance therapy for ≥6 weeks | 1539 | 52.93* | Pharma | ||
| Lipson 201728 | < 50% or 50–80% | Adults ≥40 years, FEV1/FVC <70%, COPD GOLD D (FEV1 < 50% and CAT ≥ 10, or FEV1 ≥ 50% to < 80% and CAT ≥ 10. Either at least two moderate exacerbations or at least one severe exacerbation in the past year | 1344 | 45.3 | Pharma | ||
| Lipson 201827 | <50% or 50 to 80% | Adults ≥40 years, FEV1/FVC <70%, symptomatic COPD (with CAT ≥10), FEV1<50% with one moderate exacerbation OR FEV1 of 50 to 80% with at least two moderate exacerbations or one severe exacerbation |
45.5 | Pharma | |||
| Lipworth 201829 | <80% | Aged 40–80 years, FEV1/FVC <0.70, ≥10 pack-years smoking history | Pharma | ||||
| Magnussen 201430 | <50% | Adults ≥40 years, FEV1/FVC <0.70, ≥10 pack-years smoking history, history of at least one documented exacerbation in the 12 months before screening | 980 | 34.2 | |||
| Maltais 201931 | ≥30 and ≤80% | Adults ≥40 years, FEV1/FVC <0.70, ≥10 pack-years smoking, CAT ≥ 10, ≤1 moderate exacerbation and no severe exacerbations in the previous year | 1595 | 55.4% | 52 | Pharma | |
| Papi 201732 | ≤50% | Adults ≥40 years, FEV1/FVC <0.70, ≥10 pack-year smoking history, at least 1 moderate or severe COPD exacerbation in the last 12 months (requiring systemic corticosteroids and/or antibiotics and/or hospitalization), and a minimum 10 pack-year smoking history | 35.6–35.9† | 41.4–42.3† | Pharma | ||
| Singh 201433 | ≥30% but <80% | Adults ≥40 years, FEV1/FVC <70%, ≥10 pack-year smoking history | 1410 | 54.3 | Pharma | ||
| Vestbo 201734 | <50% | Adults ≥40 years, FEV1/FVC <70%, CAT>10 had at least one moderate or severe COPD exacerbation in the last 12 months; and used an ICS plus LABA, or ICS plus LAMA, or LABA/LAMA, or LAMA | 1100 | 36.6 | 2700 | 40 | Pharma |
| Vestbo 201635 | >50% and < 70% | Aged 40–80 years, FEV1/FVC <70%, ≥10-pack-year history, mMRC ≥2, a history of or at increased risk of cardiovascular disease | 1700 | 59.7 | Pharma | ||
| Vogelmeier 201736 | ≥50% and <80% | Adults ≥40 years, FEV1/FVC <70%, ≥10 pack-year smoking history, mMRC score≥1, stable dose of baseline treatment with any SABA and/or SAMA or LABA or LAMA or LABA + ICS for at least 3 months before screening | 1786 | 63.7 | Pharma | ||
| Wedzicha 201338 | <50% | Adults ≥40 years, FEV1/FVC <70%, 10 pack-year smoking history, GOLD Stage III or IV, a history of at least one exacerbation in the previous 12 months requiring systemic corticosteroids and/or antibiotics. | 1040 | 18.3 | 39.3 | Pharma |
Notes: †Data was provided in treatment groups and not available for entire study population. *Blank values denotes that the data was not listed in the paper.
At the title and abstract screening phase, the percent agreement was 96.8% with a Cohen’s Kappa of 0.70 [95% CI 0.58–0.81]. During full-text review, there was a 94.4% observed agreement with Cohen’s Kappa of 0.88 [95% CI 0.73–1.0]. Interpretation of these kappa values represent substantial and almost perfect agreement.
Quality of Included Studies
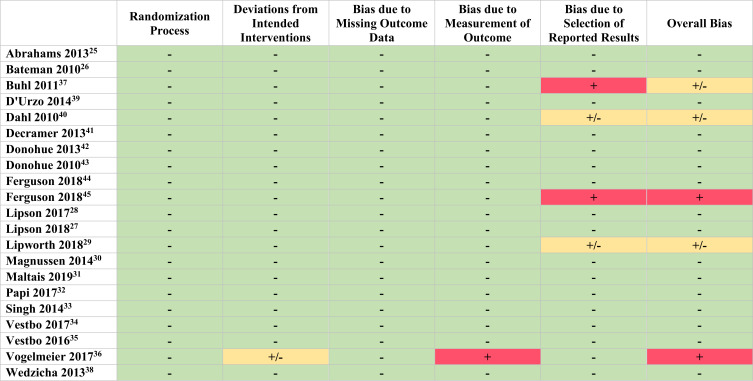
The overall quality of the included studies involved 17 (81%) studies with a low risk-of bias, three (14%) studies with some concern for bias and one (5%) with a high concern for bias, as determined by the Cochrane RoB2 tool (see Figure 2). The most prevalent domain of bias was bias due to the selection of reported results, with two (10%) of studies having some concern for bias and two (10%) studies having a high concern for bias.
Figure 2.
Risk of Bias Assessment of included Randomized Control Trials. Low concern for bias: (-). Some concern for bias: (±). High concern for bias: (+).
Baseline Data on Race and Ethnicity
Of the 21 included studies, 15 reported data on race within baseline demographics. Nine studies (43%) had a study population comprising greater than 85% of white individuals (see Table 2). Two studies (Ferguson 2018a and Lipworth 2018) were predominantly based in Asia and the remainder were multinational studies spanning across multiple continents with a large proportion of participants from the US, Canada, and Europe. The studies predominantly based in Asia had approximately 50% white and 40% Asian participants, and the remainder of RCTs (61.9%) had 77 to 97% of white individuals. The proportion of black study participants within the RCTs ranged from <1% to 4.7%. There were limited data on other ethnic groups such as American Indian or Alaska Native groups. No studies specifically reported the proportion of Hispanic/Latino participants. These other racial groups were reported as <6% of the study population.
Table 2.
Race-Based Correction and Ethnicity Data
| Author Year | Sample Size | Sex | Was Race-based Correction Used? | Race-specific Equation, Variation Compared to White Individuals | Race | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M (%) | With Data Published Publicly in the Article | Including Data Obtained by Emailing Authors | Including Data Citing ATS/ERS Guidelines | White | Asian | Black or African American | American Indian or Alaska Native | Other* | |||
| Abrahams 201325 | 2080 | 64.5% | Not reported | Not reported | Yes | 88.5% | |||||
| Bateman 201026 | 3991 | 77.5% | Not reported | Not reported | Not reported | ||||||
| Buhl 201137 | 1598 | 68.4% | Not reported | Not reported | Yes | 95% | |||||
| D’Urzo 201439 | 1692 | 53.1% | Not reported | Not reported | Yes | 93.2% | |||||
| Dahl 201040 | 1732 | 79.7% | Not reported | Not reported | Not reported | ||||||
| Decramer 201341 | 3444 | 77.0% | Not reported | Not reported | Yes | 77% | 16% | 0.3% | 2% | 5% | |
| Donohue 201342 | 1536 | 70.2% | Yes | Yes | Yes | Hankinson 1999 and 2010, 12–15% for Blacks 12% for Asians |
|||||
| Donohue 201043 | 2059 | 62.8% | Not reported | Not reported | Yes | ||||||
| Ferguson 201844** | 1902 | 71.2% | Yes | Yes | Yes | Hankinson 1999 and 2010, 12–15% for Blacks 12% for Asians |
50.1% | 44.9% | 4.7% | 0.2% | |
| Ferguson 201845 | 2389 | 60.5% | Not reported | Yes | Yes | 96.6% | 3% | 0.4% | |||
| Lipson 201728 | 1810 | 74.1% | Not reported | Yes | Yes | Quanjer 2012, 13–15% for Blacks 3–13% for Asians |
|||||
| Lipson 201827** | 10,355 | 66.3% | Not reported | Yes | Yes | Quanjer 2012, 13–15% for Blacks 3–13% for Asians |
78% | 16% | 3% | 2% | <1% |
| Lipworth 201829 | 1756 | 74.1% | Yes | Yes | Yes | 56.7% | 40.2% | 3.0% | <0.1% | ||
| Magnussen 201430 | 2485 | 82.5% | Not reported | Not reported | Yes | 81.4% | 12.4% | 0.4% | |||
| Maltais 201931 | 2431 | 59.3% | Not reported | Not reported | Not reported | 95% | <1% | 3% | 2% | <1% | |
| Papi 201732 | 1765 | 72.6–75.9% | Not reported | Not reported | Not reported | 95.9–98.1% | |||||
| Singh 201433 | 1729 | 67.6% | Not reported | Not reported | Not reported | 94.9% | |||||
| Vestbo 201734 | 2691 | 76.4% | Not reported | No | No | 99.3% | <1% | <1% | |||
| Vestbo 201635 | 16,568 | 74.5% | Yes | No | Not reported | 81.0% | 16.5% | 2.4% | |||
| Vogelmeier 201736 | 4389 | 65.1% | Not reported | Yes | Yes | Quanjer 2012, 13–15% for Blacks 3–13% for Asians |
|||||
| Wedzicha 201338 | 2224 | 74.8% | Not reported | Not reported | Yes | 82.1% | 11.8% | 0.7% | 5.3% | ||
Notes: An empty cell denotes that the data was not provided or unavailable. *Other includes studies that specified other, unknown, native Hawaiian or pacific islander. There were no studies that reported Hispanic/latino. **Note that Lipson 2018 and Ferguson 2018a were predominantly based in Asia. All other studies were multinational across several continents and based predominantly in US, Canada and Europe.
Race-Based Correction Usage Within Clinical Trials
Four RCTs explicitly mentioned whether race-based correction was utilized, and the remainder of the studies did not report any details on whether race-based correction was utilized for pulmonary function testing. The corresponding authors of the articles were contacted for additional information regarding race-based correction and additional details were obtained for six articles (see Table 2). With this information, eight of nine studies were confirmed to have utilized race-based correction and one study did not utilize race-based correction. Note that there was one discrepancy between author reported information and the publicly available data.46 There were 12 studies with unknown data regarding whether race-based correction was utilized. Among studies with unknown data, seven studies cited that spirometry was performed according to previous ATS or ERS guidelines, which recommend and endorse the usage of race-based correction. Assuming that studies who cited the previous ATS or ERS guidelines utilized race-based correction, 15 studies were estimated to have utilized race-based correction, one study did not utilize race-based correction and five studies remaining with unknown data.
The studies cited three main references for race-based correction (see Table 2).47–49 In the study published by Hankinson et al, race-specific equations were proposed, where percent predicted values varied in comparison to White individuals of up to 12% to 15% for African-Americans and Black individuals and 6% for Asian individuals.48,50 Hankinson published an update to these reference values in 2010, where a correction factor of around 12% was utilized for Asian populations and other reference values were maintained consistent.49 Quanjer et al examined multi-ethnic values within a global population.47,50 They proposed race-specific equations with variations in percent predicted lung function compared to White individuals of approximately 13–15% for Black individuals, 1–3% for North East Asians (Korea and China north of the Huaihe River and Qinling Mountains), and 12–13% for South East Asians (Thailand, Japan, and the remainder of China).
Impact of Race-Based Correction on Inclusion of Minority Groups
All of the studies utilized FEV1 cutoffs to include participants into trials based on their COPD severity. Seven of twenty-one studies utilized some clinical markers of COPD severity to determine study eligibility such as COPD exacerbations, the modified Medical Research Council Dyspnea Scale (mMRC), COPD Assessment Test (CAT). The studies reported FEV1 cutoffs of 80% or lower to include participants in clinical trials (see Table 1). With race-based corrections of 15% for Black individuals, this would require a 15% lower absolute value in FEV1 in milliliters in order to be included within the trial. This may contribute to the low proportions of black participants in the trials as study eligibility. The correction for Asian participants ranged from 3% to 13% in the RCTs. There were varying rates of Asian participants in clinical trials, with around 10–16% in most clinical trials and upwards of 50% when the trials were predominantly based in Asia. There was minimal reporting on other ethnic groups. Given only one study did not utilize race-based correction as well as the heterogeneity between studies, there was insufficient data to perform a statistical analysis for the differences between groups.
Discussion
This systematic review presents novel data regarding the usage of race-based correction among major COPD trials. Firstly, race-based correction may be frequently used in COPD clinical research trials, but there remains inconsistent reporting surrounding data on ethnicity and race-based correction. There was limited reporting within clinical trials on whether race-based correction was utilized, with only four studies mentioning race-based correction and six studies providing no ethnicity data regarding study participants. After gathering additional data by contacting the authors or by examining their citations of ATS/ERS guidelines, the majority of articles were found to employ race-based correction even when not explicitly reported. Furthermore, minority groups remain underrepresented, with black study participants representing <5% of the study participants and other racial minorities representing <6% of the study population. Given the frequent usage of FEV1 criteria to determine study eligibility, race-based correction may contribute to the exclusion of minority groups within COPD research trials.
Three main sets of lung function reference and race-based correction values were cited by the included RCTs, all of which apply correction factors to Asian and Black populations that assume that these populations have lower lung function and may result in the overestimation of lung function. Recent re-analyses of these race-based correction formulas on new datasets suggest there is no improvement in prediction of clinical outcomes such as mortality and chronic lower respiratory disease events, with usage of race-based corrections.15 The early studies did not report correction for socioeconomic factors when developing these algorithms. In one of the studies examining these race-based corrections, they showed a 4% range of error when applying the correction factor for African-Americans.48
In light of the emerging research regarding the impact of race-specific equations, the ATS released a statement recommending the usage of race-neutral equations in pulmonary function testing.12 One analysis which was performed on a Pennsylvania cohort demonstrated that removal of race-based corrections led to a 20% increase in the number of lung disease diagnoses in Black patients.51 A scoping review demonstrated the estimated severity of lung disease in African Americans was decreased with race-specific equations, resulting in the misclassification of COPD in this population, however was not associated with improved prediction of clinical outcomes.52 White individuals were found to have increased severity of lung disease with race-specific equations.52 This raises questions regarding the validity of the COPD severity classification itself in the context of race-based correction. Furthermore, race is a social construct, one which is applied and defined differently based upon the context. Previous literature demonstrates that the majority of studies examining lung function and race provide no definition for each racial group, which raises concern given the significant variability in defining racial groups as well as persons in mixed racial groups.18 Our results were consistent with this, given the limited reporting around definitions of racial groups. There is a limited biological basis for these race-based corrections, with no biological mechanism regarding the difference in lung function between groups, adjusting for social factors such as income, socioeconomic status, and other factors.
There is a possibility that the race-neutral approach could result in lower lung function estimates in racial populations and perhaps affect the lower limit of FEV1 inclusion into trials. It is unclear whether patients would be excluded because their lung function is estimated to be too low for inclusion, although the cut-off for many of these included trials is very low and unlikely be restrictive. Further research in this area is required to examine this effect, which requires clinical trials to gather valuable data regarding the excluded participants from trials including their race and socioeconomic factors.
Representation of Minority Groups in Research
The topic of representation of minority groups in research is complex and there are a multitude of factors contributing, including recruitment processes, language, lack of time and financial resources, stigma, and other socioeconomic barriers.53 While race-based correction may not be the only factor resulting in these differences, this study is the first to examine race-based correction as a potential contributor. Given significant gaps in representation and difficulties in recruitment strategies for racial minorities, even a minor contribution of race-based correction in exclusion of minority groups warrants examination and research into this area.
Strengths and Limitations of the Review
This review was a high-quality systematic review in which study selection, data extraction, and critical appraisal was performed rigorously in duplicate. There was excellent agreement between reviewers as highlighted by the Cohen’s kappa values during screening. One of the limitations was that only one database was searched, however this review was designed to provide an overview of major COPD trials guiding clinical practice rather than a comprehensive review of all trials. Furthermore, given the vast realm of COPD research, this review was focused on large-scale COPD bronchodilator trials in order to conduct a relevant and feasible review. However, COPD research also includes many forms including epidemiologic, qualitative and translational research, and thus future research can consider examining the impact of race-based correction in other areas of COPD research. There was minimal reporting regarding the usage of race-based correction within the published and thus a limitation of this study was that data was retrieved from other authors regarding usage of race-based correction which was not included in the protocol a priori. Limited data was available to analyze the proportion of individuals who would be excluded due to race-based correction. FEV1 was included as an outcome in the inclusion/exclusion criteria to allow assessment of the effect of race-based correction on the number of participants labelled as “responders” and “non-responders”. However, due to the lack of data reporting, there was insufficient data to assess this. We attempted to contact the authors on further data on excluded populations including race and their pulmonary function testing data, however this data was unavailable.
Implications for Future Research, Policy and Practice
This review highlights the need for standard reporting guidelines to increase transparency surrounding the use of race-based correction within clinical trials. In this study, we demonstrate that that race-based correction may be frequently used within current research and propose race-based correction as a potential contributor to the exclusion of these populations from major COPD research studies. The majority of articles did not report any details regarding race-based correction, despite using it within their pulmonary function testing. Furthermore, we attempted to contact authors of clinical trials to gather data on parameters such as race and lung function of excluded participants, however this was not collected or available. This review therefore calls for improved data collection on race in clinical trials, to consider parameters of race in excluded participants for future analyses. Future research can also examine the usage of race-based correction within clinical applications to assess its impact on misdiagnoses or delayed diagnoses as well as clinical outcomes in patients with COPD.
The ATS recently released a statement in 2023 supporting the use of race-neutral equations in clinical practice. Alongside the release of the 2023 statement, this review establishes the baseline usage of race-based correction in current research and highlights the need to develop standard reporting guidelines to increase transparency surrounding its usage clinical trials. While guideline recommendations often take time to translate into clinical practice, standardized reporting will allow future research to analyze the number of studies following society recommendations as well as to quantify the effect of race-based correction of on inclusion of racial minorities in clinical trials.
In summary, this systematic review presents novel findings regarding race-based correction in pulmonary function testing within major COPD clinical trials. There remains inconsistent reporting surrounding the usage of race-based correction within randomized controlled trials and when details are provided, the majority of clinical trials utilize race-based correction. The proportion of racial minority groups remains low across clinical trials in COPD. This systematic review suggests that race-based correction may be one of the contributing factors to the exclusion of minority groups in clinical trials.
Acknowledgments
The authors would like to thank Eman Mugami (The Ottawa Hospital Research Institute) for providing contextual feedback regarding a lens on equity, diversity, and inclusion. We would also like to thank Tim Ramsay and Ranjeeta Mallick (The Ottawa Hospital Research Institute) for their technical expertise regarding statistical analysis. Presented in the Canadian Respiratory Conference, Montreal QC, April 20, 2023.
Abbreviations
ATS, American Thoracic Society; COPD, Chronic Obstructive Pulmonary Disease; CAT, COPD Assessment Tool; ERS, European Respiratory Society; FEV1, Forced Expiratory Volume in One Second; FVC, Forced Vital Capacity; mMRC, Modified Medical Research Council Dyspnea Scale; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, Randomized Control Trial.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi: 10.1183/13993003.00164-2019 [DOI] [PubMed] [Google Scholar]
- 2.Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10(5):447–458. doi: 10.1016/S2213-2600(21)00511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisner MD, Blanc PD, Omachi TA, et al. Socioeconomic status, race and COPD health outcomes. J Epidemiol Community Heal. 2011;65(1):26–34. doi: 10.1136/jech.2009.089722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dransfield MT, Bailey WC. COPD: racial disparities in susceptibility, treatment, and outcomes. Clin Chest Med. 2006;27(3):463–471. doi: 10.1016/j.ccm.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 5.Han MLK, Curran-Everett D, Dransfield MT, et al. Racial differences in quality of life in patients with COPD. Chest. 2011;140(5):1169–1176. doi: 10.1378/chest.10-2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ejike CO, Woo H, Galiatsatos P, et al. Contribution of individual and neighborhood factors to racial disparities in respiratory outcomes. Am J Respir Crit Care Med. 2021;203(8):987–997. doi: 10.1164/rccm.202002-0253OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamary AJ, Stewart JI, Kinney GL, et al. Race and gender disparities are evident in COPD underdiagnoses across all severities of measured airflow obstruction. Chronic Obstr Pulm Dis. 2018;5(3):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etti M, Fofie H, Razai M, Crawshaw AF, Hargreaves S, Goldsmith LP. Ethnic minority and migrant underrepresentation in Covid-19 research: causes and solutions. EClinicalMedicine. 2021;36:100903. doi: 10.1016/j.eclinm.2021.100903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, Eggly S. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016;23(4):327–337. doi: 10.1177/107327481602300404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lujan HL, DiCarlo SE. Science reflects history as society influences science: brief history of “race”, “race correction”, and the spirometer. Adv Physiol Educ. 2018;42(2):163–165. doi: 10.1152/advan.00196.2017 [DOI] [PubMed] [Google Scholar]
- 11.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 12.Bhakta NR, Bime C, Kaminsky DA, et al. Race and ethnicity in pulmonary function test interpretation: an official American Thoracic Society statement. Am J Respir Crit Care Med. 2023;207(8):978–995. doi: 10.1164/rccm.202302-0310ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson MA, Malhotra A, Non AL. Could routine race-adjustment of spirometers exacerbate racial disparities in COVID-19 recovery? Lancet Respir Med. 2021;9(2):124–125. doi: 10.1016/S2213-2600(20)30571-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun L. Race, ethnicity and lung function: a brief history. Can J Respir Ther. 2015;51(4):99. [PMC free article] [PubMed] [Google Scholar]
- 15.Elmaleh-Sachs A, Balte P, Oelsner EC, et al. Race/ethnicity, spirometry reference equations, and prediction of incident clinical events: the multi-ethnic study of atherosclerosis (mesa) lung study. Am J Respir Crit Care Med. 2022;205(6):700–710. doi: 10.1164/rccm.202107-1612OC [DOI] [PubMed] [Google Scholar]
- 16.Non AL, Gravlee CC. Biology and culture beyond the genome: race, racism, and health. Am Anthropol. 2015;117(4):737–738. doi: 10.1111/aman.12365 [DOI] [Google Scholar]
- 17.Bhakta NR, Kaminsky DA, Bime C, et al. Addressing race in pulmonary function testing by aligning intent and evidence with practice and perception. Chest. 2022;161(1):288–297. doi: 10.1016/j.chest.2021.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun L, Wolfgang M, Dickersin K. Defining race/ethnicity and explaining difference in research studies on lung function. Eur Respir J. 2013;41(6):1362–1370. doi: 10.1183/09031936.00091612 [DOI] [PubMed] [Google Scholar]
- 19.Braun L. Spirometry, measurement, and race in the nineteenth century. J Hist Med Allied Sci. 2005;60(2):135–169. doi: 10.1093/jhmas/jri021 [DOI] [PubMed] [Google Scholar]
- 20.Roberts DE. Abolish race correction. Lancet. 2021;397(10268):17–18. doi: 10.1016/S0140-6736(20)32716-1 [DOI] [PubMed] [Google Scholar]
- 21.Thakur N, Holguin F, Alvidrez J, et al. Enhancing recruitment and retention of minority populations for clinical research in pulmonary, critical care, and sleep medicine: an official American Thoracic Society research statement. Am J Respir Crit Care Med. 2021;204(3):E26–50. doi: 10.1164/rccm.202105-1210ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geller SE, Koch AR, Roesch P, Filut A, Hallgren E, Carnes M. The more things change, the more they stay the same: a study to evaluate compliance with inclusion and assessment of women and minorities in randomized controlled trials. Acad Med. 2018;93(4):630. doi: 10.1097/ACM.0000000000002027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JZ, Shin M, Frances R, Chow R, Yang S, Pakhale S. The usage and impact of race-based correction in pulmonary function testing within major randomized controlled trials in COPD: a systematic review protocol [internet]. Open Science Framework. 2022. Available from: https://osf.io/ea5h4/. Accessed October 09,2024.
- 24.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366(l4898). [DOI] [PubMed] [Google Scholar]
- 25.Abrahams R, Moroni-Zentgraf P, Ramsdell J, Schmidt H, Joseph E, Karpel J. Safety and efficacy of the once-daily anticholinergic BEA2180 compared with tiotropium in patients with COPD. Respir Med. 2013;107(6):854–862. doi: 10.1016/j.rmed.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 26.Bateman ED, Tashkin D, Siafakas N, et al. A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med. 2010;104(10):1460–1472. doi: 10.1016/j.rmed.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 27.Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 28.Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446. doi: 10.1164/rccm.201703-0449OC [DOI] [PubMed] [Google Scholar]
- 29.Lipworth BJ, Collier DJ, Gon Y, et al. Improved lung function and patient-reported outcomes with co-suspension delivery technology glycopyrrolate/formoterol fumarate metered dose inhaler in COPD: a randomized Phase III study conducted in Asia, Europe, and the USA. Int J Chron Obs Pulmon Dis. 2018;13:2969–2984. doi: 10.2147/COPD.S171835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi: 10.1056/NEJMoa1407154 [DOI] [PubMed] [Google Scholar]
- 31.Maltais F, Bjermer L, Kerwin EM, et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the EMAX randomised trial. Respir Res. 2019;20(1):238. doi: 10.1186/s12931-019-1193-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papi A, Dokic D, Tzimas W, et al. Fluticasone propionate/formoterol for COPD management: a randomized controlled trial. Int J Chron Obs Pulmon Dis. 2017;12:1961–1971. doi: 10.2147/COPD.S136527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh D, Jones PW, Bateman ED, et al. Efficacy and safety of Aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm Med. 2014;14(1):178. doi: 10.1186/1471-2466-14-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389(10082):1919–1929. doi: 10.1016/S0140-6736(17)30188-5 [DOI] [PubMed] [Google Scholar]
- 35.Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387(10030):1817–1826. doi: 10.1016/S0140-6736(16)30069-1 [DOI] [PubMed] [Google Scholar]
- 36.Vogelmeier CF, Gaga M, Aalamian-Mattheis M, et al. Efficacy and safety of direct switch to indacaterol/glycopyrronium in patients with moderate COPD: the CRYSTAL open-label randomised trial. Respir Res. 2017;18(1):140. doi: 10.1186/s12931-017-0622-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buhl R, Dunn LJ, Disdier C, et al. Blinded 12-week comparison of once-daily indacaterol and tiotropium in COPD. Eur Respir J. 2011;38(4):797–803. doi: 10.1183/09031936.00191810 [DOI] [PubMed] [Google Scholar]
- 38.Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209. doi: 10.1016/S2213-2600(13)70052-3 [DOI] [PubMed] [Google Scholar]
- 39.D’Urzo AD, Rennard SI, Kerwin EM, et al. Efficacy and safety of fixed-dose combinations of Aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir Res. 2014;15(1):123. doi: 10.1186/s12931-014-0123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahl R, Jadayel D, Alagappan VK, Chen H, Banerji D. Efficacy and safety of QVA149 compared to the concurrent administration of its monocomponents indacaterol and glycopyrronium: the BEACON study. Int J Chron Obs Pulmon Dis. 2013;8(PG–501–8):501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1(7):524–533. doi: 10.1016/S2213-2600(13)70158-9 [DOI] [PubMed] [Google Scholar]
- 42.Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107(10):1538–1546. doi: 10.1016/j.rmed.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 43.Donohue JF, Fogarty C, Lotvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182(2):155–162. doi: 10.1164/rccm.200910-1500OC [DOI] [PubMed] [Google Scholar]
- 44.Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, Phase 3 randomised controlled tr. Lancet Respir Med. 2018;6(10):747–758. doi: 10.1016/S2213-2600(18)30327-8 [DOI] [PubMed] [Google Scholar]
- 45.Ferguson GT, Papi A, Anzueto A, et al. Budesonide/formoterol MDI with co-suspension delivery technology in COPD: the TELOS study. Eur Respir J. 2018;52(3):1801334. doi: 10.1183/13993003.01334-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vestbo J, Anderson J, Brook RD, et al. The study to understand mortality and morbidity in COPD (SUMMIT) study protocol. Eur Respir J. 2013;41(5):1017–1022. doi: 10.1183/09031936.00087312 [DOI] [PubMed] [Google Scholar]
- 47.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 49.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Graham Barr R. Performance of American thoracic society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (mesa) lung study. Chest. 2010;137(1):138–145. doi: 10.1378/chest.09-0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramsey NB, Apter AJ, Israel E, Louisias M. Deconstructing the way we use pulmonary function test race-based adjustments. J Allergy Clin Immunol Pract. 2022;10(4):972–978. doi: 10.1016/j.jaip.2022.01.023 [DOI] [PubMed] [Google Scholar]
- 51.Moffett AT, Eneanya ND, Halpern SD, Weissman GE. The impact of race correction on the interpretation of pulmonary function testing among black patients. In: A7. A007 Impact of Race, Ethnicity, and Social Determinants on Individuals with Lung Diseases. American Thoracic Society; 2021:A1030–A1030. [Google Scholar]
- 52.Davidson SR, Idris MY, Awad CS, et al. Race adjustment of pulmonary function tests in the diagnosis and management of COPD: a scoping review. Int J Chron Obstruct Pulmon Dis. 2024;19:969–980. doi: 10.2147/COPD.S430249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104(2):e16. doi: 10.2105/AJPH.2013.301706 [DOI] [PMC free article] [PubMed] [Google Scholar]