Abstract
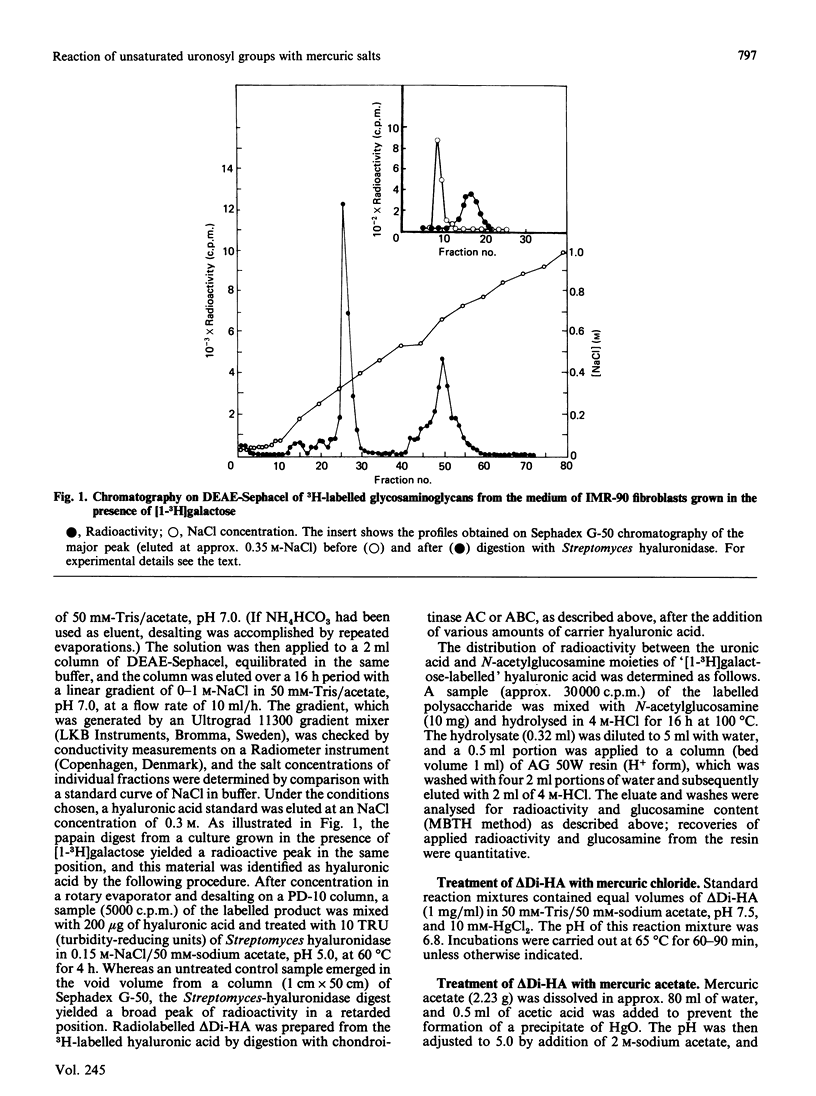
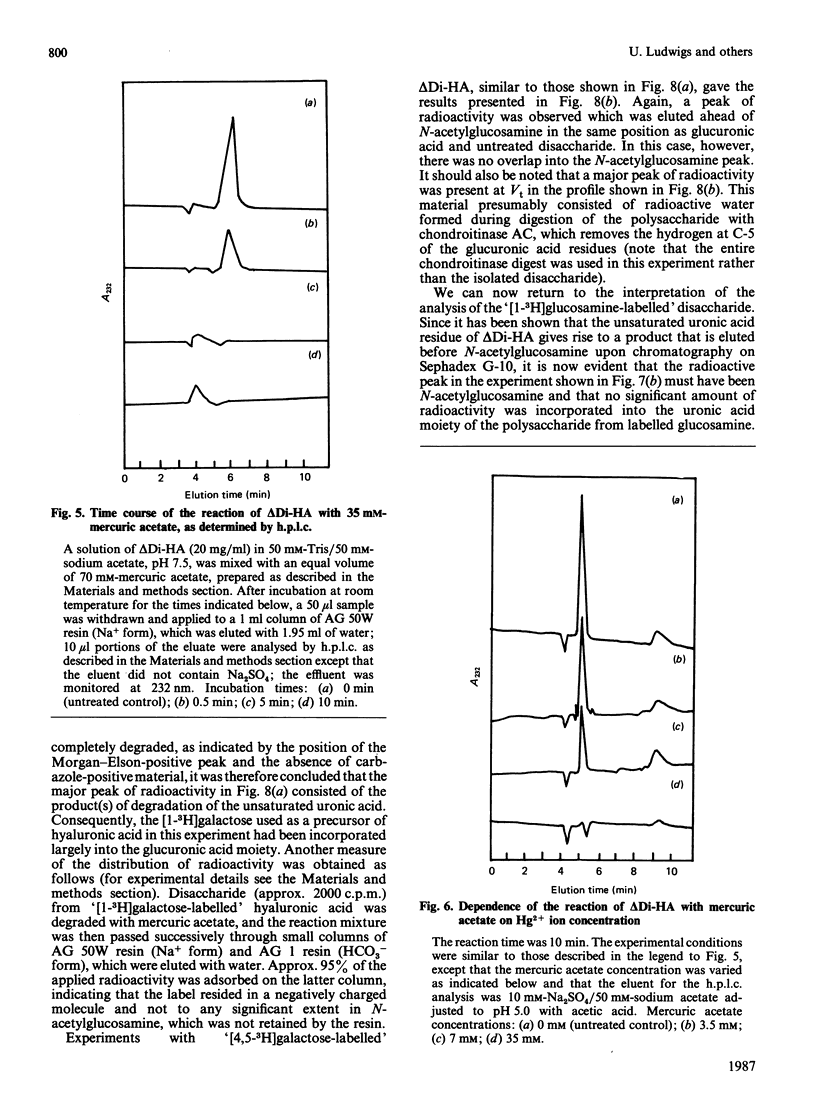
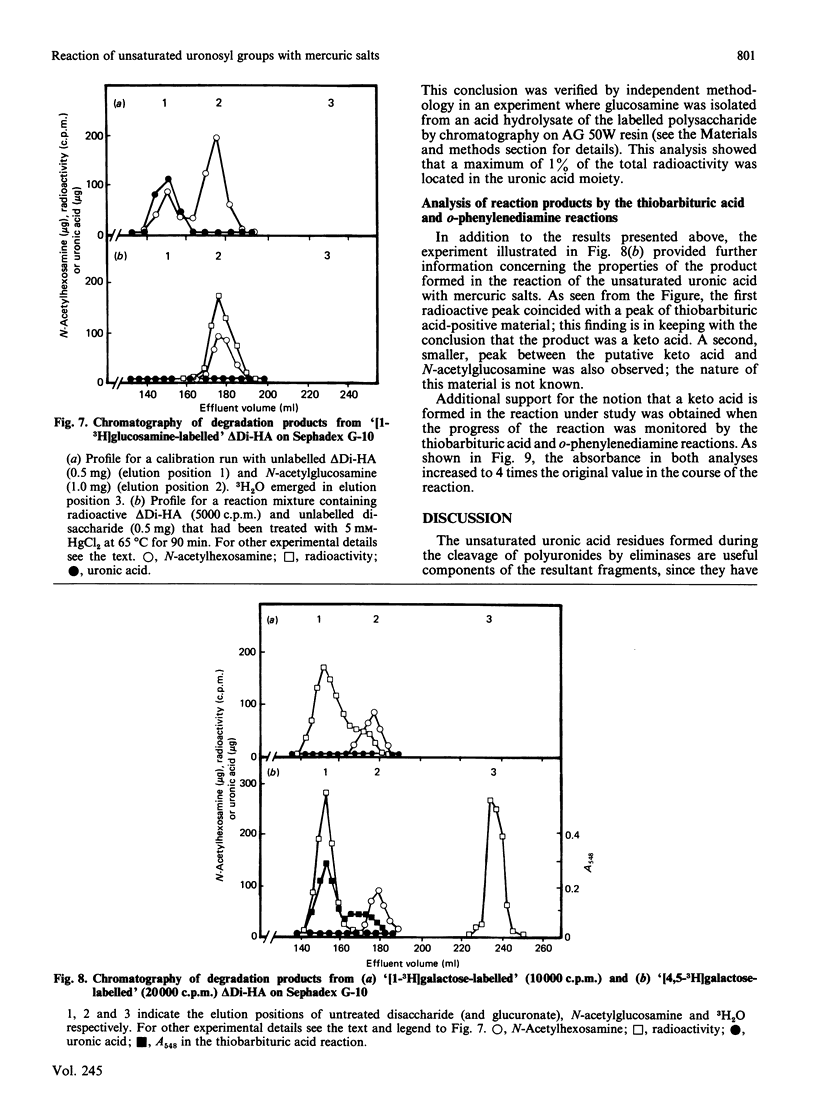
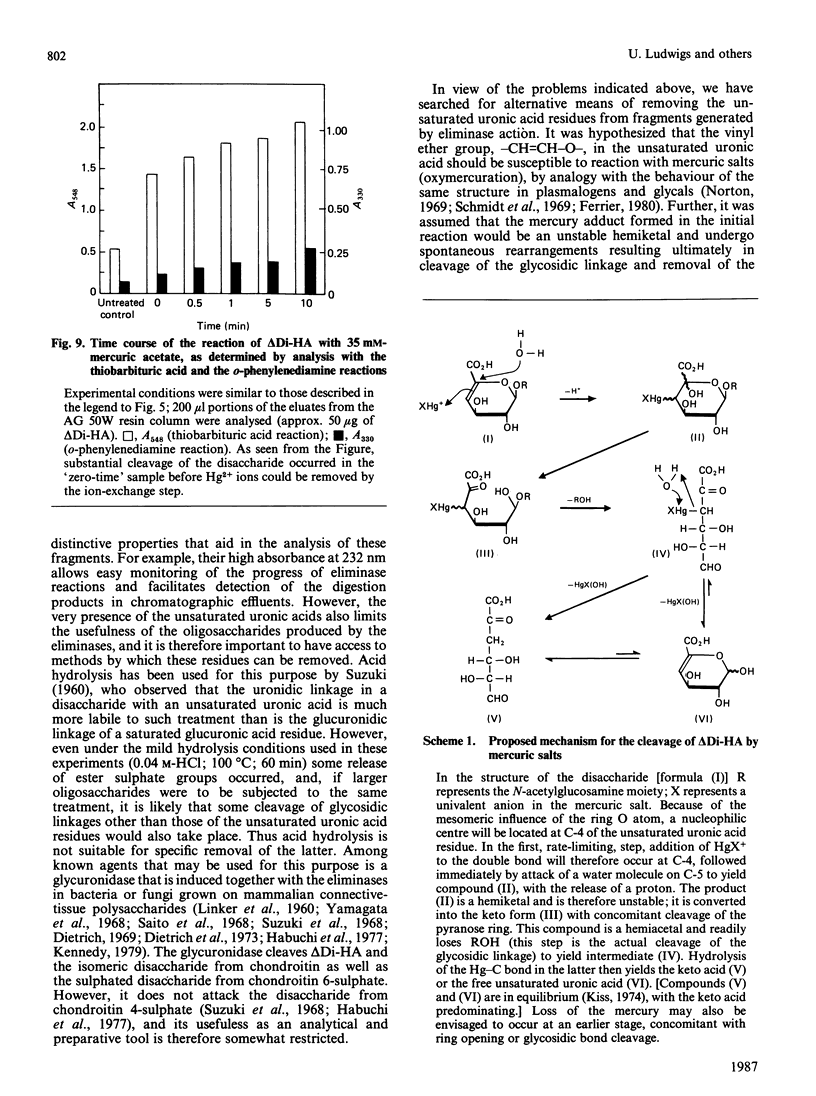
Degradation of connective-tissue polysaccharides with bacterial or fungal eliminases and subsequent characterization of the reaction products are now part of standard methodology for the analysis of these compounds. However, the scope of preparative and analytical work based on the use of eliminases has been limited by the lack of procedures for specific removal of the unsaturated uronic acid residues generated in the eliminase reactions. In the present investigation, we have shown that these residues are cleaved by mercuric salts under mild conditions that are not likely to affect other structures in an oligo- or poly-saccharide molecule. Thus the disaccharide generated from hyaluronic acid by digestion with chondroitinase AC or ABC was cleaved into a keto acid and free N-acetylglucosamine within 10 min at room temperature upon exposure to 14 mM-mercuric acetate at pH 5. The reaction of the disaccharide with mercuric salts was used for ready determination of the distribution of radioactivity between the glucuronic acid and N-acetylglucosamine moieties in radioactive hyaluronic acid that had been synthesized by IMR-90 fibroblasts from 3H-labelled monosaccharides. When the precursor was [3H]galactose, over 95% of the incorporated radioactivity was found in the glucuronic acid moiety. In contrast, cells grown in the presence of [3H]glucosamine synthesized a polysaccharide in which almost all of the label was located in the N-acetylglucosamine units. It is apparent from these experiments that the reaction of unsaturated uronic acid residues with mercuric salts provides a new tool with potential for many applications in the study of the structure and metabolism of connective-tissue polysaccharides.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOUDOROFF M., PALLERONI N. J. Characterization and properties of 2-keto-3-deoxy-D-arabonic acid. J Biol Chem. 1956 Nov;223(1):499–508. [PubMed] [Google Scholar]
- Dietrich C. P. Enzymic degradation of heparin. A glucosaminidase and a glycuronidase from Flavobacterium heparinum. Biochemistry. 1969 May;8(5):2089–2094. doi: 10.1021/bi00833a046. [DOI] [PubMed] [Google Scholar]
- Dietrich C. P., Silva M. E., Michelacci Y. M. Sequential degradation of heparin in Flavobacterium heparinum. Purification and properties of five enzymes involved in heparin degradation. J Biol Chem. 1973 Sep 25;248(18):6408–6415. [PubMed] [Google Scholar]
- Elgavish A., Smith J. B., Pillion D. J., Meezan E. Sulfate transport in human lung fibroblasts (IMR-90). J Cell Physiol. 1985 Nov;125(2):243–250. doi: 10.1002/jcp.1041250211. [DOI] [PubMed] [Google Scholar]
- Ford J. D., Baker J. R. Procedures for the automated analyses of proteoglycans and glycosaminoglycans. Anal Biochem. 1978 Feb;84(2):539–550. doi: 10.1016/0003-2697(78)90073-8. [DOI] [PubMed] [Google Scholar]
- HOFFMAN P., LINKER A., LIPPMAN V., MEYER K. The structure of chondroitin sulfate B from studies with Flavobacterium enzymes. J Biol Chem. 1960 Nov;235:3066–3069. [PubMed] [Google Scholar]
- Habuchi O., Sugiura K., Kawai N. Glucose branches in chondroitin sulfates from squid cartilage. J Biol Chem. 1977 Jul 10;252(13):4570–4576. [PubMed] [Google Scholar]
- Hascall V. C., Riolo R. L., Hayward J., Jr, Reynolds C. C. Treatment of bovine nasal cartilage proteoglycan with chondroitinases from Flavobacterium heparinum and Proteus vulgaris. J Biol Chem. 1972 Jul 25;247(14):4521–4528. [PubMed] [Google Scholar]
- Hay R. J., Williams C. D., Macy M. L., Lavappa K. S. Cultured cell lines for research on pulmonary physiology available through the American type culture collection. Am Rev Respir Dis. 1982 Feb;125(2):222–232. doi: 10.1164/arrd.1982.125.2.222. [DOI] [PubMed] [Google Scholar]
- Hiyama K. Action of chondroitinases. II. Numerical calculation of the degree of multiple attack. J Biochem. 1976 Dec;80(6):1209–1214. doi: 10.1093/oxfordjournals.jbchem.a131391. [DOI] [PubMed] [Google Scholar]
- Hiyama K., Okada S. Action of chondroitinases. I. The mode of action of two chondroitinase-AC preparations of different origin. J Biochem. 1976 Dec;80(6):1201–1207. doi: 10.1093/oxfordjournals.jbchem.a131390. [DOI] [PubMed] [Google Scholar]
- Hjerpe A., Antonopoulos C. A., Engfeldt B. Determination of sulphated disaccharides from chondroitin sulphates by high-performance liquid chromatography. J Chromatogr. 1979 Apr 1;171:339–344. doi: 10.1016/s0021-9673(01)95313-0. [DOI] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- LANNING M. C., COHEN S. S. The detection and estimation of 2-ketohexonic acids. J Biol Chem. 1951 Mar;189(1):109–114. [PubMed] [Google Scholar]
- LINKER A., HOFFMAN P., MEYER K., SAMPSON P., KORN E. D. The formation of unsaturated disacharides from mucopoly-saccharides and their cleavage to alpha-keto acid by bacterial enzymes. J Biol Chem. 1960 Nov;235:3061–3065. [PubMed] [Google Scholar]
- LINKER A., MEYER K., HOFFMAN P. The production of unsaturated uronides by bacterial hyaluronidases. J Biol Chem. 1956 Mar;219(1):13–25. [PubMed] [Google Scholar]
- Linker A., Hovingh P. The enzymatic degradation of heparin and heparitin sulfate. I. The fractionation of a crude heparinase from flavobacteria. J Biol Chem. 1965 Oct;240(10):3724–3728. [PubMed] [Google Scholar]
- NORTON W. T. Reaction of mercuric chloride with plasmalogen. Nature. 1959 Oct 10;184(Suppl 15):1144–1145. doi: 10.1038/1841144a0. [DOI] [PubMed] [Google Scholar]
- Nichols W. W., Murphy D. G., Cristofalo V. J., Toji L. H., Greene A. E., Dwight S. A. Characterization of a new human diploid cell strain, IMR-90. Science. 1977 Apr 1;196(4285):60–63. doi: 10.1126/science.841339. [DOI] [PubMed] [Google Scholar]
- PREISS J., ASHWELL G. Polygalacturonic acid metabolism in bacteria. I. Enzymatic formation of 4-deoxy-L-threo-5-hexoseulose uronic acid. J Biol Chem. 1963 May;238:1571–1583. [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- SALTON M. R. An improved method for the detection of N-acetylamino sugars on paper chromatograms. Biochim Biophys Acta. 1959 Aug;34:308–312. doi: 10.1016/0006-3002(59)90284-7. [DOI] [PubMed] [Google Scholar]
- SCHMIDT G., OTTENSTEIN B., SPENCER W. A., KECK K., BLIETZ R., PAPAS J., PORTER D., LEVIN M. L., THANNHAUSER S. J. The partition of tissue phospholipides by phosphorus analysis. AMA J Dis Child. 1959 May;97(5 Pt 2):691–708. doi: 10.1001/archpedi.1959.02070010693007. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Smith R. L., Gilkerson E. Quantitation of glycosaminoglycan hexosamine using 3-methyl-2-benzothiazolone hydrazone hydrochloride. Anal Biochem. 1979 Oct 1;98(2):478–480. doi: 10.1016/0003-2697(79)90170-2. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Saito H., Yamagata T., Anno K., Seno N., Kawai Y., Furuhashi T. Formation of three types of disulfated disaccharides from chondroitin sulfates by chondroitinase digestion. J Biol Chem. 1968 Apr 10;243(7):1543–1550. [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]


