Abstract
The G protein-coupled cholecystokinin 1 receptor (CCK1R) is activated permanently by type II photodynamic action (i.e., by singlet oxygen) in the freshly isolated rat pancreatic acini, in contrast to reversible activation by CCK. But how CCK1R is photodynamically activated is not known. Therefore, in the present work, we subjected membrane proteins extracted from isolated rat pancreatic acini to photodynamic action with photosensitiser sulphonated aluminium phthalocyanine (SALPC), and used reducing gel electrophoresis and Western blot to detect possible changes in CCK1R oligomerization status. Photodynamic action (SALPC 1 µM, light 36.7 mW cm− 2 × 10 min) was found to convert dimeric CCK1R nearly quantitatively to monomers. Such conversion was dependent on both irradiance (8.51–36.7 mW cm− 2) and irradiation time (1–20 min). Minimum effective irradiance was found to be 11.1 mW cm− 2 (× 10 min, with SALPC 1 µM), and brief photodynamic action (SALPC 1 µM, 36.7 mW cm− 2 × 1 min) was effective. Whilst CCK stimulation of purified membrane proteins alone had no effect on CCK1R dimer/monomer balance, sub-threshold photodynamic action (SALPC 100 nM, 36.7 mW cm− 2 × 10 min) plus CCK revealed a bell-shaped CCK dose response curve for CCK1R monomerization, which was remarkably similar to the dose response curve for CCK-stimulated amylase secretion in isolated rat pancreatic acini. These two lines of evidence together suggest that during photodynamic CCK1R activation, CCK1R is permanently monomerized, thus providing a unique approach for permanent G protein-coupled receptor (GPCR) activation which has not been achieved before.
Keywords: Cholecystokinin 1 receptor (CCK1R), Permanent activation, Photodynamic pharmacology, G protein-coupled receptor activated by singlet oxygen (GPCR-ABSO)
Introduction
Cholecystokinin (CCK) 1 receptors (CCK1R) mediate physiological functions such as exocrine pancreatic secretion, gallbladder contraction, gastrointestinal motility (Desai et al. 2014; Ozaki et al. 2013; Dong et al. 2015), satiety and other sensations (Ozaki et al. 2013; Broberger et al. 2001; Kaczynska and Szereda-Przestaszewska 2015; Li et al. 2011). Importantly, CCK1R are expressed in hippocampus and defined extracortical sites (Nishimura et al. 2015; Ballaz 2017).
We have previously found that CCK1R is permanently activated by type II photodynamic action with photosensitisers such as sulphonated aluminium phthalocyanine (SALPC) by generating singlet oxygen (1O2) at the plasma membrane in the freshly isolated rat pancreatic acini (Cui and Kanno 1997; Cui et al. 1997; An et al. 2003; Jiang et al. 2017). CCK1R is permanently activated also by SALPC photodynamic action in rat pancreatic acinar tumor cell AR4-2J, and permanently activated after ectopical expression in cell lines by photodynamic action with SALPC and with genetically encoded protein photosensitisers KillerRed and miniSOG (Jiang et al. 2018). Such permanent photodynamic CCK1R activation leads to persistent physiological-like calcium oscillations (Cui and Kanno 1997; Cui et al. 1997; An et al. 2003; Jiang et al. 2018) and enhanced amylase secretion with no detrimental effect on cellular physiology (Matthews and Cui 1989, 1990a, b; Cui and Matthews 1998; An et al. 2003). Although permanent photodynamic CCK1R activation is now firmly established, the detailed activation mechanism is not known.
Most G protein-coupled receptors (GPCR) are known to form dimers or oligomers in situ in living cells (Terrillon and Bouvier 2004; Milligan 2004; Bai 2004; Gurevich and Gurevich 2008; Pétrin and Hébert 2012; Møller et al. 2017). CCK1R over-expressed in certain cell lines are dimeric under resting conditions but dissociate into monomers after CCK stimulation (Cheng and Miller 2001; Cheng et al. 2003). Therefore, to elucidate the mechanism of permanent photodynamic CCK1R activation, we examined whether photodynamic action would have any effect on CCK1R oligomerization status.
Therefore, in the present work, we extracted membrane proteins from the freshly isolated rat pancreatic acini, subjected the extracted membrane proteins to SALPC photodynamic action, before reducing gel electrophoresis and Western blot, to detect possible SALPC photodynamic effects on CCK1R dimer/monomer balance. Most remarkably, SALPC photodynamic action was found to convert resting dimeric CCK1R almost quantitatively to monomers at the photodynamic intensity known to permanently activate CCK1R in the freshly isolated rat pancreatic acini, in rat pancreatic acinar tumor cell AR4-2J, and in ectopically CCK1R-expressing cell lines! In addition, sub-threshold SALPC photodynamic action was found to unmask (transfix) a dose response curve for CCK-stimulated CCK1R monomerization nearly identical to the dose response curve for CCK-stimulated amylase secretion in the isolated rat pancreatic acini. Our data provide two strong lines of evidence to indicate that permanent photodynamic CCK1R activation is due to direct and permanent CCK1R dimer-to-monomer conversion. To the best of our knowledge, this is the only case of permanent activation of a G protein-coupled receptor (GPCR), achieved by permanent photodynamic monomerization. This intrinsic photo-oxidative activation property of CCK1R has important implications for CCK1R physiology.
Materials and Methods
Reagents
Cholecystokinin sulphated octapeptide (CCK) and soybean trypsin inhibitor were from Sigma–Aldrich (St. Louis, MO, USA). MEM amino acids mixture (50×), DMEM/F12 (1:1) medium, trypsin-EDTA were from InVitrogen. Cell-Tak was from BD Biosciences (Bedford, MA, USA). Fura-2 AM was from AAT Bioquest (Sunnyvale, CA, USA). Sulphonated aluminium phthalocyanine (SALPC, also named aluminium phthalocyanine chloride tetrasulfonic acid, or tetrasulphonated aluminium phthalocyanine chloride, formula: C32H16AlClN8O12S4) was from Frontier Scientific Inc. (Cat. No. AlPcS-834, Logan, UT, USA). Collagenase P was from Roche (Mannheim, Germany). Rabbit anti-CCK1R and membrane protein extraction kit were from Abcam (Cambridge, UK). HRP-conjugated goat anti-mouse IgG, HRP-conjugated goat anti-rabbit IgG, monoclonal mouse anti-β-actin, PVDF membrane and ECL Western blot kit were from Beijing Kangweishiji (Beijing, China).
Isolation of Rat Pancreatic Acini
Male rats of the Sprague–Dawley strain (body weight 250–450 g) (Vital River/HuaFuKang Experimental Animals, Beijing, China) were housed with free access to food and water with natural light cycles. Rats were killed by CO2 asphyxia, then abdominal cavity was opened, pancreas was excised and used for pancreatic acini isolation by collagenase P (2 g L− 1) digestion as reported before (Cui and Kanno 1997; An et al. 2003; Liang et al. 2013). This procedure was approved by The Animal Ethics and Welfare Committee at Beijing Normal University School for Life Sciences. Buffer for acini isolation had the following composition (in mM): NaCl 118, KCl 4.7, MgCl2 1.13, CaCl2 2.5, NaH2PO4 1.0, d-glucose 5.5, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 10, l-glutamine 2.0, bovine serum albumin (BSA) 2%, minimum essential medium (MEM) amino acids mixture 2%, soybean trypsin inhibitor 0.1 g L− 1, with pH adjusted to 7.4.
SALPC Photodynamic Action
Total membrane proteins were extracted from isolated rat pancreatic acini as reported in other cell types (Subbarayal et al. 2015). Extracted membrane proteins were incubated with SALPC for 10 min in the dark, before light irradiation (λ > 580 nm, 20,500 lx/36.7 mW cm− 2) from a halogen light source (Hoya-Schott, HL100R, Tokyo, Japan), equipped with a condenser (HLL201) and a filter (R60, λ > 580 nm). Note that 580–710 nm would cover the whole Q band of the SALPC absorption spectrum, with a peak at 675 nm. In the future, a laser or LED emitting at around 675 nm may also be used for such experiments.
Western Blot
Protein samples were subject to SDS–PAGE under reducing conditions. The separated proteins were visualized and quantified by Western blot, with band intensity normalized to that of β-actin. For SDS–PAGE, samples each containing 30 µg of solubilized membrane proteins were incubated with SALPC in reducing loading buffer for 10 min at 37 °C before light irradiation. Irradiated samples were then loaded onto 12% Tris-glycine polyacrylamide gels and run at 120 V in the presence of 0.1% SDS. Western blots were performed as described in the literature (Li et al. 2007; Hu et al. 2013). Blots were probed with a rabbit anti-rat CCK1R polyclonal antibody; β-actin was probed as internal controls with mouse monoclonal anti-rat β-actin antibody. Immunoreactive proteins were visualized by SuperSignal West Pico Chemiluminescent Substrate (KangWeiShiJi, Beijing, China) and quantified.
Statistical Analysis
All experiments described here have been done at least three times, each time with extracted membrane proteins from different rats. Western blot images were analyzed with ChemiAnalysis; quantified data are given as mean ± SEM from ≥ 3 independent experiments from different rats, and plotted with SigmaPlot. Student’s t test was used, with asterisk (*) indicates statistical significance at p < 0.05.
Results and Discussions
Photodynamic Dimer-to-Monomer Conversion of Rat Pancreatic Acinar Cell CCK1R: SALPC Concentration Dependence
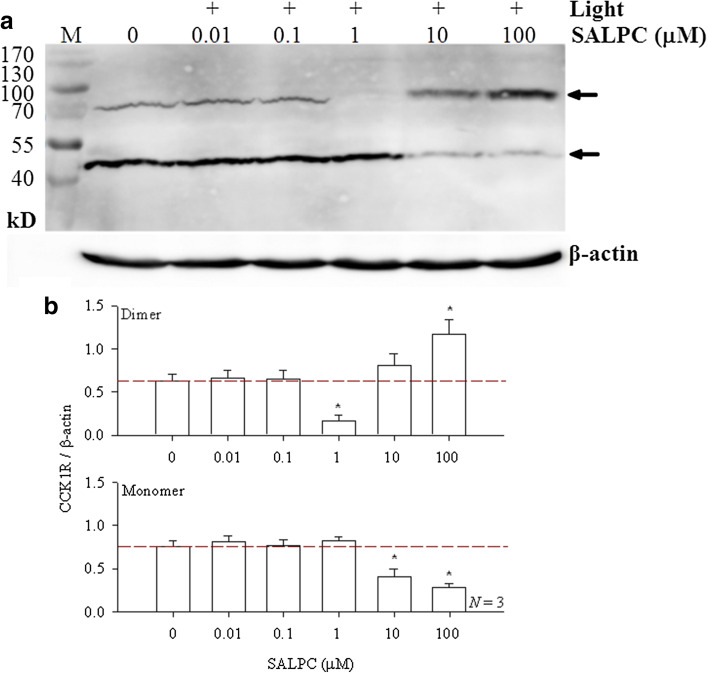
Membrane proteins extracted from isolated rat pancreatic acini were incubated with SALPC in the dark. Since in live cell physiology with either the freshly isolated rat pancreatic acini, with AR4-2J cells or with ectopical CCK1R-expressing cell lines, SALPC was used at 1 μM to trigger permanent CCK1R activation (Cui and Kanno 1997; An et al. 2003; Jiang et al. 2018), the SALPC concentrations used in vitro with extracted membrane proteins varied from 0.01 to 100 µM (two orders lower and two orders higher than 1 µM) to cover possible variations either way, red light (λ > 580 nm) irradiation was applied at irradiance of 36.7 mW cm− 2 (illuminance 20, 500 lx) for 10 min. Photodynamically treated membrane proteins were then subject to reducing gel electrophoresis and Western blot. In the absence of SALPC and light, monomer predominated over dimeric CCK1R (Fig. 1a, b). Photodynamic action with lower concentrations of SALPC (0.01 and 0.1 µM) had no apparent effect on CCK1R dimer/monomer balance (Fig. 1a, b). Most remarkably, photodynamic action with SALPC 1 µM led to near complete disappearance of the dimeric form of CCK1R (Fig. 1a, b, p < 0.05), the monomeric CCK1R was correspondingly increased slightly (Fig. 1a, b). At higher SALPC concentration of 10 µM, the monomeric CCK1R decreased significantly (Fig. 1a, b, p < 0.05), with corresponding slight increase in dimer content. Photodynamic action with SALPC 100 µM resulted in marked increase in the dimeric CCK1R and corresponding marked decrease in monomeric CCK1R (Fig. 1a), changes in both directions were statistically significant (Fig. 1b, p < 0.05).
Fig. 1.
Photodynamic monomerization of rat pancreatic acinar cell CCK1R: SALPC concentration dependence. a Extracted rat pancreatic acinar cell membrane proteins were incubated with SALPC (0.01, 0.1, 1, 10, 100 μM) for 10 min, then exposed to light (λ > 580 nm, 20,500 lx/36.7 mW cm− 2) for 10 min, before electrophoresis and Western blot. SALPC concentrations and light irradiation are as indicated. Note the β-actin internal standards. Positions of CCK1R monomer (46 kD) and dimer (92 kD) are as indicated by the arrows. M molecular weight markers (in kD). A representative experiment is shown. b Quantitative analysis was done, and ratios of CCK1R dimer/β-actin or CCK1R monomer/β-actin are plotted (N = 3). Asterisks (*) indicate p < 0.05
These data indicate that extracted CCK1R protein of rat pancreatic acini exists in vitro in both monomeric and dimeric forms, with the monomer predominating. Lower photodynamic intensities (SALPC 0.01, 0.1 μM, 36.7 mW cm− 2, 10 min) did not seem to have any apparent effect on the monomer/dimer balance, but moderate photodynamic intensity (SALPC 1 μM, 36.7 mW cm− 2, 10 min), which leads to permanent CCK1R activation in live cells (SALPC 1 μM, 53,000–55,000 lx/72.39 mW cm− 2, see Cui and Kanno 1997; An et al. 2003; Jiang et al. 2018), led to near total disappearance of CCK1R dimers.
Although it is not clear how photo-oxidation leads to CCK1R monomerization, obliteration of certain hydrophobic patches important for inter-molecular association may play a role. This could involve the oxidation of the hydrophobic Met residues important for CCK1R activation (Cheng et al. 2003; Gigoux et al. 1998; Escrieut et al. 2002; Archer-Lahlou et al. 2005). Photodynamic oxidation may also interfere with interactions between TM5 and TM6, which are known to be important for µ-opioid receptor dimer formation (Manglik et al. 2012)—such TM interfaces are destabilized by agonist binding (Kuszak et al. 2009). Synthetic peptides of CCK1R-TM6 inhibit competitively CCK1R oligomerization. Alanine scanning revealed that I317, V321, and L325 in TM6 are important for CCK1R-BRET/dimer formation (Harikumar et al. 2006), but these 3 residues are not singlet oxygen targets, therefore, are unlikely to be involved in photodynamic CCK1R monomerization.
Increased photodynamic strength (SALPC 10, 100 μM, 36.7 mW cm− 2, 10 min) led to a reversion of CCK1R dimer/monomer ratios. This latter effect could probably be due to cross-linking of CCK1R proteins but at the pancreatic acinar cell level such high intensity photodynamic action will permeabilize plasma membrane (Matthews and Cui 1990a, b), therefore, is of no significance for normal cellular physiology and will not be explored further. Nonetheless, it may be noted here that photodynamic protein cross-linking has been reported before. Rose Bengal photodynamic action was found to lead to cross-linking of His (P-Acap-His)- or Lys (P-Acap-Lys)-containing N-(2-hydroxypropyl)methacrylamide, due to His–His and His–Lys cross-linkage (Shen et al. 1996a). Rose Bengal photodynamic action also generated dimeric and oligomeric forms of bovine pancreatic RNase A by His–His/His–Lys linkage without involving any Met or Cys residues (Shen et al. 1996b). Photodynamic cross-link has been found with STAT-3, to form inactivated STAT-3 homo-oligomers (Liu et al. 2004; Henderson et al. 2007; Zheng et al. 2009). Photofrin photodynamic action in cultured human epidermoid skin carcinoma cell A431 and directly on recombinant epidermal growth factor receptor (EGFR) protein led to EGFR cross-linkage with complete abolition of EGFR kinase activity (Hsieh et al. 2017).
The oligomerization status of GPCR could be investigated by a number of means depending on whether the receptor is studied in situ (Mishra et al. 2016; Song et al. 2018), or in vitro outside of the cellular environment, as is the case in the present work. Extracted membrane proteins and purified receptor proteins could be studied directly without interference from the lipid bilayer. Which method is used also depends on the purification status of the extracted membrane/receptor proteins (Matthews and Sunde 2012; Mathiasen et al. 2014; Gong et al. 2017).
In the present work, photodynamic CCK1R dimer-to-monomer conversion was achieved in extracted membrane proteins in solution and this is directly correlated to permanent photodynamic activation of CCK1R. Therefore, we further characterize the process of photodynamic CCK1R monomerization.
Photodynamic Dimer-to-Monomer Conversion of Pancreatic Acinar Cell CCK1R: Dependence on Irradiance and Irradiation Duration
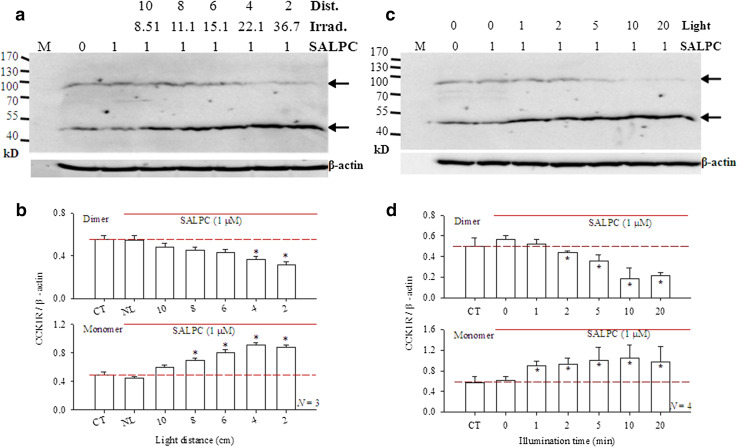
The photodynamic CCK1R dimer-to-monomer transition was found to be dependent on light irradiance. In this series of experiments, the extracted rat pancreatic acinar cell membrane proteins were subject to SALPC photodynamic action with irradiance from 0 to 36.7 mW cm− 2. As before, SALPC 1 μM in the absence of light had no effect on CCK1R dimer/monomer balance (Fig. 2a, b). Keep SALPC concentration constant at 1 µM, and light (λ > 580 nm) irradiation duration at 10 min, light irradiance was varied at 0, 8.51, 11.1, 15.1, 22.1, 36.7 mW cm− 2 (Fig. 2a, b). Low irradiance of 8.51 mW cm− 2 (with corresponding solution-to-light head distance of 10 cm) did not lead to statistically significant changes in CCK1R dimer/monomer balance (Fig. 2a, b). But light at 11.1 and 15.1 mW cm− 2 (solution-to-light head distances of 8 and 6 cm, respectively) increased the monomer content significantly (Fig. 2a, b, p < 0.05). At higher irradiances of 22.1 mW cm− 2 (4 cm from light head) and 36.7 mW cm− 2 (2 cm from light head), the CCK1R receptor dimer decreased significantly, with simultaneous increases in monomer content (Fig. 2a, b, p < 0.05).
Fig. 2.
Photodynamic monomerization of rat pancreatic acinar cell CCK1R: dependence on irradiation duration and irradiance. a Extracted rat pancreatic acini membrane proteins were incubated with SALPC 1 µM for 10 min, then exposed to light of different irradiance (λ > 580 nm, 8.51–36.7 mW cm− 2) for 10 min, before electrophoresis and Western blot. SALPC concentration (1 μM), light irradiance (0, 8.51, 11.1, 15.1, 22.1, 36.7 mWatt cm− 2), and corresponding distances between membrane protein solution and light head (in cm) are as indicated. Note the β-actin internal standards. Positions of CCK1R monomer (46 kD) and dimer (92 kD) are as indicated by the arrows. M molecular weight markers (in kD). A representative experiment is shown. b Quantitative analysis was done, ratios of CCK1R dimer/β-actin or CCK1R monomer/β-actin are plotted (N = 3). CT control, no light or SALPC. NL with SALPC 1 μM but no light. Asterisks (*) indicate P < 0.05. c Extracted rat pancreatic acini membrane proteins were incubated with SALPC 1 µM for 10 min, then exposed to light (λ > 580 nm, 20,500 lx/36.7 mW cm− 2) for 1–20 min, before electrophoresis and Western blot. Note the β-actin internal standards. Positions of CCK1R monomer (46 kD) and dimer (92 kD) are as indicated by the arrows. M molecular weight markers (kD). A representative experiment is shown. d Quantitative analysis was done, ratios of CCK1R dimer/β-actin or CCK1R monomer/β-actin are plotted (N = 4). CT control, no light or SALPC. Asterisks (*) indicate P < 0.05
These data show clearly that photodynamic CCK1R monomerization was dependent on light irradiance, very low light level (8.51 mWatt cm− 2) could exert distinguishable tendency for photodynamic CCK1R monomerization, although statistical significance was not reached until light was increased to 11.1 mWatt cm− 2. These data illustrate the high sensitivity of photodynamic CCK1R activation.
Photodynamic CCK1R dimer-to-monomer transition was dependent also on the duration of light irradiation. In this series of experiments, the photosensitiser concentration was set at 1 μM and irradiance set at 36.7 mW cm− 2 but with varied irradiation time (0–20 min). SALPC 1 μM alone in the dark was without any effect on CCK1R dimer/monomer balance (Fig. 2c, d). Very brief photodynamic action (SALPC 1 μM, light 36.7 mW cm− 2 × 1 min) of the SALPC-incubated membrane proteins led to significant increase in monomeric CCK1R (Fig. 2c, d, p < 0.05), although simultaneous decrease in dimer was still not detected (Fig. 2c, d). Double the time of the photodynamic action to 2 min, simultaneous decrease in dimer and increase in monomer were seen (Fig. 2c, d, p < 0.05). Longer photodynamic action (5, 10, 20 min) led to progressively more dimer-to-monomer conversion, with nearly complete obliteration of the dimer content (Fig. 2c, d, p < 0.05).
These data clearly indicate that with fixed photodynamic intensity (SALPC 1 µM, light 36.7 mW cm− 2), the photodynamic monomerization process was very rapid. This correlates very well to the photodynamic CCK1R activation time course/kinetics we have reported previously. Very brief (1–1.5 min) photodynamic action with SALPC 1 μM (illuminance 53,000–55,000 lx/irradiance 72.39 mW cm− 2) activated permanently CCK1R in the freshly isolated rat pancreatic acini (1 min) (Cui and Kanno 1997; An et al. 2003) and in the cultured rat pancreatic acinar tumor cell line AR4-2J (SALPC 0.5 µM, red light > 580 nm, 36.7 mWatt cm− 2 × 90 s)(Jiang et al. 2018). Furthermore, photodynamic activation of CCK1R with the genetically encoded protein photosensitisers KillerRed or miniSOG was similarly very rapid (Jiang et al. 2018). Therefore, permanent photodynamic activation by monomerization of CCK1R requires only brief irradiation at low irradiance.
CCK-Stimulated Pancreatic Acinar Cell CCK1R Monomerization Revealed by Sub-threshold SALPC Photodynamic Action
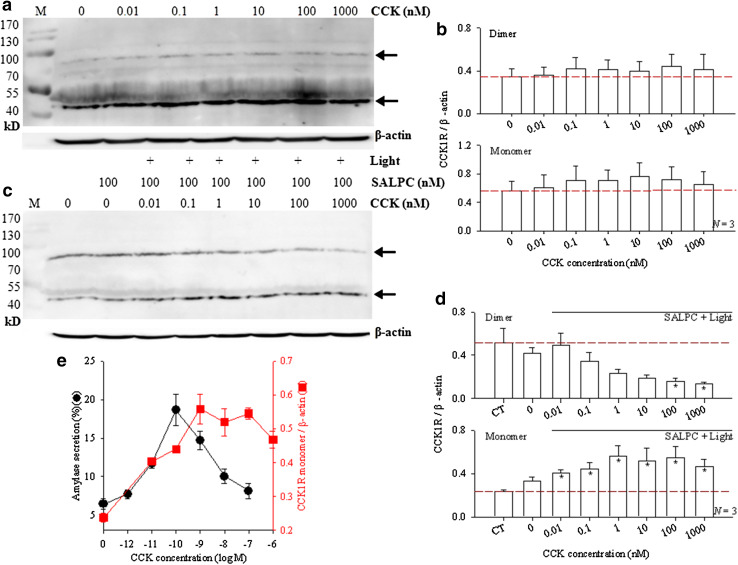
Although agonist-stimulated CCK1R monomerization has been demonstrated with ectopically expressed CCK1R in cell lines by BRET in situ (Cheng et al. 2003; Harikumar et al. 2006), such reversible CCK1R monomerization was not detected with extracted membrane proteins from rat pancreatic acini in vitro. Figure 3a and b show that CCK (10 pM − 1000 nM) had no effect on CCK1R dimer/monomer balance (p > 0.05). In this series of experiments, extracted rat pancreatic acini membrane proteins containing CCK1R were incubated (for 15 min) with CCK, then subject to reducing gel electrophoresis and Western blot. No apparent CCK perturbation of the CCK1R dimer/monomer balance was found (Fig. 3a, b). Such lack of an obvious CCK dose response relationship with CCK stimulation alone notwithstanding, we were able, however, to demonstrate a very nice “bell-shaped” CCK concentration dependence when simultaneous sub-threshold SALPC photodynamic action was applied.
Fig. 3.
A “bell-shaped” CCK dose response curve for pancreatic acinar cell CCK1R monomerization revealed by sub-threshold SALPC photodynamic action. a Extracted rat pancreatic acinar cell membrane proteins were incubated with CCK (0, 0.01, 0.1, 1, 10, 100, 1000 nM) for 15 min, before electrophoresis and Western blot. b Quantitative analysis of experiments as shown in a, with ratios of CCK1R dimer/β-actin or CCK1R monomer/β-actin plotted (N = 3). P > 0.05 at all data points (N = 3). c Extracted rat pancreatic acinar cell membrane proteins were incubated with CCK (0, 0.01, 0.1, 1, 10, 100 nM) for 15 min, SALPC 100 nM was then added and incubated for another 10 min (in the presence of CCK), before light irradiation (λ > 580 nm, 36.7 mW cm− 2) for 10 min, and subsequent electrophoresis/Western blot. CCK concentrations, SALPC (100 nM) and light are as indicated. d Quantitative analysis of experiments as shown in c, with ratios of CCK1R dimer/β-actin or CCK1R monomer/β-actin plotted. Asterisks (*) indicate p < 0.05 (N = 3). CT control, no CCK, SALPC, or light. Column 2 from left: SALPC 100 nM, no CCK, no light. Positions of CCK1R monomer (46 kD) and dimer (92 kD) are as indicated by the arrows in a and c M MWt markers (in kD), β-actin internal standards are also shown (a, c). Representative experiments are shown (a, c). e A comparison of the dose response curves of CCK-stimulated amylase secretion from freshly isolated rat pancreatic acini and of CCK-stimulated rat pancreatic acinar cell CCK1R monomerization under sub-threshold SALPC photodynamic action (SALPC 100 nM, 36.7 mWatt.cm− 2 × 10 min). The curve for CCK-stimulated amylase secretion in isolated rat pancreatic acini (circles on left ordinate) was re-drawn from data we published previously as Fig. 1a in Xiao and Cui (2004). The curve for CCK1R monomerization (squares on right ordinate) was re-drawn from d lower panel. Note the complete overlap of the two curves on the left side, one order of magnitude difference (right-hand shift for CCK1R monomerization) in maximal CCK concentrations, and elevated right-side shoulder of the CCK1R monomerization curve
The extracted pancreatic acinar cell membrane proteins were incubated with CCK (0.01–1000 nM) for 15 min, followed by the addition of SALPC 100 nM for another 10 min (with CCK still present), light irradiation was then applied for 10 min. This latter part of the treatment (SALPC 100 nM, 36.7 mW cm− 2 × 10 min) was sub-threshold photodynamic action, which was without any effect on CCK1R dimer/monomer balance (see Fig. 1a, b). But CCK plus the sub-threshold SALPC photodynamic action revealed a very clear-cut “bell-shaped” CCK dose response curve.
As before, SALPC 100 nM in the dark had no effect on CCK1R dimer/monomer balance (Fig. 3c, d). Upon stimulation with CCK 0.01 nM (10 pM), sub-threshold photodynamic action (SALPC 100 nM, light 36.7 mWatt.cm− 2 × 10 min) led to a marked increase in CCK1R monomer content (Fig. 3c, d, p < 0.05). Higher CCK concentrations led to higher CCK1R monomer content (Fig. 3c, d). Maximal increase in CCK1R monomer content was seen with CCK 1 nM, the effect of further increases in CCK concentrations gradually leveled off, before supra-maximal inhibition was seen at CCK 1000 nM (Fig. 3c, d). The increases in monomer content with increasing CCK concentrations formed a typical “bell-shaped” dose response curve. The corresponding decrease in CCK1R dimer content followed a similar dose relationship, although statistically significant decreases were not seen until CCK concentrations were increased to 100 or 1000 nM (Fig. 3c, d). The “bell-shaped” curve of CCK dose response observed with monomer content here in the presence of sub-threshold SALPC photodynamic action resembles very closely the dose response curve of CCK-stimulated amylase secretion in freshly isolated rat pancreatic acini that we have reported previously (Rogers et al. 1988; Xiao and Cui 2004; Wang et al. 2009).
The CCK concentration for maximal stimulation of amylase secretion from freshly isolated rat pancreatic acini has been found to be 100 pM (Rogers et al. 1988; Xiao and Cui 2004; Wang et al. 2009), whereas the maximal CCK concentration for stimulation of CCK1R monomerization (under sub-threshold photodynamic action) was found to be 1 nM (Fig. 3e). It is most remarkable that the left-hand sides of the 2 curves almost completely overlapped, meaning little sensitivity towards physiological CCK stimulations (pM range) is lost even after CCK1R is extracted from the cellular environment. On the other hand, there is one order of magnitude of rightward-shift in maximal CCK stimulation when CCK1R is extracted in comparison with CCK1R in situ in intact pancreatic acini. Also note the elevated right-side shoulder of the curve for CCK1R monomerization (Fig. 3e), which suggests that the cellular environment is indeed required for complete supra-maximal inhibition of CCK-stimulated amylase secretion to occur.
Conclusions and Perspectives
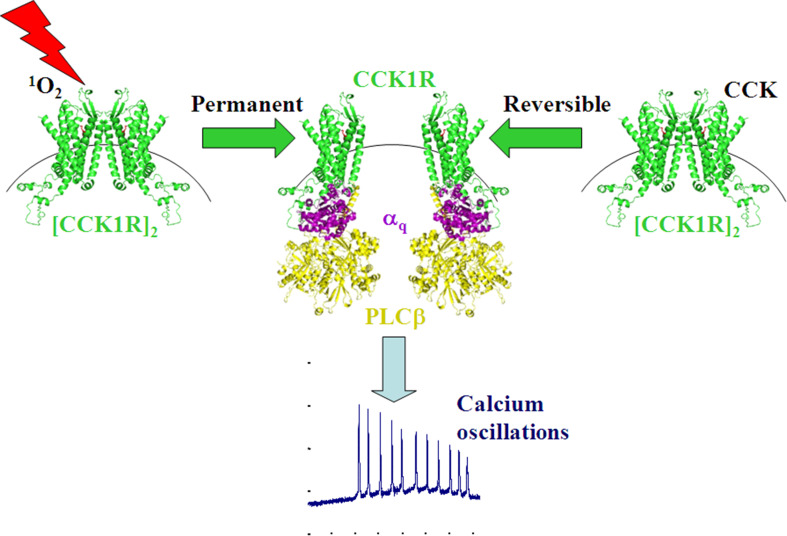
In summary, it was found in the present work that SALPC photodynamically converted normal rat pancreatic acinar cell CCK1R protein dimer to monomer, dependent upon SALPC concentration, light irradiance and irradiation duration. The rapid response time and low light irradiance needed to achieve such photodynamic CCK1R monomerization are consistent with parameters for permanent photodynamic CCK1R activation in isolated rat pancreatic acini, in cultured rat pancreatic acinar tumor cell line, and in ectopically CCK1R-expressing cell lines. Further, sub-threshold photodynamic action revealed a “bell-shaped” CCK dose response relationship similar to the dose response curve for CCK-stimulated amylase secretion in isolated rat pancreatic acini. These two lines of evidence strongly suggest that permanent photodynamic CCK1R activation is achieved by irreversible photodynamic CCK1R monomerization, to trigger physiological-like cytosolic calcium oscillations (Fig. 4). This intrinsic photo-oxidative activation property of CCK1R has important implications for photodynamic physiology. For a recent review article on this latter point, please refer to Jiang et al. 2017. In addition, in the clinic, photodynamic therapy of primary pancreatic cancer if not strictly restricted to the lesion site might, by permanently activating the CCK1R, actually stimulate normal pancreatic acinar cells to secrete digestive enzymes through the normal duct system to the duodenum. The much reduced doses of photosensitiser and light in neighboring normal tissue might, therefore, help to improve digestion. This photo-oxidative activation (also with fused genetically encoded protein photosensitisers such as KillerRed or miniSOG, see Jiang et al. 2018) property of CCK1R may also render it a useful photodynamic tool kit for in vivo confirmation of CCK1R function both in the central nervous system and in the periphery tissues.
Fig. 4.
CCK1R—a G protein-coupled receptor activated by singlet oxygen (GPCR-ABSO). Dimer-to-monomer conversion of CCK1R by photodynamic action (SALPC 1 µM, light 36.7 mW cm− 2) or by CCK (20 pM) leads to permanent or reversible CCK1R activation, respectively, both can trigger physiological cytosolic calcium oscillations. CCK1R, Gαq, phospholipase Cβ (PLCβ) are represented by 3-dimensional structures. The three-dimensional model of CCK1R is obtained from Swiss-model, with input CCK-1R (green) sequence from Kazmi et al. (2016). Structures of Gαq (purple) and PLCβ(yellow) are obtained with PyMOL after input from PDB database (1TAG, 2ZKM respectively)
Acknowledgements
This work was supported by The Natural Science Foundation of China (Grant Nos. 31670856, 31270892) and by The Ministry of Science and Technology of China (Grant No. 2011CB809101). We would like to thank the anonymous Referee for very insightful comments which have helped to improve our MS.
Author Contributions
ZJC conceived the idea of the project, supervised the experiments and finalized the MS. WYJ performed the experiments and wrote the initial drafts. YL and ZYL helped in the design and interpretation of data, YL drew the 3-D structures in Fig. 4. All authors read and approved the final version for publication.
Compliance with Ethical Standards
Conflict of interest
The authors (WYJ, YL, ZYL, ZJC) declare that there are no conflicts of interest.
References
- An YP, Xiao R, Cui H, Cui ZJ ZJ (2003) Selective activation by photodynamic action of cholecystokinin receptor in the freshly isolated rat pancreatic acini. Br J Pharmacol 139:872–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer-Lahlou E, Escrieut C, Clerc P, Martinez J, Moroder L, Logsdon C, Kopin C, Seva C, Dufresne M, Pradayrol L, Maigret B, Fourmy D (2005) Molecular mechanism underlying partial and full agonism mediated by the human cholecystokinin-1 receptor. J Biol Chem 280:10664–10674 [DOI] [PubMed] [Google Scholar]
- Bai M (2004) Dimerization of G-protein-coupled receptors: roles in signal transduction. Cell Signal 16:175–186 [DOI] [PubMed] [Google Scholar]
- Ballaz S (2017) The unappreciated roles of the cholecystokinin receptor CCK1 in brain functioning. Rev Neurosci 28:573–585 [DOI] [PubMed] [Google Scholar]
- Broberger C, Holmberg K, Shi TJ, Dockray G, Hokfelt T (2001) Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res 903:128–140 [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Miller LJ (2001) Agonist-dependent dissociation of oligomeric complexes of G protein-coupled cholecystokinin receptors demonstrated in living cells using bioluminescence resonance energy transfer. J Biol Chem 276:48040–48047 [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Harikumar KG, Holicky EL, Miller LJ (2003) Heterodimerization of type A and B cholecystokinin receptors enhance signaling and promote cell growth. J Biol Chem 278:52972–52979 [DOI] [PubMed] [Google Scholar]
- Cui ZJ, Kanno T (1997) Photodynamic triggering of calcium oscillation in the isolated rat pancreatic acini. J Physiol 504:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui ZJ, Matthews EK (1998) Photodynamic modulation of cellular function. Acta Pharmacol Sin/Zhongguo Yao Li Xue Bao 19:297–303 [PubMed] [Google Scholar]
- Cui ZJ, Habara Y, Wang DY, Kanno T (1997) A novel aspect of photodynamic action: induction of recurrent spikes in cytosolic calcium concentration. Photochem Photobiol 65:382–386 [DOI] [PubMed] [Google Scholar]
- Desai AJ, Harikumar KG, Miller LJ (2014) A type 1 cholecystokinin receptor mutant that mimics the dysfunction observed for wild type receptor in a high cholesterol environment. J Biol Chem 289:18314–18326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Vattelana AM, Lam PC, Orry AJ, Abagyan R, Christopoulos A, Sexton PM, Haines DR, Miller LJ (2015) Development of a highly selective allosteric antagonist radioligand for the type 1 cholecystokinin receptor and elucidation of its molecular basis of binding. Mol Pharmacol 87:130–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escrieut C, Gigoux V, Archer E, Verrier S, Maigret B, Behrendt R, Moroder L, Bignon E, Silvente-Poirot S, Pradayrol L, Fourmy D (2002) The biologically crucial C terminus of cholecystokinin and the nonpeptide agonist SR-146, 131 share a common binding site in the human CCK1 receptor. Evidence for a crucial role of Met-121 in the activation process. J Biol Chem 277:7546–7555 [DOI] [PubMed] [Google Scholar]
- Gigoux V, Escrieut C, Silvente-Poirot S, Maigret B, Gouilleux L, Fehrentz JA, Gully D, Moroder L, Vaysse N, Fourmy D (1998) Met-195 of the cholecystokinin-A receptor interacts with the sulfated tyrosine of cholecystokinin and is crucial for receptor transition to high affinity state. J Biol Chem 273:14380–14386 [DOI] [PubMed] [Google Scholar]
- Gong Z, Liu Z, Dong X, Ding YH, Dong MQ, Tang C (2017) Protocol for analyzing protein ensemble structures from chemical cross-links using DynaXL. Biophys Rep 3(4–6):100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2008) Rich tapestry of G protein-coupled receptor signaling and regulatory mechanisms. Mol Pharmacol 74:312–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar KG, Dong M, Cheng Z, Pinon DI, Lybrand TP, Miller LJ (2006) Transmembrane segment peptides can disrupt cholecystokinin receptor oligomerization without affecting receptor function. Biochemistry 45:14706–14716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BW, Daroqui C, Tracy E, Vaughan LA, Loewen GM, Cooper MT, Baumann H (2007) Cross-linking of signal transducer and activator of transcription 3-a molecular marker for the photodynamic reaction in cells and tumors. Clin Cancer Res 13:3156–3163 [DOI] [PubMed] [Google Scholar]
- Hsieh YJ, Chien KY, Yang IF, Lee IN, Wu CC, Huang TY, Yu JS (2017) Oxidation of protein-bound methionine in Photofrin-photodynamic therapy-treated human tumor cells explored by methionine-containing peptide enrichment and quantitative proteomics approach. Sci Rep 7:1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Hu K, Liu T, Stern MK, Mistry R, Challiss RA, Costanzi S, Wess J (2013) Novel structural and functional insights into M3 muscarinic receptor dimer/oligomer formation. J Biol Chem 288:34777–34790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HN, Li Y, Cui ZJ (2017) Photodynamic physiology—photonanomanipulations in cellular physiology with protein photosensitisers. Front Physiol 8:191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HN, Li Y, Jiang WY, Cui ZJ (2018) Cholecystokinin 1 receptor—a unique G protein-coupled receptor activated by singlet oxygen (GPCR-ABSO). Front Physiol 9:497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczynska K, Szereda-Przestaszewska M (2015) Contribution of CCK1 receptors to cardiovascular and respiratory effects of cholecystokinin in anesthetized rats. Neuropeptides 54:29–34 [DOI] [PubMed] [Google Scholar]
- Kazmi HR, Chandra A, Nigam J, Baghel K, Srivastava M, Maurya SS, Parmar D (2016) Polymorphism and expression profile of cholecystokinin type A receptor in relation to gallstone disease susceptibility. Biochem Genet 54:665–675 [DOI] [PubMed] [Google Scholar]
- Kuszak AJ, Pitchiaya S, Anand JP, Mosberg HI, Walter NG, Sunahara RK (2009) Purification and functional reconstitution of monomeric µ-opioid receptors: allosteric modulation of agonist binding by Gi2. J Biol Chem 284:26732–26741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Han SJ, Hamdan FF, Kim SK, Jacobson KA, Bloodworth LM, Zhang X, Wess J (2007) Distinct structural changes in a G protein-coupled receptor caused by different classes of agonist ligands. J Biol Chem 282:26284–26293 [DOI] [PubMed] [Google Scholar]
- Li Y, Wu X, Zhou S, Owyang C (2011) Low-affinity CCK-A receptors are coexpressed with leptin receptors in rat nodose ganglia: implications for leptin as a regulator of short-term satiety. Am J Physiol Gastrointest Liver Physiol 300:G217-G227 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liang HY, Song ZM, Cui ZJ (2013) Lasting inhibition of receptor-mediated calcium oscillations in pancreatic acini by neutrophil respiratory burst-A novel mechanism for secretory blockade in acute pancreatitis? Biochem Biophys Res Commun 437:361–367 [DOI] [PubMed] [Google Scholar]
- Liu W, Oseroff AR, Baumann H (2004) Photodynamic therapy causes cross-linking of signal transducer and activator of transcription proteins and attenuation of interleukin-6 cytokine responsiveness in epithelial cells. Cancer Res 64:6579–6587 [DOI] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S (2012) Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature 485:321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiasen S, Christensen SM, Fung JJ, Rasmussen SG, Fay JF, Jorgensen SK, Veshaguri S, Farrens DL, Kiskowski M, Kobilka B, Stamou D (2014) Nanoscale high-content analysis using compositional heterogeneities of single proteoliposomes. Nat Methods 11:931–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EK, Cui ZJ (1989) Photodynamic action of rose bengal on isolated rat pancreatic acini: stimulation of amylase release. FEBS Lett 256:29–32 [DOI] [PubMed] [Google Scholar]
- Matthews EK, Cui ZJ (1990a) Photodynamic action of sulphonated aluminium phthalocyanine (SALPC) on isolated rat pancreatic acini. Biochem Pharmacol 39:1445–1457 [DOI] [PubMed] [Google Scholar]
- Matthews EK, Cui ZJ (1990b) Photodynamic action of sulphonated aluminium phthalocyanine (SALPC) on AR4-2J cells, a carcinoma cell line of rat exocrine pancreas. Br J Cancer 61:695–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JM, Sunde M (2012) Dimers, oligomers, everywhere. Adv Exp Med Biol 747:1–18 [DOI] [PubMed] [Google Scholar]
- Milligan G (2004) G protein-coupled receptor dimerization: function and ligand pharmacology. Mol Pharmacol 66:1–7 [DOI] [PubMed] [Google Scholar]
- Mishra AK, Gragg M, Stoneman MR, Biener G, Oliver JA, Miszta P, Filipek S, Raicu V, Park PS (2016) Quaternary structures of opsin in live cells revealed by FRET spectrometry. Biochem J 473:3819–3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller TC, Moreno-Delgado D, Pin JP, Kniazeff J (2017) Class C G protein-coupled receptors: reviving old couples with new partners. Biophys Rep 3(4–6):57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Bilgüvar K, Ishigame K, Sestan N, Günel M, Louvi A (2015) Functional synergy between cholecystokinin receptors CCKAR and CCKBR in mammalian brain development. PLoS ONE 10:e0124295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki T, Mohammad S, Morioka E, Takiguchi S, Ikeda M (2013) Infant satiety depends on transient expression of cholecystokinin-1 receptors on ependymal cells lining the third ventricle in mice. J Physiol 591:1295–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétrin D, Hébert TE (2012) The functional size of GPCR-monomers, dimers or tetramers? Subcell Biochem 63:67–81 [DOI] [PubMed] [Google Scholar]
- Rogers J, Hughes RG, Matthews EK (1988) Cyclic GMP inhibits protein kinase C-mediated secretion in rat pancreatic acini. J Biol Chem 263:3713–3719 [PubMed] [Google Scholar]
- Shen HR, Spikes JD, Kopeceková P, Kopecek J (1996a) Photodynamic crosslinking of proteins. I. Model studies using histidine- and lysine-containing N-(2-hydroxypropyl)methacrylamide copolymers. J Photochem Photobiol B Biol 34:203–210 [DOI] [PubMed] [Google Scholar]
- Shen HR, Spikes JD, Kopecková P, Kopecek J (1996b) Photodynamic crosslinking of proteins. II. Photocrosslinking of a model protein-ribonuclease A. J Photochem Photobiol B Biol 35:213–219 [DOI] [PubMed] [Google Scholar]
- Song Y, Ge B, Lao J, Wang Z, Yang B, Wang X, He H, Li J, Huang F (2018) Regulation of the oligomeric status of CCR3 with binding ligands revealed by single-molecule fluorescence imaging. Biochemistry 57:852–860 [DOI] [PubMed] [Google Scholar]
- Subbarayal P, Karunakaran K, Winkler AC, Rother M, Gonzalez E, Meyer TF, Rudel T (2015) EphrinA2 receptor (EphA2) is an invasion and intracellular signaling receptor for Chlamydia trachomatis. PLoS Pathog 11:e1004846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M (2004) Roles of G-protein-coupled receptor dimerization. EMBO Rep 5:30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BJ, Liang HY, Cui ZJ (2009) Duck pancreatic acinar cell as a unique model for independent cholinergic stimulation-secretion coupling. Cell Mol Neurobiol 29:747–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Cui ZJ (2004) Mutual dependence of VIP/PACAP and CCK receptor signaling for a physiological role in duck exocrine pancreatic secretion. Am J Physiol Regul Integr Comp Physiol 286:R189-R198 [DOI] [PubMed] [Google Scholar]
- Zheng X, Morgan J, Pandey SK, Chen Y, Tracy E, Baumann H, Missert JR, Batt C, Jackson J, Bellnier DA (2009) Conjugation of 2-(1′-hexyloxyethyl)-2-devinylpyropheophorbide-a (HPPH) to carbohydrates changes its subcellular distribution and enhances photodynamic activity in vivo. J Med Chem 52:4306–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]