Abstract
This study was designed to examine differential expression of miR-338-5p in gliomas and the role of miR-338-5p in glioma cell invasion via its potential target gene TSHZ3 encoding Teashirt zinc finger homobox 3, predicted by bioinformatics, and matrix metallopeptidase 2 (MMP2), the key pro-invasive protease overexpressed in gliomas. Quantitative real-time reverse transcription PCR (qRT-PCR) and Spearman correlation analysis were used to determine differential expressions of miR-338-5p and TSHZ3 in astrocytic gliomas of different grades (n = 35) and glioblastoma cell lines (U87 and U251) in comparison to non-neoplastic brain (NNB) tissues (n = 6). Western blotting was used to determine the protein levels of TSHZ3 and MMP2 in glioblastoma cell lines and Matrigel invasion assay to examine the role of miR-338-5p in cell invasiveness. The results showed that the expression of miR-338-5p, normalized to hsnRNA U6, was significantly higher in grade III and IV gliomas and glioblastoma cell lines compared to that in NNB and grade II gliomas, whereas TSHZ3 expression, normalized to GAPDH, was inversely related to miR-338-5p (R = −0.636, P < 0.01). Luciferase assays showed TSHZ3 to be a target gene of miR-338-5p. In both U87 and U251 cells, miR-338-5p mimics increased MMP2 and invasiveness of the cells. Overexpression of ectopic TSHZ3 suppressed the cell invasiveness and attenuated the pro-invasive effect of miR-338-5p mimics. Overall, our results showed that miR-338-5p has a function in promoting glioma cell invasion by targeting TSHZ3 suppression on MMP2. In conclusion, miR-338-5p is a possible potential biomarker for the diagnosis and target for therapy of high-grade glioma.
Keywords: Glioma, MiR-338-5p, TSHZ3, MMP2, Invasion
Introduction
Glioma is the primary malignant brain tumor. The survival rate of glioma patients has been marginally improved recently by total tumor resection followed by combined radio- and chemotherapies. However, the prognosis of patients with highly invasive gliomas remains poor (Liu et al. 2015). There is no clinical drug that can effectively target glioma invasion. Hence, treating malignant gliomas without a deep understanding of the molecular mechanisms underlying the invasiveness of glioma cells remains a challenge.
The development and progression of gliomas are associated with accumulations of genetic and epigenetic alterations to suppress tumor suppressors and/or activate oncogenes. In the past decades, deregulation of microRNAs (miRNAs) has been discovered in the initiation, progression and metastasis of many human cancers (Yin et al. 2015). They are a class of small endogenous non-coding RNAs of 18–25 nucleotides with functions of inducing mRNA degradation and/or suppressing protein translation via binding of the matching sequences in the 3′-untranslational region (UTR) of mRNAs, hence regulating protein translation from the targeted mRNAs. As a new regulatory factor class, miRNA involvement has been widely found in the control of cell survival, proliferation and motility of both normal developmental processes and malignancies (Peng et al. 2014).
MiR-338 is a family of brain-specific miRNA precursors found in mammals, including humans. MiR-338-5p (approximately 22 nucleotides) is a mature miRNA of MiR-338 excised from the precursor hairpin by the enzyme Dicer. It has been reported that miR-338-5p is involved in the tumorigenesis of colorectal cancer, H. pylori-negative gastric cancer, follicular lymphomas and hepatocellular carcinoma (Yong et al. 2013; Chang et al. 2015; Takei et al. 2014; Chen et al. 2015). The expression status and role of miR-338-5p in the tumorigenesis, diagnosis and prognosis of glioma were unknown prior this study.
In our previous study, we isolated invasive cells that migrated into normal mouse brain tissues from xenografts developed in orthotopic mouse models from seven clinical gliomas. Through comparison of miRNA expression profiles in the invasive cells with the matched cells from the tumor mass, we identified a panel of differentially expressed miRNAs in tumor core cells and their corresponding invasive cells of the same tumor origin (Huang et al. 2012). Among these differentially expressed miRNAs, miR-338-5p was highly expressed in invasive cells in comparison to cells in the tumor mass. Data from miRNA chip assays suggest that miR-338-5p may play a role in promoting glioma invasion. In this study, we determined the expression of miR-338-5p in a panel of 35 clinical gliomas of different malignant grades and explored the role of miR-338-5p in glioma cell invasion and its underling mechanisms in two glioblastoma cell lines, U87 and U251.
Materials and Methods
Human Tissue Samples
Thirty-five glioma samples were obtained from patients who underwent surgical treatment at the Department of Neurosurgery in the First Affiliated Hospital of Soochow University (Suzhou, China). These tumor samples were classified into different astrocytic glioma grades according to the 2007 CNS WHO classification system: glioblastoma (GBM, WHO grade IV, n = 14), anaplastic astrocytoma (AAST, WHO grade III, n = 12) and diffuse astrocytoma (AST, WHO grade II, n = 9). Six non-neoplastic brain (NNB) tissues were obtained from patients with craniocerebral trauma in which partial resections of brain tissues were performed to reduce intracranial pressure. Demographic data of glioma patients are shown in Table 1. All samples were flash-frozen in liquid nitrogen after resection. This study was approved by the Institutional Review Board of The First Affiliated Hospital of Soochow University. Written informed consent for the use of these samples for research was obtained from each patient.
Table 1.
Clinico-pathologic features of 35 glioma patients considered in the PCR assay
| Variant | Patient numbers (%) |
|---|---|
| Age | |
| ≥40 | 21 (60%) |
| <40 | 14 (40%) |
| Gender | |
| Male | 21 (60%) |
| Female | 14 (40%) |
| Tumor grade | |
| II | 9 (25.7%) |
| III | 12 (34.3%) |
| IV | 14 (40%) |
Cell Cultures and Oligonucleotide Transfection
Human glioblastoma cell lines (U87 and U251) and human kidney epithelial cell line (293T) were purchased from the Cell Bank Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were grown in DMEM/F12 medium (Corning, NY, USA) supplemented with 10% FBS (Gibco, Gaithersburg, MD, USA). To study the functions of miR-338-5p, 100 nmol/l miR-338-5p mimics or inhibitors designed and synthesized as small single-stranded RNA molecules to ascend and reduce mature endogenous miRNAs were transfected into U87 and U251 cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Their sequences were as follows: miR-338-5p mimics: 5′-AACAAUAUCCU GGUGCUGAGUG-3′; negative control mimics: 5′-UUCUCCGAACGUGUCACGUTTACG UGACACGUUCGGAGAATT-3′; miR-338-5p inhibitors: 5′-CACUCAGCACCAGGAUAU UGUU-3′; negative control inhibitors: 5′-CAGUACUUUUGUGUAGU ACAA-3′.
Real-Time PCR Analyses of mRNA and miRNA Expressions
Total RNA of tissues and cells was extracted using TRIzol RNA reagent (Invitrogen). The expression of TSHZ3 was quantified using the SYBR Green quantitative real-time RT-PCR kit (Light Cycler 480; Roche, Switzerland) and normalized to the expression of GAPDH. The primers were as follows: F-TSHZ3 (5′-CAGAGGAGCATACG GCAGAT-3′) and R-TSHZ3 (5′-TGTGTGACTCGCTGTCCATT-3′), F-GAPDH (5′-AGAAGGCTGGGGCTC ATTTG-3′) and R-GAPDH (5′-AGGGGCCATCCACAGTCTTC-3′). The expression of miR-338-5p was quantified using the All-in-One miRNA qRT-PCR Detection Kit (GeneCopoeia, Rockville, MD, USA) according to the manufacturer’s instructions and normalized to the expression of U6. The relative expression of each gene was calculated and normalized by the 2−ΔΔCT method. Each sample was tested in triplicate. MiR-338-5p mimics and its negative control oligonucleotides were synthesized by Genepharma. The experimental method was carried out according to Yang et al. (2014).
Constructs and Luciferase Reporter Assay
Predicted targets of miR-338-5p in the 3′-UTR of the TSHZ3 were located in the 589–596, 928–934 and 1253–1260 positions. The sense and anti-sense sequences of the predicted miR-338-5p targets of the wild type and mutant were synthesized, double stranded and inserted into the SacI/XhoI sites of the GP-miRGLO. A pcDNA3.1-TSHZ3 plasmid was constructed by cloning the open reading frame of TSHZ3 into vector pcDNA3.1 for the ectopic overexpression of TSHZ3.
The 293T cells were plated in 24-well plates at 104 cells per well and co-transfected with miR-338-5p and GP-miRGLO-TSHZ3-3′UTR plasmid (wild type or mutant) with Lipofectamine 2000. Twenty-four hours after transfection, cells were harvested, added to wells with 15 ml 1× Passive Lysis Buffer (Promega) and incubated for 15 min. Firefly and Renilla luciferase activities were measured sequentially using the Dual-Luciferase Reporter Assay System (Promega, E1960) by a Veritas microplate luminometer (Turner BioSystems, Sunnyvale, CA). Experiments were performed in three independent sets. Values were presented as means ± SD.
Matrigel Invasion Assay
U87 and U251 cells were transfected with negative control mimics, miR-338-5p mimics, GP-miRGLO-TSHZ3-3′UTR plasmid and miR-338-5p mimics + GP-miRGLO-TSHZ3-3′UTR plasmid and cultured for 48 h. Then, 5 × 105 cells in DMEM/F12 without serum supplement were added to the Matrigel (14× diluted from 9–10 mg/ml, 100 μl per well) coated chambers (24 well, 8-um pore size; Biosciences), and 1 ml DMEM/F12 was added to the bottom chamber. A small amount of serum (0.5%FBS) was added to bottom of the chamber to encourage cell migration through the pores without interfering with the assay by MMPs in serum, as described previously (Mayes et al. 2006).
After incubating for 24 h, the filters were fixed in 4% formaldehyde and stained with 0.2% crystal violet. Invaded cells (cells on the bottom side of the filter) were manually counted in five random fields under a microscope, and images were taken. Values were shown as means ± SD.
Protein Extraction and Western Blot Analysis
Whole-cell lysate (WCL) was extracted using RIPA lysis buffer (150 mM of NaCl, 1% NP-40, 0.5% sodium deoxycholic acid, 0.1% SDS and 50 mM Tris, pH 7.5) supplemented with a Mini Protease Inhibitor tablet (Roche). Proteins from WCL were separated in SDS-PAGE gel and transferred to nitrocellulose membranes. Primary antibodies for TSHZ3 (Abcam, England), MMP2 (Abcam) and GAPDH (Santa Cruz Biotechnology, USA) were diluted (1:1000) with blocking agent and applied to membranes for 24 h at 4 °C. Membranes were washed with PBST and incubated with HRP-conjugated anti-rabbit or anti-mouse secondary antibodies (Santa Cruz Biotechnology) for 2 h and washed with PBST. A chemiluminescent detection of horseradish peroxidase (HRP) activity was used to show the signal in the membranes, and images were taken with a chemiluminescence apparatus.
Statistical Analysis
Statistical analyses were carried out by using GraphPad Prism (GraphPad Software Inc., USA), and significance was determined by Tukey’s multiple comparison test. The Spearman rank correlation test was used to analyze the correlation between miRNA-338-5p and TSHZ3 by using SPSS13.0 software. Statistical significance was considered when P < 0.05.
Results
Differential Expression and Inverse Correlation of miR-338-5p and TSHZ3 in Gliomas
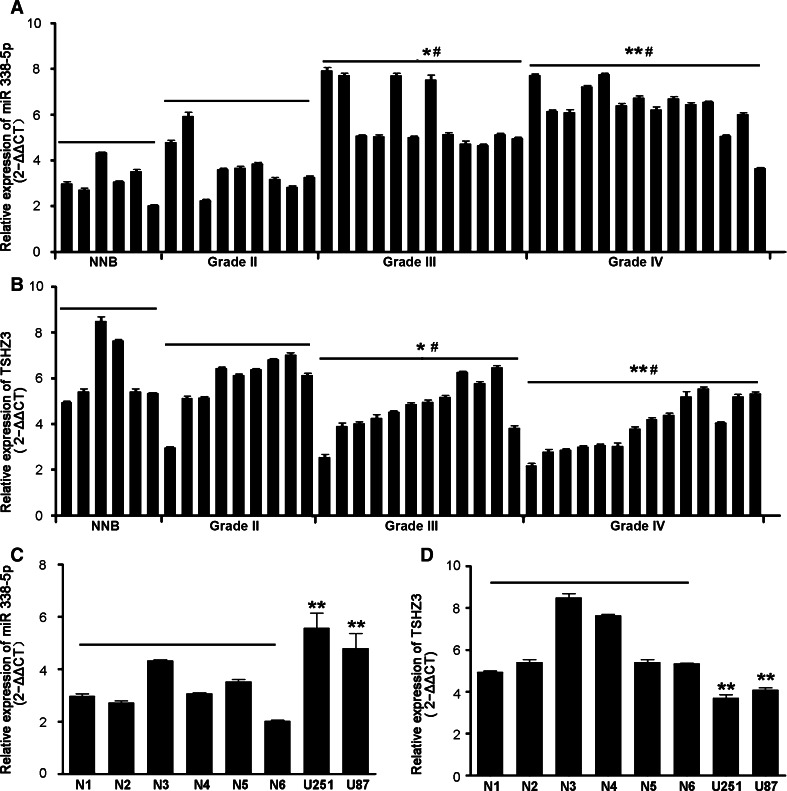
To assess the expression status of miR-338-5p in gliomas, the expression of miR-338-5p was determined by qRT-PCR with RNA samples of 35 gliomas of different grades, 6 NNB tissues and 2 glioblastoma cell lines (U87 and U251). Compared with NNB tissues, miR-338-5p expressions were unchanged in low-grade astrocytic gliomas (grade II), but increased in high-grade astrocytic gliomas (grades III and IV) in a grade-dependent manner; hence, a negative association with survival was observed, which was related to an increase of grades (Fig. 1a, c). A higher expression of miR-338-5p was also shown in U251 and U87 cells in comparison to NNB tissues.
Fig. 1.
Differential expression of miR-338-5p and TSHZ3 in normal brain tissues, gliomas and glioma cell lines. a, b Comparison of qRT-PCR results of miR-338-5p and TSHZ3 in non-neoplastic brain (NNB) tissues and glioma tissues of different grades. * P < 0.05 and ** P < 0.01 for gliomas vs. NNB tissues; # P < 0.05 for grades III and IV gliomas vs. Grade II gliomas. c, d Comparison of qRT-PCR results of miR-338-5p and TSHZ3 in glioblastoma cell lines U251 and U87 compared with the NNB tissues. ** P < 0.01 for glioma cells vs. NNB tissues
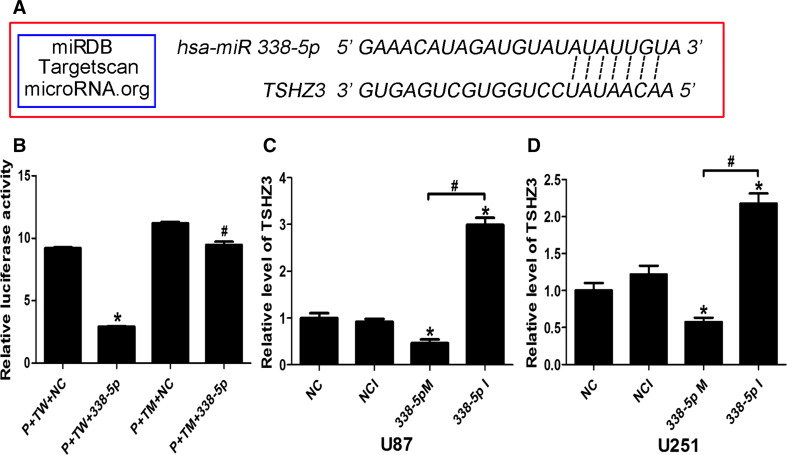
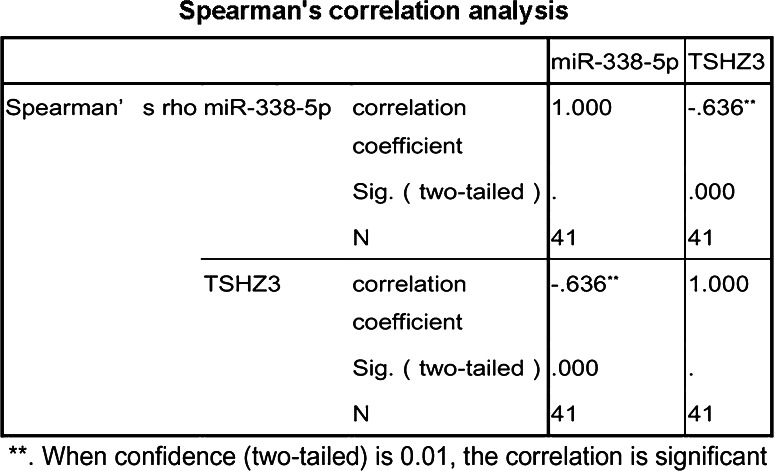
To explore the role of miR-338-5p in the glioma, we used bioinformatics software miRDB, Targetscan and microRNA.org to predict potential targets of miR-338-5p, and TSHZ3 was identified as a potential target gene of miR-338-5p (see target sequence details in Fig. 3a). To support this prediction, TSHZ3 was quantified by qRT-PCR in the same set of RNA samples as used for quantifying miR-338-5p. As shown in Fig. 1b and c, a lower level of TSHZ3 was shown in higher grade gliomas in comparison to that in NNB tissues and low-grade gliomas; TSHZ3 expressions in U251 and U87 cells were also lower compared to those in NNB tissues. The negative correlation between the levels of miR-338-5p and TSHZ3 in gliomas was verified by the Spearman coefficient value (R = −0.636) and significance P value (<0.01) (Fig. 2).
Fig. 3.
Dual-luciferase reporter assay on miR-338-5p targeting TSHZ3. a Predicted target of miR-338-5p by miRDB, Tartgetscan, microRNA.org. b Relative luciferase activity of P+TW+NC and P+TW+338-5p transfected 293T cells. c, d Quantitative RT-PCR of TSHZ3 in the transfected U87 and U251 cells. P pmirGlo vector, TW TSHZ3 wild oligonucleotides, NC negative control oligonucleotides, TM TSHZ3 mutant oligonucleotides
Fig. 2.
Spearman’s correlation for the expression of miR-338-5p and TSHZ3 in gliomas
TSHZ3 is a Direct Target Gene of microRNA-338-5p
The above data indicated that TSHZ3 was possibly the direct target gene of miR-338-5p. A dual-luciferase reporter assay was then performed to validate whether miR-338-5p directly recognized the predicted target sites in the 3′-UTR region of TSHZ3 (Fig. 3a). Plasmid GP-miRGLO-TSHZ3 (WT) +NC, GP-miRGLO-TSHZ3 (WT) + miR-338-5p, GP-miRGLO-TSHZ3 (MUT) +NC and GP-miRGLO-TSHZ3 (MUT) + miR-338-5p were co-transfected into 293T cells for 24 h followed by measuring the luciferase activity. As shown in Fig. 3b, miR-338-5p inhibited the luciferase activity of GP-miRGLO-TSHZ3 (WT), but not GP-miRGLO-TSHZ3 (MUT).
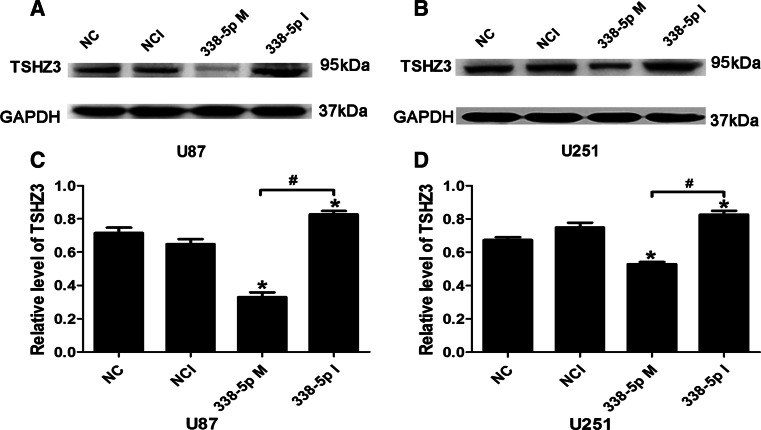
To further demonstrate the regulatory role of miR-338-5p on TSHZ3 expression, U87 and U251 cells were transfected with miR-338-5p mimics or inhibitors (100 nmol/l) in parallel with corresponding controls. The mRNA and protein levels of TSHZ3 were assessed by qRT-PCR and Western blotting, respectively. As shown in Fig. 3c and d, miR-338-5p mimics reduced TSHZ3 expression in both cell lines. In contrast, miR-338-5p inhibitor increased the mRNA level of TSHZ3. Similar effects of miR-338-5p mimics and inhibitor on TSHZ3 were shown at the protein level by Western blotting (Fig. 4a–d).
Fig. 4.
MiR-338-5p inhibited TSHZ3 expression in glioblastoma cells. a, b Western blot analysis of TSHZ3in miR-338-5p-transfected in U87 and U251 cells. c, d Densitometry of immoblots shown in (a) and (b). * P < 0.05 vs. NC, NCI; # P < 0.05 vs. 338-5p M. NC negative control, NCI negative control inhibitors, 338-5p M miR338-5p mimics
MiR-338-5p Promotes MMP2 Expression
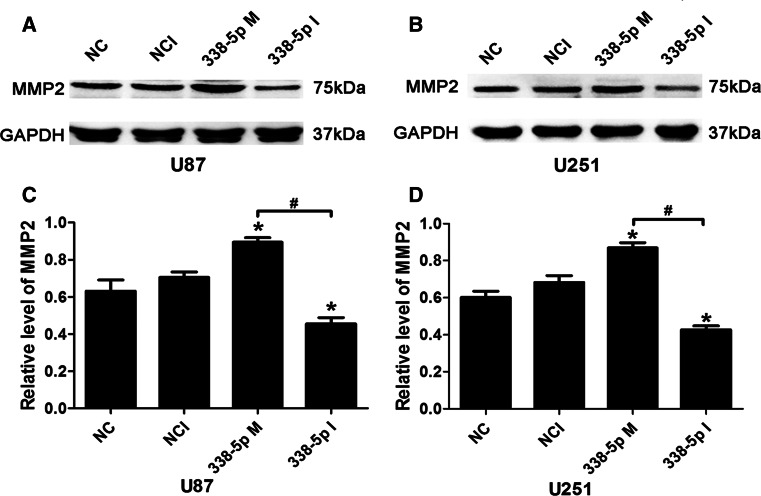
To investigate whether miR-338-5p was involved in the regulation of glioma cell invasion, we examined the effect of miR-338-5p on the expression of the key gelatinase of glioma, known as MMP2, by transfecting U87 and U251 cells with 100 nmol/l of miR-338-5p mimics or inhibitor. As shown in Fig. 5, miR-338-5p mimics increased MMP2 protein expression in both U87 and U251 cells, respectively, compared to the negative control (NC) transfected cells. In contrast, miR-338-5p inhibitor reduced MMP2. The differential effects on MMP2 between miR-338-5p mimics and inhibitor in both cell lines were significantly different (P < 0.05).
Fig. 5.
MiR-338-5p promoted MMP2 expression in glioblastoma cells. a, b Western blotting analysis of MMP2 in miR-338-5p-transfected U87 and U215 cells, respectively. c, d Densitometry of immunoblots shown in (a) and (b). * P < 0.05 vs. NC negative control, NCI negative control inhibitors; # P < 0.05 vs. 338-5p M (miR338-5p mimics)
Contrasting Roles of miR-338-5p and TSHZ3 on Regulating Glioma Cell Invasiveness
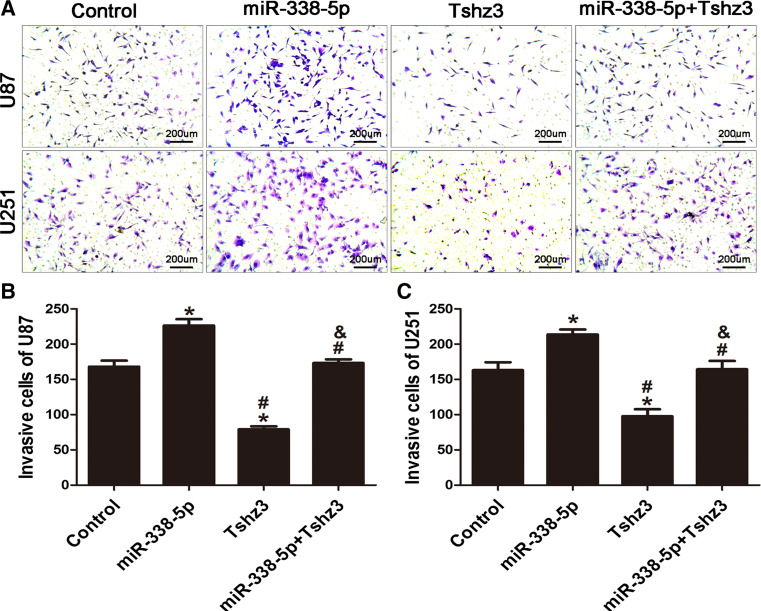
High tumor infiltration is a characteristic feature of high-grade glioma. Hence, we carried out a study to determine whether miR-338-5p has a role in modulating the invasiveness of glioma cells. U251 and U87 cells were transfected with 100 nmol/l of miR-338-5p mimics and pcDNA3.1-TSHZ3 alone or miR-338-5p mimics combined with pcDNA3.1-TSHZ3. Twenty-four hours following transfection, cells were used for Matrigel invasion assay. As shown in Fig. 6, compared with controls, both U87 and U251 cell transfected pcDNA3.1-TSHZ3 showed significant reductions (40–60%) in the number of cells crossing the Matrigel-coated membranes. In contrast, cells transfected with miR-338-5p mimics showed a significant increase in cell invasiveness, by approximately 1.3-fold in both cell lines. Co-transfection of miR-338-5p and pcDNA3.1-TSHZ3 brought the invasiveness to a comparable level as that of un-transfected cells.
Fig. 6.
MiR-338-5p promotes glioblastoma cell invasion. a Transwell assay exhibited the invasion of U87 and U251 cells transfected with miR-338-5p mimics, TSHZ3 and miR-338-5p mimics + TSHZ3 (×100). b, c Comparision of the number of invasive cells in various transfected groups of cells. *P < 0.05 vs. the control group. # P < 0.05 vs. the miR-338-5p mimics group. & P < 0.05 vs. the TSHZ3 group
Discussion
It has been widely accepted that activation of oncogenes and inhibition of tumor suppressor genes contribute to the development and progression of glioma (Reifenberger and Collins 2004). In this study, we demonstrated differential expressions of miR-338-5p and TSHZ3 with significant inverse correlations in high-grade astrocytic gliomas and glioblastoma cell lines and furthermore showed TSHZ3 to be a direct target gene of miR-338-5p via a sequence-specific target site in the 3′-UTR region of TSHZ3. In U87 and U251 cell lines, we further showed miR-338-5p’s effect on increasing MMP2 expression and cell invasiveness, while miR-338-5p’s pro-invasive activities were abolished by expressions of ectopic TSHZ3, which alone showed an inhibitory effect on invasion. Hence, miR-338-5p is a negative regulator of tumor suppressor gene TSHZ3 in glioma.
TSHZ3 encodes a zinc finger transcription factor important for the development of the trunk (Fasano et al. 1991) and muscle cell differentiation (Caubit et al. 2008; Faralli et al. 2011). In mice, Tshz3 is essential for the neural circuitry that controls breathing (Caubit et al. 2010). Tshz3 is a homolog of the Drosophila melanogaster teashirt gene, expressed in peri-urothelial cells of the proximal ureter and renal pelvis at 9 weeks of gestation (Jenkins et al. 2010), with expression detected in the brain, but strongly reduced in post-mortem elderly subjects with Alzheimer's disease (Kajiwara et al. 2009). The TSHZ3 promoter has been reported to be frequently methylated in primary breast cancers and prostate cancer cells (Yamamoto et al. 2011). Moreover, the downregulation or loss of function of TSHZ3 contributes to the pathogenesis of ovarian cancer (McBride et al. 2012). These available data suggest a tumor suppressor function of TSHZ3. Consistent with these previous findings, we discovered that TSHZ3 expression in human brain glioma tissues and in human glioma cell lines was significantly downregulated. Downregulation of TSHZ3 was negatively associated with the grades in gliomas, and overexpression of TSHZ3 suppressed the invasiveness of U87 and U251 glioblastoma cells. However, it should be noted that TSHZ3 was found to be amplified in 2.6% of breast cancers and that its silence was selectively lethal in cancer cells harboring 19q12 amplification (Natrajan et al. 2012). Thus, it is possible that TSHZ3 may play distinct roles in different tumor types.
Our data showed a pro-invasive oncogenic role of miR-338-5p and its association with high-grade glioma. Upregulation of miR-338-5p was shown in colorectal cancer (Yong et al. 2013), H. pylori-negative gastric cancer tissues (Chang et al. 2015), follicular lymphomas (Takei et al. 2014) and hepatocellular carcinoma (Chen et al. 2015), and combined detection of miR-338-5p, miR-193a-3p and miR-23a was proposed as an early detection marker for colorectal cancer (Yong et al. 2013).
Many recent therapies for glioma are ineffective against invasive cells. Tumor cell invasion and metastasis are signs of an advanced stage of tumor progression, with changes in cell-cell and cell-matrix adhesion (Yang et al. 2014). Under pathologic conditions, matrix metalloproteinases (MMPs) are activated, and the expressions of MMPs, especially MMP2 and MMP9, are correlated with the progression, tumor aggressiveness, angiogenesis and poor prognosis in glioma (Rao 2003; Tarassishin and Lee 2013; Wild-Bode et al. 2001; Kunishio et al. 2003; Bjorklund and Koivunen 2005; Kondraganti et al. 2000). MMP2s have been shown overexpressed in high-grade glioma and functions in promoting glioma cell invasion (Sato et al. 1994; Noel et al. 1997; McCawley and Matrisian 2001; Zhou et al. 2005).
MMP2 has been reported to be particularly involved in angiogenesis via the promotion of VEGFA expression and its coexpression with VEGFR2 (Chetty et al. 2010; Du et al. 2008). Our previous gelatin zymography assay revealed that MMP2, not MMP9, was expressed by U251 and U87 cells. In this study, we found miR-338-5p promoted the expression of MMP2 and had a consistently pro-invasive role in glioblastoma cells.
In summary, our results demonstrated overexpression of miR-338-5p in high-grade gliomas and its positive association with glioma grades. The oncogenic role of miR-338-5p in glioma can be attributed to the inhibition of TSHZ3 expression and consequently promotion of MMP2 expression. As glioma presents a therapeutic challenge because of the lack of molecular targets, a therapeutic miRNA might provide a new insight into the development of therapeutic strategies against glioma by inhibiting miR-338-5p as well as a new diagnostic marker for higher grade gliomas.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. NSFC81372689), the Foundation of the Health Department in Jiangsu Province (Grant No. K201106) and the Foundation of the Chinese Anti-cancer Association (CSNO-2013-MSD009).
Footnotes
Yanyan Li and Yulun Huang have contributed equally to this work.
References
- Bjorklund M, Koivunen E (2005) Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta 1755(1):37–69. doi:10.1016/j.bbcan.2005.03.001 [DOI] [PubMed] [Google Scholar]
- Caubit X, Lye CM, Martin E, Core N, Long DA, Vola C, Jenkins D, Garratt AN, Skaer H, Woolf AS, Fasano L (2008) Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development 135(19):3301–3310. doi:10.1242/dev.022442 [DOI] [PubMed] [Google Scholar]
- Caubit X, Thoby-Brisson M, Voituron N, Filippi P, Bevengut M, Faralli H, Zanella S, Fortin G, Hilaire G, Fasano L (2010) Teashirt 3 regulates development of neurons involved in both respiratory rhythm and airflow control. J Neurosci 30(28):9465–9476. doi:10.1523/JNEUROSCI.1765-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Kim N, Park JH, Nam RH, Choi YJ, Lee HS, Yoon H, Shin CM, Park YS, Kim JM, Lee DH (2015) Different microRNA expression levels in gastric cancer depending on helicobacter pylori infection. Gut Liver 9(2):188–196. doi:10.5009/gnl13371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen J, Liu Y, Li S, Huang P (2015) Plasma miR-15b-5p, miR-338-5p, and miR-764 as biomarkers for hepatocellular carcinoma. Med Sci Monit 21:1864–1871. doi:10.12659/MSM.893082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty C, Lakka SS, Bhoopathi P, Rao JS (2010) MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3 K/AKT signaling in A549 lung cancer cells. Int J Cancer 127(5):1081–1095. doi:10.1002/ijc.25134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Petritsch C, Lu K, Liu P, Haller A, Ganss R, Song H, Vandenberg S, Bergers G (2008) Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GBM. Neuro Oncol 10(3):254–264. doi:10.1215/15228517-2008-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralli H, Martin E, Core N, Liu QC, Filippi P, Dilworth FJ, Caubit X, Fasano L (2011) Teashirt-3, a novel regulator of muscle differentiation, associates with BRG1-associated factor 57 (BAF57) to inhibit myogenin gene expression. J Biol Chem 286(26):23498–23510. doi:10.1074/jbc.M110.206003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano L, Roder L, Core N, Alexandre E, Vola C, Jacq B, Kerridge S (1991) The gene teashirt is required for the development of Drosophila embryonic trunk segments and encodes a protein with widely spaced zinc finger motifs. Cell 64(1):63–79 [DOI] [PubMed] [Google Scholar]
- Huang Y, Mao H, Zhao X, Baxter P, Zhou Y, Wang Z, Li X-N (2012) Micro-RNA signature of glioblastoma multiforme invasion: an in vivo study in patient-tumor derived orthotopic xenograft mouse models. Cancer Res 72(Suppl 8):489 [Google Scholar]
- Jenkins D, Caubit X, Dimovski A, Matevska N, Lye CM, Cabuk F, Gucev Z, Tasic V, Fasano L, Woolf AS (2010) Analysis of TSHZ2 and TSHZ3 genes in congenital pelvi-ureteric junction obstruction. Nephrol Dial Transplant 25(1):54–60. doi:10.1093/ndt/gfp453 [DOI] [PubMed] [Google Scholar]
- Kajiwara Y, Akram A, Katsel P, Haroutunian V, Schmeidler J, Beecham G, Haines JL, Pericak-Vance MA, Buxbaum JD (2009) FE65 binds Teashirt, inhibiting expression of the primate-specific caspase-4. PLoS ONE 4(4):e5071. doi:10.1371/journal.pone.0005071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondraganti S, Mohanam S, Chintala SK, Kin Y, Jasti SL, Nirmala C, Lakka SS, Adachi Y, Kyritsis AP, Ali-Osman F, Sawaya R, Fuller GN, Rao JS (2000) Selective suppression of matrix metalloproteinase-9 in human glioblastoma cells by antisense gene transfer impairs glioblastoma cell invasion. Cancer Res 60(24):6851–6855 [PubMed] [Google Scholar]
- Kunishio K, Okada M, Matsumoto Y, Nagao S (2003) Matrix metalloproteinase-2 and -9 expression in astrocytic tumors. Brain Tumor Pathol 20(2):39–45 [DOI] [PubMed] [Google Scholar]
- Liu C, Liang S, Xiao S, Lin Q, Chen X, Wu Y, Fu J (2015) MicroRNA-27b inhibits Spry2 expression and promotes cell invasion in glioma U251 cells. Oncol Lett 9(3):1393–1397. doi:10.3892/ol.2015.2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes DA, Hu Y, Teng Y, Siegel E, Wu X, Panda K, Tan F, Yung WK, Zhou YH (2006) PAX6 suppresses the invasiveness of glioblastoma cells and the expression of the matrix metalloproteinase-2 gene. Cancer Res 66(20):9809–9817. doi:10.1158/0008-5472.CAN-05-3877 [DOI] [PubMed] [Google Scholar]
- McBride DJ, Etemadmoghadam D, Cooke SL, Alsop K, George J, Butler A, Cho J, Galappaththige D, Greenman C, Howarth KD, Lau KW, Ng CK, Raine K, Teague J, Wedge DC, Cancer Study Group AO, Caubit X, Stratton MR, Brenton JD, Campbell PJ, Futreal PA, Bowtell DD (2012) Tandem duplication of chromosomal segments is common in ovarian and breast cancer genomes. J Pathol 227(4):446–455. doi:10.1002/path.4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM (2001) Tumor progression: defining the soil round the tumor seed. Curr Biol 11(1):R25–R27 [DOI] [PubMed] [Google Scholar]
- Natrajan R, Mackay A, Wilkerson PM, Lambros MB, Wetterskog D, Arnedos M, Shiu KK, Geyer FC, Langerod A, Kreike B, Reyal F, Horlings HM, van de Vijver MJ, Palacios J, Weigelt B, Reis-Filho JS (2012) Functional characterization of the 19q12 amplicon in grade III breast cancers. Breast Cancer Res 14(2):R53. doi:10.1186/bcr3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel A, Gilles C, Bajou K, Devy L, Kebers F, Lewalle JM, Maquoi E, Munaut C, Remacle A, Foidart JM (1997) Emerging roles for proteinases in cancer. Invasion Metastasis 17(5):221–239 [PubMed] [Google Scholar]
- Peng Y, Yu S, Li H, Xiang H, Peng J, Jiang S (2014) MicroRNAs: emerging roles in adipogenesis and obesity. Cell Signal 26(9):1888–1896. doi:10.1016/j.cellsig.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Rao JS (2003) Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer 3(7):489–501. doi:10.1038/nrc1121 [DOI] [PubMed] [Google Scholar]
- Reifenberger G, Collins VP (2004) Pathology and molecular genetics of astrocytic gliomas. J Mol Med (Berl) 82(10):656–670. doi:10.1007/s00109-004-0564-x [DOI] [PubMed] [Google Scholar]
- Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M (1994) A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370(6484):61–65 [DOI] [PubMed] [Google Scholar]
- Takei Y, Ohnishi N, Kisaka M, Mihara K (2014) Determination of abnormally expressed microRNAs in bone marrow smears from patients with follicular lymphomas. SpringerPlus 3:288. doi:10.1186/2193-1801-3-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassishin L, Lee SC (2013) Interferon regulatory factor 3 alters glioma inflammatory and invasive properties. J Neurooncol 113(2):185–194. doi:10.1007/s11060-013-1109-3 [DOI] [PubMed] [Google Scholar]
- Wild-Bode C, Weller M, Wick W (2001) Molecular determinants of glioma cell migration and invasion. J Neurosurg 94(6):978–984. doi:10.3171/jns.2001.94.6.0978 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Cid E, Bru S, Yamamoto F (2011) Rare and frequent promoter methylation, respectively, of TSHZ2 and 3 genes that are both downregulated in expression in breast and prostate cancers. PLoS ONE 6(3):e17149. doi:10.1371/journal.pone.0017149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH, Chen GL, Xie XS, Li B, Wei YX, Guo LC, Zhang Y, Huang YL, Zhou YX, Du ZW (2014) MicroRNA-16 inhibits glioma cell growth and invasion through suppression of BCL2 and the nuclear factor-kappaB1/MMP9 signaling pathway. Cancer Sci 105(3):265–271. doi:10.1111/cas.12351 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yin F, Zhang JN, Wang SW, Zhou CH, Zhao MM, Fan WH, Fan M, Liu S (2015) MiR-125a-3p regulates glioma apoptosis and invasion by regulating Nrg1. PLoS ONE 10(1):e0116759. doi:10.1371/journal.pone.0116759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong FL, Law CW, Wang CW (2013) Potentiality of a triple microRNA classifier: miR-193a-3p, miR-23a and miR-338-5p for early detection of colorectal cancer. BMC cancer 13:280. doi:10.1186/1471-2407-13-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YH, Hess KR, Liu L, Linskey ME, Yung WK (2005) Modeling prognosis for patients with malignant astrocytic gliomas: quantifying the expression of multiple genetic markers and clinical variables. Neuro Oncol 7(4):485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]