Abstract
Coxsackievirus B3 (CVB3) is a common human pathogen that has been associated with serious diseases including myocarditis and pancreatitis. To better understand the effect of cytotoxic T-lymphocyte (CTL) responses in controlling CVB3 infection, we have inserted well-characterized CTL epitopes into the CVB3 genome. Constructs were made by placing the epitope of interest upstream of the open reading frame encoding the CVB3 polyprotein, separated by a poly-glycine linker and an artificial 3Cpro/3CDpro cleavage site. This strategy results in the foreign protein being translated at the amino- terminus of the viral polyprotein, from which it is cleaved prior to viral assembly. In this study, we cloned major histocompatibility complex class I-restricted CTL epitopes from lymphocytic choriomeningitis virus (LCMV) into recombinant CVB3 (rCVB3). In vitro, rCVB3 growth kinetics showed a 1- to 2-h lag period before exponential growth was initiated, and peak titers were ∼1 log unit lower than for wild-type virus. rCVB3 replicated to high titers in vivo and caused severe pancreatitis but minimal myocarditis. Despite the high virus titers, rCVB3 infection of naive mice failed to induce a strong CD8+ T-cell response to the encoded epitope; this has implications for the proposed role of “cross-priming” during virus infection and for the utility of recombinant picornaviruses as vaccine vectors. In contrast, rCVB3 infection of LCMV-immune mice resulted in direct ex vivo cytotoxic activity against target cells coated with the epitope peptide, demonstrating that the rCVB3-encoded LCMV-specific epitope was expressed and presented in vivo. The preexisting CD8+ memory T cells could limit rCVB replication; compared to naive mice, infection of LCMV-immune mice with rCVB3 resulted in ∼50-fold-lower virus titers in the heart and ∼6-fold-lower virus titers in the pancreas. Although the inserted CTL epitope was retained by rCVB3 through several passages in tissue culture, it was lost in an organ-specific manner in vivo; a substantial proportion of viruses from the pancreas retained the insert, compared to only 0 to 1.8% of myocardial viruses. Together, these results show that expression of heterologous viral proteins by recombinant CVB3 provides a useful model for determining the mechanisms underlying the immune response to this viral pathogen.
Coxsackieviruses are members of the family Picornaviridae and lie in the Enterovirus genus, together with polioviruses, echoviruses, and unclassified enteroviruses. Coxsackieviruses are classified, according their pathogenicity in newborn mice, into groups A and B, which comprise 24 and 6 serotypes, respectively. Type B coxsackieviruses (CVB) are common human pathogens and have been implicated in acute and chronic myocarditis; there is a strong correlation between prior CVB infection and dilated cardiomyopathy, which can be effectively treated only by heart transplantation (47). In addition to cardiovascular disease, CVB has been associated with hepatitis, encephalitis, and pancreatitis, and CVB4 infection has been suggested as an underlying cause of diabetes mellitus in humans (11, 25, 48). The outcome of CVB infection is often similar in mice and humans. For example, in both species there may be marked myocarditis followed by cardiac scarring and dilation (18, 23, 28, 55, 57), and in the absence of functional B cells, the virus establishes a long-term chronic infection (16, 20, 37).
Although picornavirus infections are very common, we have only a rudimentary understanding of the immune responses which control and clear these agents. Antibodies are important in eradicating enteroviruses, and agammaglobulinemic humans are susceptible to chronic infections with polioviruses (26), echoviruses (35, 38), and coxsackieviruses (16, 20). However, T cells also play a role in limiting viral titers (18, 55), and antibodies appear to contribute little to the protection induced by a CVB DNA vaccine (19), suggesting that virus-specific memory T cells might be important in vaccine-induced immunity. To clarify the part played by vaccine-induced CD8+ memory T cells in protecting against picornavirus challenge, we wished to develop a vaccine which would induce virus-specific CD8+ T cells in the absence of virus-specific antibody. However, CVB-specific CD8+ T-cell epitopes have not yet been mapped, making it more difficult to design the desired vaccine. As an alternative approach, we chose to incorporate well-characterized foreign CD8+ cytotoxic T-lymphocyte (CTL) epitopes into the CVB3 genome and to evaluate the ability of vaccine-induced CTL to protect against this recombinant picornavirus.
Four strategies have been used to construct recombinant picornaviruses. First, foreign sequences have been inserted within the open reading frame (ORF) of poliovirus capsid proteins such as VP1, but conformational constraints demand that these sequences be very short (6, 13). Second, dicistronic polioviruses have been constructed which contain an additional internal ribosome entry site driving a second ORF for expressing the foreign protein; however, these viruses were genetically unstable and long inserts resulted in a genome which could not be packaged (1). Third, it is possible to replace the sequences encoding poliovirus structural proteins with foreign genes of interest, generating a defective genome which can be packaged into infectious virus by cell lines that supply the missing poliovirus proteins (9); however, such recombinants are defective and cannot produce infectious progency in vivo. The fourth strategy exploits the fact that the picornavirus polyprotein is autolytically cleaved by two viral proteases, 2Apro and 3Cpro/3CDpro, to generate the individual proteins that control viral replication and virion assembly. The cloning strategy places the foreign protein in frame with the viral polyprotein, followed by an artificial protease cleavage site (4, 34). This site permits the foreign protein to be cleaved from the rest of the viral proteins during translation, allowing viral capsid formation to proceed. This strategy permits the isolation of a replication-competent recombinant virus and allows greater diversity in the size of the heterologous gene products that can be incorporated into the viral genome.
Most work with recombinant enteroviruses has focused on poliovirus, but a recent report confirmed that the above approach could be applied to an attenuated coxsackievirus (21). In the experiments described herein, CD8+ CTL epitopes from lymphocytic choriomeningitis virus (LCMV) were cloned into a cardiovirulent strain of CVB3 and viable recombinant coxsackievirus progeny (rCVB) were produced. We used the recombinant viruses to ask the following questions. (i) How does rCVB3 compare to cardiovirulent CVB in plaque morphology, growth kinetics, RNA production, and in vivo virulence? (ii) Is a rCVB3 which carries a highly -immunogenic CTL epitope able to induce strong CTL responses to that epitope? (iii) Do epitope-specific memory CTL protect against coxsackievirus challenge? Finally, we evaluate the stability of the recombinant viruses in vivo and provide data consistent with organ-specific selection; recombinant CVB present in the hearts of infected mice are devoid of LCMV epitopes, while viruses isolated from the pancreas often retain the foreign sequences.
MATERIALS AND METHODS
Mice and virus.
C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) or obtained from the breeding colony at the Scripps Research Institute. Wild-type CVB3 (wtCVB3) was produced from the plasmid pH3, which contains a full-length cDNA encoding the myocarditic strain Nancy (H3 variant); this plasmid (GenBank accession number U57056 [28]) was the kind gift of Kirk Knowlton (University of California, San Diego, Calif.).
Recombinant coxsackievirus cDNA construction.
The complete CVB3 genome in plasmid pH3 does not contain a unique restriction site into which foreign inserts could be cloned. Therefore, oligonucleotides containing a unique SfiI site and an artificial protease cleavage site were cloned into a SacI site located immediately downstream of the CVB3 polyprotein initiation codon in plasmid pH3. Since pH3 contains three SacI sites, a partial SacI digest was performed to generate a linear plasmid, and the termini were blunted using Klenow fragment. Complementary oligonucleotides containing a unique SfiI site and an artificial 3Cpro/3CDpro cleavage site were annealed, phosphorylated, and ligated into the vector, and a plasmid carrying the oligonucleotide insert in the desired SacI site was identified and designated pMKS1. The oligonucleotide was designated to maintain the ORF between the upstream ATG and the remainder of the viral polyprotein. The unique SfiI site in this plasmid was used as the recipient cloning site for preparing subsequent plasmids. pMKS2 (containing the Db-restricted LCMV GP33–41 epitope) and pMKS3 (containing the Ld-restricted LCMV NP118–126 epitope) were prepared by cutting pMKS1 with SfiI and inserting the appropriate oligonucleotide sequences, again maintaining the ORF. Each insert was completely sequenced prior to transfection into HeLa cells to generate recombinant virus stocks.
Transfection of cDNA clones to obtain infectious virus.
Virus was prepared by transfecting the infectious cDNA into HeLa cells, using Lipofectamine Plus reagent as specified by the manufacturer's directions (Gibco, Rockville, Md.). At 3 to 5 h later, complete Dulbecco minimal essential medium (DMEM) containing 10% fetal bovine serum (FBS) and l-glutamate was added, and the medium was changed after 24 h. The HeLa cell monolayers showed cytopathic effects by 3 days posttransfection, and at this time point, the cells and supernatants were collected, frozen-thawed three times, and subjected to titer determination on HeLa cells. Viruses were plaque purified twice, and working stocks were expanded on HeLa cells using a multiplicity of infection (MOI) of 10 PFU/cell. The recombinant viruses were named to reflect their plasmid of origin. Thus, rCVB3.1 was derived from pMKS1, while pMKS2 and pMKS3 gave rise to rCVB3.2 and rCVB3.3, respectively.
One-step growth curve.
HeLa cell monolayers in six-well plates were infected with either wtCVB3, rCVB3.1, rCVB3.2, or rCVB3.3 at an MOI of 10. After 60 min at 37°C, unbound virus was removed by washing the cells twice with prewarmed saline (0.9% NaCl), and 3 ml of prewarmed DMEM containing 10% FBS was added. Cells and supernatants were collected at the indicated time points by scraping the monolayers with a rubber policeman. The samples were frozen-thawed three times prior to serial dilution and plaque assay on HeLa cell monolayers.
FISH-fluorescence-activated cell sorter (FACS) analyses of intracellular RNA.
RNA probes for fluorescent in situ hybridization (FISH) were transcribed in vitro from a linearized plasmid carrying the CVB3 genome. Biotin-16-UTP (Boehringer Mannheim) was included in the transcription reaction mixture, and the probe was purified through a ChromaSpin 30 column (Clontech, Palo Alto, Calif.). Thereafter, the published protocol was followed (7), with some modifications. Cells were incubated with CVB3 (MOI = 10) or mock infected. At the designated time points, the cells were washed once with saline, trypsinized, transferred to 15-ml conical tubes, and washed once with complete DMEM and once with HH buffer (1 × Hanks' balanced salt solution, 20 mM HEPES [pH 7.2]). The cells were then resuspended in 400 μl of HH by gentle pipetting, transferred to 1.5 ml microcentrifuge tubes, and fixed by the addition of 45 μl of 10% neutral buffered formalin. After a 5-min incubation at room temperature, the cells were centrifuged at 300 × g for 5 min, washed once with HH, and resuspended in 300 μl of HH. Then 0.7 ml of absolute ethanol was added, and the cells (at a concentration of 3 × 106 cells per ml) were stored at −20°C until analyzed. For analysis, 5 × 105 cells in 160 μl were mixed with 3 μl of 10% diethylpyrocarbonate in ethanol and held for 15 min at room temperature, to inactivate endogenous RNase activity. The cells were then centrifuged at 300 × g for 5 min, resuspended in HH buffer with 0.5% Tween 20 (buffer HH-T), and held for 5 min at room temperature. Then 1 volume of 20× SSC (1×SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 2 volumes of formamide were added to this solution. Cells were pelleted by centrifugation and resuspended in 10 μl of prewarmed hybridization buffer (5× SSC, 50% formamide, 0.1% sodium dodecyl sulfate [SDS], 500 μg of tRNA per ml) containing ∼1 ng of the biotinylated RNA probe. The cells were incubated overnight at 45°C with rotation, pelleted, washed with 100 μl of prewarmed hybridization buffer (without probe) for 45 min at 45°C with rotation, centrifuged, and resuspended in prewarmed 0.1× SSC–0.1% SDS for 30 min at 45°C. After centrifugation, the cells were resuspended in 50 μl of streptavidin-phycoerythrin (PharMingen, San Diego, Calif.) (5 μg/ml) in HH-T, incubated for 30 min at 45°C, pelleted, and resuspended in 400 μl of HH-T containing 0.1× SSC and 0.1% SDS. After 2 min at 45°C, the cells were pelleted, resuspended in 200 μl of HH-T, and analyzed on a FACScan flow cytometer. Data were analyzed with CellQuest software (Becton- Dickinson, Franklin Lakes, N.J.).
Intracellular cytokine staining (ICCS).
High-pressure liquid chromatography-purified (>95% pure) major histocompatibility complex (MHC) class I peptides GP33–41 (KAVYNFATM) and NP396–404 (FQPQNGQFI) were purchased from Peptidogenic (Livermore, Calif.). Spleen cells (2 × 106 per well) from virus-infected mice were stimulated with 10−7 M peptide in the presence of 2 μg/ of brefeldin A (Sigma, St. Louis, Mo.) per ml for 6 h at 37°C under 5% CO2 in RPMI 1640 containing 10% FBS, 20 mM HEPES, l-glutamine, and antibiotics. The cells were stained overnight at 4°C with Cychrome-labeled anti-CD8 antibody (PharMingen). The cells were washed, fixed, and permeabilized using Cytofix/Cytoperm (PharMingen). Samples were stained with fluorescein isothiocyanate labeled anti-gamma interferon (IFN-γ) antibody, washed and resuspended in phosphate-buffered saline containing 2% formaldehyde, and analyzed on a FACScan flow cytometer with CellQuest software (Becton Dickinson).
Direct ex vivo cytotoxicity assay.
LCMV-immune (>8 weeks after intraperitoneal [i.p.] infection with 2 × 105 PFU of LCMV Armstrong) or naive C57BL/6 mice were infected with rCVB3.2 or rCVB3.3; 7 days later they were sacrificed and their splenocytes were assayed for CTL activity directly ex vivo (in the absence of in vitro restimulation). The CTL assays were performed as previously described (52) using targets labeled with 51Cr in the presence or absence of 10−7 M LCMV GP33–41 peptide.
Evaluating the protective effects of rCVB-specific memory T cells.
LCMV-immune mice were prepared as described above and were infected (along with naive mice) with 2 × 106 PFU of rCVB3.2 or rCVB3.3. At 2 or 4 days later, the mice were sacrificed and tissues were collected on the indicated days. The heart and pancreas were weighed and frozen at −80°C in DMEM containing 10% FBS. Samples were later thawed, homogenized, serially diluted in 10-fold increments, and subjected to titer determination on HeLa cell monolayers.
Determining the stability of rCVB in tissue culture and in vivo.
For tissue culture studies, each passage of virus was done as follows. HeLa cells were infected with rCVB3 (MOI = 10), and 7 h later virus was harvested and subjected to titer determination on HeLa cells prior to the next passage. Following passages 4 and 5, RNA was prepared using Trizol reagent (Gibco BRL, Rockville, MD.). For in vivo studies, naive C57BL/6 mice were infected with 2 × 106 PFU of rCVB3 and 4 days later were sacrificed, and their tissues were harvested. RNA was prepared from the heart and pancreas using Trizol reagent. The resulting RNAs were used as templates for reverse transcription-PCR (RT-PCR) using two CVB-specific primers. The first primer, used for reverse transcription, was 5′CGTGTAGTGAATAATGGAATTGCCGCT3′; the second primer was 5′GTTGGATTTATACCACTTAGCTTGAGAGAGG3′. The resulting PCR fragments were analyzed by agarose gel electrophoresis. In addition, the PCR fragments produced using the in vivo-derived RNA templates were cloned by the T-A method (Invitrogen, San Diego, Calif.), and bacterial colonies were replicated onto nitrocellulose membranes. Following colony lysis and nucleic acid denaturation, the colonies were analyzed by in situ hybridization, using 5′-32P-labeled single-stranded oligonucleotide probes specific for the GP33 epitope (probe 5′GGAAGGCTGTCTACAATTTTGCCACCTGTGGGGGAGGAG 3′) and for the nearby CVB sequence (probe 5′CGTGTAGTGAATAATGGAATTGCCGCT3′). After being washed, positive colonies were identified by autoradiography.
RESULTS
Incorporation of foreign genes into the rCVB3 genome.
We and others (21) have developed a system which allows foreign sequences to be expressed from recombinant CVB3. This system was first conceived for recombinant poliovirus (4, 34). The sequence of interest is placed at the N terminus of the viral polyprotein, separated from it by a picornavirus protease cleavage site which ensures that the recombinant molecule is released from the polyprotein during the course of virus replication (4, 33, 34). We used the 3Cpro/3CDpro cleavage site (consensus AXXQG, where X is any amino acid). The plasmid pH3, which encodes wtCVB3, had no suitable unique restriction site, and so, to facilitate construction of recombinant viruses, a unique SfiI cloning site (followed by a polyglycine linker and an artificial proteolytic cleavage sequence ALFQG) was inserted into pH3 immediately downstream from the ATG start codon of the viral polyprotein, generating plasmid pMKS1, which encodes the “parental” recombinant CVB, rCVB3.1. Next, minigenes containing well-characterized LCMV-specific CD8+ T-cell epitopes were inserted in frame into the unique SfiI site of this plasmid, generating infectious clones from which recombinant viruses could be prepared. rCVB3.2 contains the Db-restricted LCMV GP33–41 epitope, and rCVB3.3 contains the Ld-restricted LCMV NP118–126 epitope (51, 53). Amino acid sequences at the N termini of the above-mentioned viruses are shown in Fig. 1. In all cases, the sequences of the regions at and around the cloning sites were confirmed by DNA sequencing prior to transfection into HeLa cells to produce live recombinant virus.
FIG. 1.
rCVB cloning strategy and amino acid sequences at the N termini of wild-type and recombinant coxsackieviruses. pH3 encodes the full-length CVB3 polyprotein (black arrow). As detailed in Materials and Methods, this plasmid was partially digested with SacI and an oligonucleotide (thin box) encoding a unique SfiI restriction site followed by the coding sequence for a polyglycine linker and a typical 3Cpro/3CDpro cleavage site was inserted into the (circled) SacI site immediately downstream of the CVB3 polyprotein start codon. The resulting plasmid (pMKS1) was used to generate rCVB3.1; it acted also as the parental cloning vector for construction of new rCVB3 viruses, by cutting with SfiI and inserting antigen expression cassettes including the LCMV GP33–41 Db epitope (resulting in pMKS2 and rCVB3.2) or the LCMV NP118–126 Ld epitope (resulting in pMKS3 and rCVB3.3). The amino acid sequences at the N termini of wtCVB and of the three recombinant viruses are shown. The artificial cleavage site is denoted by an arrowhead, and the LCMV epitopes are highlighted in bold type.
In vitro growth kinetics of rCVB3.
All rCVB3 constructs yielded viable, replication-competent viruses after cDNA transfection of HeLa cell monolayers. After partial cytopathic effect was observed (24 to 48 h post transfection), the cells and supernatant were collected and subjected to three rounds of freezing-thawing prior to plaque assay on HeLa cell monolayers. Virus stocks were plaque purified twice before being expanded on HeLa cells (MOI 10). The rCVB3 viruses formed plaques within 48 h, but the plaques were smaller than in wtCVB3 (Fig. 2A). An in vitro one-step growth curve was generated to compare the growth rates of the rCVB3 strains to that of wtCVB3 (Fig. 2B). Exponential growth of rCVB3 viruses was delayed by about 1 to 2 h compared to that of wtCVB3, but once viral replication was initiated, the growth rates of wtCVB3 and recombinant CVB3 were similar. However, the maximum titer of rCVB3 was ∼108 PFU/ml, about 1 log unit lower than the wild-type titer.
FIG. 2.
Plaque morphology and growth kinetics of wild-type and recombinant coxsackieviruses. (A) Plaque assays of wtCVB (strain H3) (28) and rCVB3.1, rCVB3.2, and rCVB3.3 were performed in parallel on HeLa cell monolayers. The numbers indicate the average plaque diameter of 40 to 60 individual plaques ± the standard deviation. (B) A one-step growth curve was generated to compare the in vitro growth kinetics of rCVB3 and wtCVB. HeLa cell monolayers were infected at an MOI of 10, and the amount of infectious virus was determined at each time point by a plaque assay. p.i., postinfection.
Delayed initiation of replication by rCVB3.
The 1 to 2 h delay in production of infectious particles might have been attributable to early (attachment/entry/uncoating), intermediate (genomic RNA replication/proteolytic processing), or late (maturation/packaging/egress) events. We used the technique of FISH-FACS (7) to evaluate the kinetics of viral RNA production in infected cells. This method employs a biotinylated CVB-specific RNA probe which is incubated with infected cells under in situ hybridization conditions, and after being washed, the cells are incubated with fluorescently labeled streptavidin (see Materials and Methods). After further washes, the cells are analyzed on a flow cytometer. In Fig. 3, the y axis shows the number of recorded events and the x axis shows their fluorescence intensity (log10 scale). In cells infected with wtCVB, a slight increase in fluorescence (average ∼2-fold) was detectable at 3 h postinfection; by 4 h, most cells showed a ∼100-fold increase; and by 5 to 6 h, almost all of the cells showed strong fluorescence. In contrast, cells infected with rCVB3 showed a delay in fluorescence acquisition; this was most evident at the 4-h time point, when the majority of cells fluoresced but did so at much lower intensity than cells infected with wtCVB. These data are consistent with a defect in an early event, which delays the onset of viral RNA synthesis by ∼1 h. At 5 h, many rCVB-infected cells have a level of fluorescence similar to that observed in wtCVB-infected cells, indicating that once cells are infected by rCVB, their viral RNA content appears similar to that observed during wtCVB infection; this agrees with the observed similarities in exponential growth rates (Fig. 2). Therefore, there is no obvious defect in replication of the recombinant RNA genome.
FIG. 3.
FISH-FACS analysis of RNA synthesis by wtCVB and rCVB. HeLa cells were incubated with wtCVB (left column) or with rCVB encoding GFP (right column) (both at an MOI of 10) or were mock infected (UNINF). At the indicated times, the cells were harvested, processed as described in Materials and Methods, and incubated under in situ hybridization conditions with a biotinylated RNA probe specific for the genomic strand of CVB3. After being washed to remove unbound probe, the cells were incubated with streptavidin-phycoerythrin, washed, and analyzed on a flow cytometer. The y axes show the number of recorded events, and the x axes indicate their fluorescence (arbitrary units, log10 scale).
In vivo replication and pathogenesis of rCVB3.
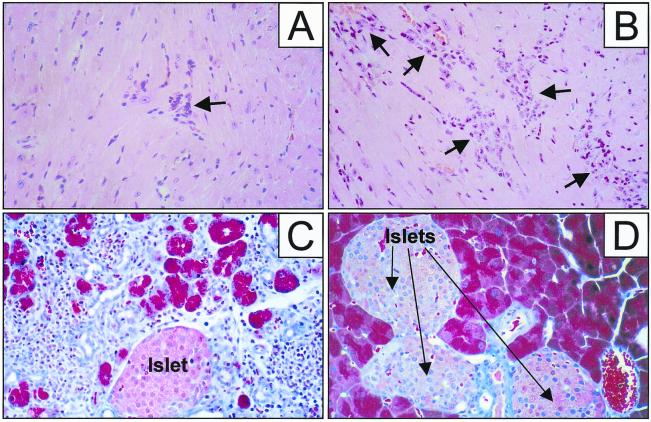
Infection of mice with wtCVB results in widespread viral replication; the highest titers usually are found in the heart and pancreas (∼108 and ∼1010 PFU/g, respectively), with accompanying myocarditis (18, 22, 56) and pancreatitis (36, 45, 46). Therefore, we infected BALB/c and C57BL/6 mice with various doses of rCVB3 and evaluated the viral titers in these organs. As shown in Fig. 4, both rCVB3.2 and rCVB3.3 grew to high titers in the pancreas of BALB/c and C57BL/6 mice; the pancreatic titers were very similar to those seen during wtCVB infection. The rCVB strains grew somewhat less well in the heart but still yielded 106 PFU/g. As might be expected, given the high viral titers, histological analyses of the heart and pancreas revealed inflammatory infiltrates. At 9 days postinfection, rCVB3.2 caused minimal myocarditis (Fig. 5A); the reduced cardiovirulence of the recombinant virus is clearly demonstrated by comparison with the more severe myocarditis caused by wtCVB (Fig. 5B). However, rCVB3 showed little if any attenuation of virulence in the pancreas, causing a severe pancreatitis which was detectable as early as 2 days postinfection (not shown) and resulted in marked infiltration and acinar cell destruction by 9 days postinfection (Fig. 5C). The pancreatic pathology caused by rCVB was similar to that previously described following wtCVB3 infection (36); only pancreatic acinar cells were infected, and no destruction of islet cells was observed. However, despite high virus titers and severe pancreatitis, mortality was much reduced following rCVB infection; wtCVB3 has a 50% lethal dose of ∼100 PFU for C57BL/6 mice (18), but doses of up to 107 PFU of rCVB3.1, rCVB3.2, or rCVB3.3 per mouse were nonlethal. In summary, the recombinant CVB3 strains used in these studies replicated to high titers in vivo and were less cardiovirulent than the parental virus but remained able to cause severe but nonfatal pancreatitis.
FIG. 4.
In vivo growth kinetics of rCVB3. BALB/c or C57BL/6 mice were infected with the indicated virus doses. Four days later, the heart and pancreas were harvested and the virus titers were determined. Means and standard deviations are shown.
FIG. 5.
rCVB3.2 causes mild myocarditis and severe pancreatitis. C57BL/6 mice were infected i.p. with 106 PFU of rCVB3.2, and 9 days later the heart and pancreas were examined histologically; the heart was stained with hematoxylin and eosin, and the pancreas was stained with trichrome. (A) Mild myocarditis was noted in the rCVB3.2-infected heart. (B) The more marked myocarditis occurring 9 days after infection of C57BL/6 mice with wtCVB is shown. Myocardial infiltrates are indicated by arrows. (C) rCVB3.2 infection resulted in massive destruction of the exocrine tissue of the pancreas, leaving only occasional acinar cells and the islets of Langerhans intact. (D) Normal pancreas. Islets of Langerhans are labeled and arrowed. Magnification, ×80. The samples are representative of at least four animals, and similar results were obtained with rCVB3.1 and rCVB3.3 (data not shown).
The LCMV GP33 epitope is expressed in vivo by rCVB3.2.
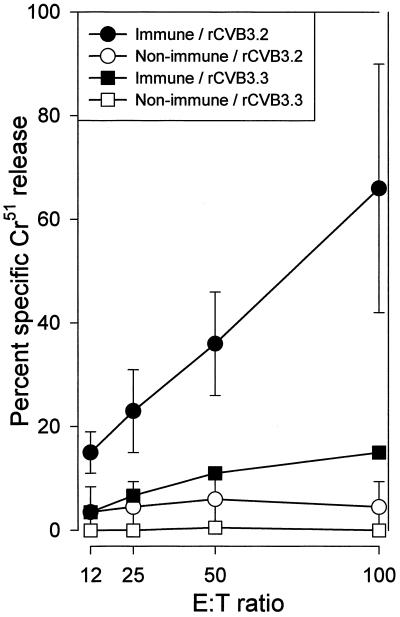
Next, we investigated whether the encoded foreign T-cell epitope was expressed in vivo by rCVB. Naive and LCMV-immune C57BL/6 (H-2b) mice were infected with either rCVB3.2 (carrying the Db-restricted GP33 epitope) or rCVB3.3 (as a negative control, carrying the Ld-restricted NP118 epitope). Seven days later, CTL activity was measured directly ex vivo against peptide-coated H-2b target cells, using a standard 51Cr release assay. CTL activity was below the level of detection in naive mice following infection with rCVB3.2 (Fig. 6), indicating that rCVB3.2 does not induce a strong enough CD8+ T-cell response to be detected directly ex vivo. The failure to induce primary CTL activity directly ex vivo is consistent with previous observations using recombinant poliovirus, in which detection of CTL activity required a 5-day secondary in vitro restimulation (30, 49). To determine if rCVB infection could stimulate memory T cells, LCMV-immune C57BL/6 (H-2b) mice were infected with rCVB3.2 (containing the Db-restricted LCMV CTL epitope). LCMV-immune C57BL/6 mice contain a high proportion of GP33-specific memory T cells, which, on secondary exposure to antigen, increase in number and acquire lytic activity. As a control to ensure that the development of lytic activity was specific for the encoded Db epitope, a group of these LCMV-immune H-2b mice were challenged with rCVB3.3 (containing the Ld-restricted LCMV CTL epitope). Strong GP33-specific lytic activity was detectable 7 days after infection of LCMV-immune H-2b mice infected with rCVB3.2 (Fig. 6). In contrast, infection of these mice with rCVB3.3 did not result in an appreciable level of CTL activity, indicating that the CTL response observed after rCVB3.2 infection required the expression of the Db-restricted LCMV epitope and was not due to nonspecific “bystander” activation. Together, these results demonstrate that rCVB3 can express heterologous CD8+ T-cell epitopes in vivo and can stimulate a cytolytic response in antigen-specific CD8+ memory T cells.
FIG. 6.
rCVB3.2 expresses an LCMV T-cell epitope in vivo. To determine if the artificial LCMV-specific Db-restricted CTL epitope encoded by rCVB3.2 was expressed in vivo, we challenged naive and LCMV-immune C57BL/6 mice with rCVB3.2 and compared the ex vivo epitope-specific CTL response to that in C57BL/6 mice infected with rCVB3.3 expressing an Ld-restricted CTL epitope. At 7 days postinfection, naive mice that were challenged with rCVB3.2 did not exhibit direct ex vivo CTL activity but rCVB3.2 infection of LCMV-immune mice resulted in readily detectable peptide-specific cytotoxicity. This result was not due to bystander activation, since infection of LCMV-immune mice with rCVB3.3 (in this case, encoding an irrelevant T-cell epitope) did not elicit peptide-specific cytolytic activity. The data show the average and standard deviation for two mice per group. Spontaneous lysis of target cells was <20%. E:T ratio, effector-to-target-cell ratio.
Poor primary CD8+ T-cell response to rCVB3.
We show above (Fig. 6) that an rCVB carrying a strong foreign epitope failed to induce primary CTL responses detectable directly ex vivo, although the virus could stimulate preexisting CD8+ memory T cells. However, in vitro cytotoxicity assays are less sensitive than ICCS, so we next used this assay to determine whether rCVB3 could induce primary CD8+ T-cell responses detectable directly ex vivo. Naive C57BL/6 mice were infected with 2 × 106 PFU of rCVB3.2 or rCVB3.3 and 7 days later were sacrificed, and their epitope-specific CD8+ T-cell responses were evaluated directly ex vivo by ICCS. No epitope-specific IFN-γ-producing CD8+ T cells were detected (data not shown). Therefore, to expand any epitope-specific T cells which had been induced by rCVB, rCVB-immunized mice were infected with LCMV and 4 days later were analyzed by ICCS. The results are shown in Fig. 7. LCMV-immune mice, upon reinfection with LCMV, mount strong responses to both the GP33 and NP396 epitopes. In contrast, mice infected with rCVB3.2, which expresses the GP33 epitope, failed to mount detectable responses even after 4 days of in vivo restimulation by LCMV.
FIG. 7.
rCVB do not induce strong CD8+ T-cell responses. Twelve C57BL/6 mice were infected as indicated (1° infections; four mice per group) with either LCMV (2 × 105 PFU i.p.), rCVB3.2, or rCVB3.3 (2 × 106 PFU i.p.), and 4 weeks later, all mice were challenged with LCMV (2 × 105 PFU i.p.). Four days later, the mice were sacrificed and their spleens were harvested. CD8+ T-cell responses to the Db-restricted LCMV epitopes GP33 and NP396 were evaluated by ICCS. Representative results are shown from a single mouse in each of the three groups. The oval indicates CD8+ IFN-γ+ cells.
rCVB3.2 replication is reduced in mice which have virus-specific CD8+ memory T cells.
If antigen-specific CD8+ memory T cells play a role in limiting rCVB3 replication, one would expect to see a reduction in rCVB3 titers in LCMV-immune mice challenged with an rCVB3 strain which expressed the correct MHC-restricted CTL epitope; in contrast, one would predict little to no difference in virus titers if the rCVB3 strain of virus expressed an irrelevant CTL epitope. To test this hypothesis, we challenged naive and LCMV-immune C57BL/6 mice with rCVB3.2 or rCVB3.3 and measured the virus titers in the heart and the pancreas at 2 and 4 days postinfection (Fig. 8). rCVB3.2 infection of naive mice (black bars) led to high titers in the heart and pancreas, consistent with the data reported in Fig. 4. In LCMV-immune H-2b mice, which have CD8+ memory cells specific for the LCMV GP epitope, myocardial rCVB3.2 titers were reduced by 4-fold at 2 days postinfection and by 50-fold at 4 days postinfection. rCVB3.2 titers in the pancreas were also consistently reduced in these mice, by five to sixfold compared to those in nonimmune animals. In contrast, rCVB3.3 titers were not altered by the prior immune status of the mouse, indicating that the observed inhibition of rCVB3.2 was epitope specific. These results demonstrate that a preexisting epitope-specific CD8+ memory T-cell response can reduce the viral load of rCVB3 infection in both heart and pancreatic tissue.
FIG. 8.
Virus-specific CD8+ memory T cells contribute to the control of picornavirus infection. Naive and LCMV-immune C57BL/6 mice were challenged with 2 × 106 PFU of rCVB3.2 (left column) or rCVB3.3 (right column), and the amount of infectious virus in the heart (top row) and pancreas (bottom row) 2 and 4 days later was determined by a plaque assay. As expected, rCVB3.3 grew equally well in naive and LCMV-immune mice. In contrast, rCVB3.2 titers were consistently lower in the heart and pancreas of LCMV-immune mice than is those of naive controls, indicating that the preexisting virus-specific memory CD8+ T cells recognized rCVB3.2-infected cells in vivo and reduced the viral burden in both heart and pancreas. The data show the average for four mice per group and the standard deviation.
In vivo stability of recombinant coxsackieviruses.
Some studies with recombinant polioviruses, performed using the same cloning strategy used here, suggested that the viruses were extremely unstable, even in tissue culture, and rapidly discarded the inserted sequences (42). In this light, the failure of rCVB3.2 to induce a strong CD8+ T-cell response might be explained by loss of the inserted epitope during viral replication in vivo. Indeed, one might argue that the loss of a CTL epitope would be accelerated in vivo by immune selection in favor of revertant CVB. Therefore we estimated the stability of the inserted epitope in tissue culture and in vivo in the heart and pancreas. Viral RNAs were amplified by RT-PCR, and the reaction products were evaluated in two ways: by agarose gel electrophoresis and by cloning and colony hybridization. As shown in Fig. 9A, the viral RNA was stable in tissue culture through passage 4, but by the next passage a significant proportion of PCR products had lost either the GP33 epitope (rCVB3.2 → rCVB3.1) or the entire insert including the SfiI cloning site (resulting in the wild-type band). By day 4 post infection, essentially all of the viral RNA present in the hearts of two mice appeared to be wild type; in contrast, the viral RNA in the pancreata comprised a mixture of the three populations. As a second means of quantitating the in vivo stability of the inserted epitope in rCVB3.2, the PCR fragments encompassing the SfiI site were cloned, and transformed bacteria were analyzed by colony hybridization with probes specific for (i) the GP33 epitope and (ii) the CVB sequence; this allowed the calculation of the percentage of cloned fragments which had retained the inserted epitope. As shown in Fig. 9B, at 4 days postinfection in two mice, a very low proportion (0 to 1.8%) of viral RNA from the heart had retained the GP33 sequence. In contrast, a much higher proportion (9 to 55%) of pancreatic CVB RNA was GP33+. Thus, at 4 days postinfection, a substantial proportion of rCVB3 expresses the LCMV GP33 epitope.
FIG. 9.
Tissue-specific loss of the LCMV epitope during rCVB3 infection. Two naive C57BL/6 mice were infected with 2 × 106 PFU of rCVB3.2; 4 days later, their hearts or pancreata were harvested and RNA was prepared. Alternatively, RNA was prepared from tissue culture cells after four or five viral passages (P4 and P5, respectively). The RNA was used as a template for RT-PCR with primers flanking the SfiI cloning site (see Materials and Methods). (A) PCR products were subjected to agarose gel electrophoresis, and bands were stained with ethidium bromide. Arrows indicate the expected band sizes for products from rCVB3.2 (containing the GP33 epitope), rCVB3.1 (lacking the epitope but containing the SfiI cloning site), and wtCVB (lacking both the epitope and the SfiI site). (B) PCR products were cloned by the T-A method. The resulting bacterial plates were duplicated, and the bacterial colonies were analyzed by hybridization with radiolabeled probes specific for CVB or for the GP33 sequence. Following autoradiography, positive colonies were counted; the data from two independent experiments are shown. ND, not done.
DISCUSSION
The availability of infectious cDNA clones of CVB3 not only permits the production of genetically pure virus stocks but also allows the development of a genetic system to prepare rCVB. Here we have exploited this by incorporating LCMV-specific CD8+ CTL epitopes into the amino terminus of the CVB3 polyprotein to evaluate the efficiency with which picornaviruses induce CD8+ T cells and to determine the role of CD8+ memory T cells in controlling rCVB3 infection. Others have recently reported the successful expression of an adenovirus antibody epitope in rCVB made from an attenuated CVB3 (21); here we focus on T-cell responses. The recombinant strains of virus used in this study had smaller plaques than wtCVB3, and an in vitro one-step growth curve lagged 1 to 2 h behind that for the parental wtCVB3 strain (Fig. 2). Several things could explain this lag and the ∼1-log-unit reduction in output of infectious virus. For example, there may be less efficient cell binding, entry, and uncoating or diminished replication of recombinant genomic RNA. The FISH-FACS data (Fig. 3) point to a defect in an early event in the rCVB3 life cycle and indicate that once rCVB3 succeeds in infecting a cell, it can produce genomic RNA in quantities similar to those synthesized by wild-type virus; consistent with this conclusion, once rCVB3 replication is initiated, the production of infectious particles proceeds at a rate very similar to that of wtCVB (Fig. 2). It is possible that the method of cloning has contributed to a defect in the virion, which in turn has led to a delay in virus entry. Picornaviruses, including poliovirus (10, 41, 44) and CVB (41), have a myristate moiety covalently attached to the glycine residue which immediately follows the initiation codon of the native polyprotein (see the sequence of wtCVB in Fig. 1). Mutation of the glycine or, in some circumstances, of other nearby residues prevents or reduces myristoylation and is often lethal (31). In the absence of myristoylation, RNA transcription appears unaffected (31), but there are multiple defects in the viral life cycle, including disruption of polyprotein processing and virus assembly, and mutant particles show reduced infectivity (5, 29, 32, 39, 40). Recombinant polioviruses made using this cloning strategy showed a delay in myristoylation, although it did eventually proceed by a novel pathway; the viruses showed reduced infectivity (33). Thus, our findings with rCVB are consistent with substandard myristoylation, which may somewhat reduce viral infectivity and delay the onset of replication.
The rCVB described here replicated well in vivo; viral titers in the pancreas were comparable to those seen during wtCVB infection, and the rCVB caused severe pancreatitis. Nevertheless, the viruses were markedly attenuated, as indicated by a much higher 50% lethal dose and minimal myocarditis. Several studies have shown that changes in the untranslated 5′ region of CVB3 attenuate the virus and decrease its cardiovirulence (50); we therefore consider it possible that our insertion of foreign sequences into the amino terminus of the polyprotein might have altered the secondary structure of the viral RNA in the 5′ untranslated region, thus decreasing viral cardiovirulence and lethality. We are currently constructing new recombinant strains of CVB3 in which the cloning site lies inside farther the polyprotein ORF, and therefore farther from the 5′ untranslated region, to determine if we can isolate cardiovirulent rCVB3.
Several reports have shown that CVB3-induced myocarditis is the result of triggering of a cross-reactive autoimmune response against cardiac tissue (12, 14, 24, 54). The results of our study do not support such a mechanism. The rCVB3 used in this study replicated well in vivo; replication in the pancreas was similar for rCVB3 and wtCVB3 (Fig. 4) and differed from that of the parental (cardiovirulent) strain of virus only by the addition of heterologous viral sequences in the amino terminus of the polyprotein (Fig. 1). Therefore, since these recombinant constructs still encode and express all of the native viral proteins, they should be able to induce autoreactivity against heart tissue. The absence of severe myocarditis during rCVB infection suggests that no such autoimmunity has been induced. Instead, the mild myocarditis is consistent with the reduced viral titers in the heart of rCVB-infected mice (Fig. 4). We (15, 18) and others (22, 27), have demonstrated that CD8+ T cells are involved with the induction of myocarditis, and we speculate that myocarditis is caused by a combination of host and viral factors in which the virus must replicate to high titers in the heart and induce both substantial damage and the accumulation of a large number of virus-specific lymphocytes.
The failure of rCVB3.2 to induce a detectable GP33-specific response following infection of naive mice seems to reflect a general inability of CVB to induce CD8+ T-cell responses. Activated T cells can be identified by incubating splenocytes with anti-CD3 antibody; the activated cells produce IFN-γ (43). Using this method, >50% of CD8+ T cells from LCMV-infected mice (day 7 postinfection) produce IFN-γ after anti-CD3 stimulation; in contrast, 7 days after rCVB3 infection, <4% respond to anti-CD3 stimulation, a response similar to that seen after stimulation of naive CD8+ T cells (data not shown). Although others have presented unequivocal proof that recombinant polioviruses can induce CD8+ T cells, these responses were not identified directly ex vivo and instead required 5 days of secondary in vitro restimulation to expand them to detectable levels (30, 49). This need for restimulation contrasts with other approaches to vaccination; for example, following a single immunization with plasmid DNA, antigen-specific CD8+ responses can be readily detected directly ex vivo and can constitute 0.4 to ∼3% of the total CD8+ T-cell population (2, 3, 17), and antigen-specific responses can be detected directly ex vivo following vaccination with recombinant vaccinia viruses (17). Taken together, these findings suggest that picornavirus-based vaccines may be limited in their ability to induce CD8+ T-cell responses.
Our data raise questions about the biological importance of “cross-priming” (also termed cross-presentation), a process in which exogenous antigens are taken up by antigen-presenting cells APCs, and processed into the MHC class I pathway. Elegant studies using poliovirus receptor transgenic mice (49) led the authors to conclude that recombinant polioviruses induced CTL by this means, and it has been suggested that this route of antigen delivery is the dominant means by which intracellular organisms induce CTL (8). If this is the case, one might expect that rCVB3.2 would induce good CTL responses, since (i) the recombinant virus encodes a strong CD8+ T-cell epitope, (ii) it replicates to high titer in vivo, and (iii) a significant proportion of the viruses retain the epitope at 4 days postinfection, a time point beyond which virus titers rapidly decline. However, although rCVB3.2 expresses the GP33 epitope in vivo (Fig. 6), it is unable to induce detectable responses to the GP33 epitope, which, when expressed by LCMV, induces a massive response (Fig. 7). Therefore, it appears that cross-priming is not an efficient way by which to present all proteins made during virus infection.
An important goal of this study was to determine the efficacy of preexisting picornavirus-specific CD8+ T cells in combating subsequent viral challenge. By immunizing mice with LCMV prior to rCVB3 infection, we were able to determine whether vaccination with a single CD8+ T-cell epitope could play a role in viral clearance. As shown in Fig. 8, LCMV-specific memory cells had no effect on rCVB3.3 (which does not encode an H-2b epitope) but substantially reduced the titers of rCVB3.2. These effects were seen as early as 2 days postinfection and became more apparent by 4 days postinfection, when rCVB3.2 titers in the heart were almost 50-fold lower in LCMV-immune animals. The difference in virus titers in the pancreas (∼six fold lower by 2 days postinfection) was less pronounced than that observed in the heart and may be related to the massive destruction of acinar cells that is observed during CVB3 infection; since CVB3 caused severe destruction of the exocrine pancreas (Fig. 5), it is possible that the number of available acinar cells was a limiting factor for viral replication in this organ. Alternatively, CVB-specific CD8+ T cells may play a more important antiviral role in the heart than in the pancreas (see below). Our demonstration that picornavirus-specific CD8+ T cells reduce the virus load 6-fold to 50-fold is consistent with our previous study using mice depleted of CD8+ T cells, in which virus titers were increased ∼20-fold (18).
One might expect that the reduced infectivity of rCVB would provide a selective pressure favoring revertant viruses which had jettisoned the inserted sequences. However, in tissue culture, some 99% of viruses retained the insert after four passages and ∼76% retained it after five (Fig. 9). This contrasts with a recent analysis of recombinant polioviruses made using this cloning strategy, which were found to be extremely unstable (42). These authors suggested that longer inserts might be less stable; thus, the stability of the inserts in the rCVB described here might be attributed to their brevity. However, we have prepared as rCVB expressing green fluorescent protein (GFP), and this virus maintains GFP expression through several passages in tissue culture and to at least day 4 in vivo (I. Mena, R. Feuer, and J. L. Whitton, unpublished data). Remarkably, when we analyzed the in vivo stability, we uncovered an organ-specific effect; almost all virus isolated from the heart had lost the insert by 4 days postinfection, while a significant proportion of pancreatic virus had retained the insert. Perhaps the reduced infectivity of rCVB3, seen in tissue culture (Fig. 2B), has a more marked inhibitory effect on infection of myocardial cells than of acinar cells; if so, the selective pressure favoring revertant virus would be stronger in the heart than in the pancreas. Alternatively, it is tempting to suggest that the selective pressure exerted on the virus by GP33-specific CD8+ T cells is stronger in the heart than in the pancreas. We have previously identified a difference in the roles played by CVB-specific CD8+ T cells in these organs; perforin-mediated CD8+ T-cell activity contributes to destruction of myocardial cells (15) but not to acinar cell death (36). Experiments are under way to distinguish among these and other possible explanations for the organ-specific selection of viral variants.
This study demonstrates the feasibility of engineering rCVB3 strains for elucidating the immunological mechanisms of viral clearance and disease. We have shown that rCVB3 can be generated that express heterologous CTL epitopes in vivo and that CD8+ T cells play a role in controlling rCVB3 infection in target organs such as the heart and pancreas. In addition to characterizing the role of CD8+ T cells, similar rCVB3 constructs could be made that express well-characterized CD4+ T-cell epitopes alone or in combination with CD8+ epitopes. Thus, the genetic system described here provides a useful tool for studying many questions regarding CVB3 infection, pathogenesis, and immunity.
ACKNOWLEDGMENTS
We are grateful to Annette Lord for excellent secretarial support.
This work was supported by NIH grant AI-32134.
Footnotes
Manuscript 12836-NP from the Scripps Research Institute.
REFERENCES
- 1.Alexander L, Lu H H, Wimmer E. Polioviruses containing picornavirus type 1 and/or type 2 internal ribosomal entry site elements: genetic hybrids and the expression of a foreign gene. Proc Natl Acad Sci USA. 1994;91:1406–1410. doi: 10.1073/pnas.91.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen T M, Vogel T U, Fuller D H, Mothe B R, Steffen S, Boyson J E, Shipley T, Fuller J, Hanke T, Sette A, Altman J D, Moss B, McMichael A J, Watkins D I. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J Immunol. 2000;164:4968–4978. doi: 10.4049/jimmunol.164.9.4968. [DOI] [PubMed] [Google Scholar]
- 3.An L L, Rodriguez F, Harkins S, Zhang J, Whitton J L. Quantitative and qualitative analyses of the immune responses induced by a multivalent minigene DNA vaccine. Vaccine. 2000;18:2132–2141. doi: 10.1016/s0264-410x(99)00546-0. [DOI] [PubMed] [Google Scholar]
- 4.Andino R, Silvera D, Suggett S D, Achacoso P L, Miller C J, Baltimore D, Feinberg M B. Engineering poliovirus as a vaccine vector for the expression of diverse antigens. Science. 1994;265:1448–1451. doi: 10.1126/science.8073288. [DOI] [PubMed] [Google Scholar]
- 5.Ansardi D C, Porter D C, Morrow C D. Myristylation of poliovirus capsid precursor P1 is required for assembly of subviral particles. J Virol. 1992;66:4556–4563. doi: 10.1128/jvi.66.7.4556-4563.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke K L, Dunn G, Ferguson M, Minor P D, Almond J W. Antigen chimaeras of poliovirus as potential new vaccines. Nature. 1988;332:81–82. doi: 10.1038/332081a0. [DOI] [PubMed] [Google Scholar]
- 7.Cao J, Vescio R A, Hong C H, Kim A, Lichtenstein A K, Berenson J R. Identification of malignant cells in multiple myeloma bone marrow with immunoglobulin VH gene probes by fluorescent in situ hybridization and flow cytometry. J Clin Investig. 1995;95:964–972. doi: 10.1172/JCI117805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbone F R, Kurts C, Bennett S R, Miller J F, Heath W R. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol Today. 1998;19:368–373. doi: 10.1016/s0167-5699(98)01301-2. [DOI] [PubMed] [Google Scholar]
- 9.Choi W S, Pal-Ghosh R, Morrow C D. Expression of human immunodeficiency virus type 1 (HIV-1) gag, pol, and env proteins from chimeric HIV-1-poliovirus minireplicons. J Virol. 1991;65:2875–2883. doi: 10.1128/jvi.65.6.2875-2883.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow M, Newman J F, Filman D, Hogle J M, Rowlands D J, Brown F. Myristylation of picornavirus capsid protein VP4 and its structural significance. Nature. 1987;327:482–486. doi: 10.1038/327482a0. [DOI] [PubMed] [Google Scholar]
- 11.Clements G B, Galbraith D N, Taylor K W. Coxsackie B virus infection and onset of childhood diabetes. Lancet. 1995;346:221–223. doi: 10.1016/s0140-6736(95)91270-3. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham M W, Antone S M, Gulizia J M, McManus B M, Fischetti V A, Gauntt C J. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci USA. 1992;89:1320–1324. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans D J, McKeating J, Meredith J M, Burke K L, Katrak K, John A, Ferguson M, Minor P D, Weiss R A, Almond J W. An engineered poliovirus chimaera elicits broadly reactive HIV-1 neutralizing antibodies. Nature. 1989;339:385–388. doi: 10.1038/339385a0. [DOI] [PubMed] [Google Scholar]
- 14.Gauntt C J, Arizpe H M, Higdon A L, Wood H J, Bowers D F, Rozek M M, Crawley R. Molecular mimicry, anti-coxsackievirus B3 neutralizing monoclonal antibodies, and myocarditis. J Immunol. 1995;154:2983–2995. [PubMed] [Google Scholar]
- 15.Gebhard J R, Perry C M, Harkins S, Lane T, Mena I, Asensio V C, Campbell I L, Whitton J L. Coxsackievirus B3-induced myocarditis: perforin exacerbates disease, but plays no detectable role in virus clearance. Am J Pathol. 1998;153:417–428. doi: 10.1016/S0002-9440(10)65585-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geller T J, Condie D. A case of protracted coxsackie virus meningoencephalitis in a marginally immunodeficient child treated successfully with intravenous immunoglobulin. J Neurol Sci. 1995;129:131–133. doi: 10.1016/0022-510x(94)00261-l. [DOI] [PubMed] [Google Scholar]
- 17.Hassett D E, Slifka M K, Zhang J, Whitton J L. Direct ex vivo kinetic and phenotypic analyses of CD8+ T-cell responses induced by DNA immunization. J Virol. 2000;74:8286–8291. doi: 10.1128/jvi.74.18.8286-8291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henke A, Huber S A, Stelzner A, Whitton J L. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J Virol. 1995;69:6720–6728. doi: 10.1128/jvi.69.11.6720-6728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henke A, Wagner E, Whitton J L, Zell R, Stelzner A. Protection of mice against lethal coxsackievirus B3 infection by using DNA immunization. J Virol. 1998;72:8327–8331. doi: 10.1128/jvi.72.10.8327-8331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hertel N T, Pedersen F K, Heilmann C. Coxsackie B3 virus encephalitis in a patient with agammaglobulinaemia. Eur J Pediatr. 1989;148:642–643. doi: 10.1007/BF00441520. [DOI] [PubMed] [Google Scholar]
- 21.Hofling K, Tracy S, Chapman N, Kim K S, Smith L J. Expression of an antigenic adenovirus epitope in a group B coxsackievirus. J Virol. 2000;74:4570–4578. doi: 10.1128/jvi.74.10.4570-4578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber S A, Job L P. Cellular immune mechanisms in Coxsackievirus group B, type 3 induced myocarditis in Balb/C mice. Adv Exp Med Biol. 1983;161:491–508. doi: 10.1007/978-1-4684-4472-8_29. [DOI] [PubMed] [Google Scholar]
- 23.Huber S A, Kupperman J, Newell M K. Hormonal regulation of CD4+ T-cell responses in coxsackievirus B3-induced myocarditis in mice. J Virol. 1999;73:4689–4695. doi: 10.1128/jvi.73.6.4689-4695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber S A, Lodge P A. Coxsackievirus B-3 myocarditis in Balb/c mice. Evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984;116:21–29. [PMC free article] [PubMed] [Google Scholar]
- 25.Kang Y, Chatterjee N K, Nodwell M J, Yoon J W. Complete nucleotide sequence of a strain of coxsackie B4 virus of human origin that induces diabetes in mice and its comparison with nondiabetogenic coxsackie B4 JBV strain. J Med Virol. 1994;44:353–361. doi: 10.1002/jmv.1890440408. [DOI] [PubMed] [Google Scholar]
- 26.Kew O M, Sutter R W, Nottay B K, McDonough M J, Prevots D R, Quick L, Pallansch M A. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J Clin Microbiol. 1998;36:2893–2899. doi: 10.1128/jcm.36.10.2893-2899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klingel K, Kandolf R. The role of enterovirus replication in the development of acute and chronic heart muscle disease in different immunocompetent mouse strains. Scand J Infect Dis Suppl. 1993;88:79–85. [PubMed] [Google Scholar]
- 28.Knowlton K U, Jeon E S, Berkley N, Wessely R, Huber S A. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996;70:7811–7818. doi: 10.1128/jvi.70.11.7811-7818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krausslich H G, Holscher C, Reuer Q, Harber J, Wimmer E. Myristoylation of the poliovirus polyprotein is required for proteolytic processing of the capsid and for viral infectivity. J Virol. 1990;64:2433–2436. doi: 10.1128/jvi.64.5.2433-2436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandl S, Sigal L J, Rock K L, Andino R. Poliovirus vaccine vectors elicit antigen-specific cytotoxic T cells and protect mice against lethal challenge with malignant melanoma cells expressing a model antigen. Proc Natl Acad Sci USA. 1998;95:8216–8221. doi: 10.1073/pnas.95.14.8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marc D, Drugeon G, Haenni A L, Girard M, van der Werf S. Role of myristoylation of poliovirus capsid protein VP4 as determined by site-directed mutagenesis of its N-terminal sequence. EMBO J. 1989;8:2661–2668. doi: 10.1002/j.1460-2075.1989.tb08406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marc D, Masson G, Girard M, van der Werf S. Lack of myristoylation of poliovirus capsid polypeptide VPO prevents the formation of virions or results in the assembly of noninfectious virus particles. J Virol. 1990;64:4099–4107. doi: 10.1128/jvi.64.9.4099-4107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattion N M, Reilly P A, Camposano E, Wu S L, DiMichele S J, Ishizaka S T, Fantini S E, Crowley J C, Weeks-Levy C. Characterization of recombinant polioviruses expressing regions of rotavirus VP4, hepatitis B surface antigen, and herpes simplex virus type 2 glycoprotein D. J Virol. 1995;69:5132–5137. doi: 10.1128/jvi.69.8.5132-5137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattion N M, Reilly P A, DiMichele S J, Crowley J C, Weeks-Levy C. Attenuated poliovirus strain as a live vector: expression of regions of rotavirus outer capsid protein VP7 by using recombinant Sabin 3 viruses. J Virol. 1994;68:3925–3933. doi: 10.1128/jvi.68.6.3925-3933.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKinney R E J, Katz S L, Wilfert C M. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev Infect Dis. 1987;9:334–356. doi: 10.1093/clinids/9.2.334. [DOI] [PubMed] [Google Scholar]
- 36.Mena I, Fischer C, Gebhard J R, Perry C M, Harkins S, Whitton J L. Coxsackievirus infection of the pancreas: evaluation of receptor expression, pathogenesis, and immunopathology. Virology. 2000;271:276–288. doi: 10.1006/viro.2000.0332. [DOI] [PubMed] [Google Scholar]
- 37.Mena I, Perry C M, Harkins S, Rodriguez F, Gebhard J R, Whitton J L. The role of B lymphocytes in coxsackievirus B3 infection. Am J Pathol. 1999;155:1205–1215. doi: 10.1016/S0002-9440(10)65223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Misbah S A, Spickett G P, Ryba P C, Hockaday J M, Kroll J S, Sherwood C, Kurtz J B, Moxon E R, Chapel H M. Chronic enteroviral meningoencephalitis in agammaglobulinemia: case report and literature review. J Clin Immunol. 1992;12:266–270. doi: 10.1007/BF00918150. [DOI] [PubMed] [Google Scholar]
- 39.Moscufo N, Chow M. Myristate-protein interactions in poliovirus: interactions of VP4 threonine 28 contribute to the structural conformation of assembly intermediates and the stability of assembled virions. J Virol. 1992;66:6849–6857. doi: 10.1128/jvi.66.12.6849-6857.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moscufo N, Simons J, Chow M. Myristoylation is important at multiple stages in poliovirus assembly. J Virol. 1991;65:2372–2380. doi: 10.1128/jvi.65.5.2372-2380.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muckelbauer J K, Kremer M, Minor I, Diana G, Dutko F J, Groarke J, Pevear D C, Rossmann M G. The structure of coxsackievirus B3 at 3.5 A resolution. Structure. 1995;3:653–667. doi: 10.1016/s0969-2126(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 42.Mueller S, Wimmer E. Expression of foreign proteins by poliovirus polyprotein fusion: analysis of genetic stability reveals rapid deletions and formation of cardioviruslike open reading frames. J Virol. 1998;72:20–31. doi: 10.1128/jvi.72.1.20-31.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen K B, Biron C A. Synergism for cytokine-mediated disease during concurrent endotoxin and viral challenges: roles for NK and T cell IFN-gamma production. J Immunol. 1999;162:5238–5246. [PubMed] [Google Scholar]
- 44.Paul A V, Schultz A, Pincus S E, Oroszlan S, Wimmer E. Capsid protein VP4 of poliovirus is N-myristoylated. Proc Natl Acad Sci USA. 1987;84:7827–7831. doi: 10.1073/pnas.84.22.7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramsingh A, Slack J, Silkworth J, Hixson A. Severity of disease induced by a pancreatropic Coxsackie B4 virus correlates with the H-2Kq locus of the major histocompatibility complex. Virus Res. 1989;14:347–358. doi: 10.1016/0168-1702(89)90027-0. [DOI] [PubMed] [Google Scholar]
- 46.Ramsingh A I. Coxsackieviruses and pancreatitis. Front Biosci. 1997;2:e53–e62. doi: 10.2741/a227. [DOI] [PubMed] [Google Scholar]
- 47.Riecansky I, Schreinerova Z, Egnerova A, Petrovicova A, Bzduchova O. Incidence of Coxsackie virus infection in patients with dilated cardiomyopathy. Cor Vasa. 1989;31:225–230. [PubMed] [Google Scholar]
- 48.Roivainen M, Knip M, Hyoty H, Kulmala P, Hiltunen M, Vahasalo P, Hovi T, Akerblom H K. Several different enterovirus serotypes can be associated with prediabetic autoimmune episodes and onset of overt IDDM. Childhood Diabetes in Finland (DiMe) Study Group. J Med Virol. 1998;56:74–78. doi: 10.1002/(sici)1096-9071(199809)56:1<74::aid-jmv12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 49.Sigal L J, Crotty S, Andino R, Rock K L. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 50.Tracy S, Chapman N M, Romero J, Ramsingh A I. Genetics of coxsackievirus B cardiovirulence and inflammatory heart muscle disease. Trends Microbiol. 1996;4:175–179. doi: 10.1016/0966-842x(96)10026-3. [DOI] [PubMed] [Google Scholar]
- 51.Whitton J L, Gebhard J R, Lewicki H, Tishon A, Oldstone M B A. Molecular definition of a major cytotoxic T-lymphocyte epitope in the glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1988;62:687–695. doi: 10.1128/jvi.62.3.687-695.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitton J L, Tishon A. Use of CTL clones in vitro to map CTL epitopes. In: Oldstone M B A, editor. Animal virus pathogenesis: a practical approach. Oxford, United Kingdom: Oxford University Press; 1990. pp. 104–115. [Google Scholar]
- 53.Whitton J L, Tishon A, Lewicki H, Gebhard J R, Cook T, Salvato M S, Joly E, Oldstone M B A. Molecular analyses of a five-amino-acid cytotoxic T-lymphocyte (CTL) epitope: an immunodominant region which induces nonreciprocal CTL cross-reactivity. J Virol. 1989;63:4303–4310. doi: 10.1128/jvi.63.10.4303-4310.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolfgram L J, Beisel K W, Rose N R. Heart-specific autoantibodies following murine coxsackievirus B3 myocarditis. J Exp Med. 1985;161:1112–1121. doi: 10.1084/jem.161.5.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woodruff J F. Lack of correlation between neutralizing antibody production and suppression of coxsackievirus B-3 replication in target organs: evidence for involvement of mononuclear inflammatory cells in host defense. J Immunol. 1979;123:31–36. [PubMed] [Google Scholar]
- 56.Woodruff J F. Viral myocarditis. A review. Am J Pathol. 1980;101:425–484. [PMC free article] [PubMed] [Google Scholar]
- 57.Woodruff J F, Woodruff J J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974;113:1726–1734. [PubMed] [Google Scholar]