Abstract
Isocitrate dehydrogenase 1 (IDH1), one member of the IDH family can convert isocitrate to α-ketoglutarate (α-KG) via oxidative decarboxylation. IDH1 and IDH2 mutations have been identified in multiple tumor types and the mutations confer neomorphic activity in the mutant protein, resulting in the conversion of α-KG to the oncometabolite, D-2-hydroxyglutarate (2-HG). The subsequent accumulation of 2-HG results in epigenetic dysregulation via inhibition of α-KG-dependent histone and DNA demethylase. And the glutamate levels are reduced in IDH mutant cells compared to wild-type. We have known that diffuse gliomas contain a high frequency of mutations in the IDH1 gene. However, the expression of IDH1 and its roles in Intracranial hemorrhage (ICH) remain largely unknown. We observed increased expression of IDH1 in neurons after intracerebral hemorrhage. Up-regulation of IDH1 was found to be accompanied by the increased expression of active caspase-3 and pro-apoptotic Bcl-2-associated X protein and decreased expression of anti-apoptotic protein B cell lymphoma-2 in vivo and vitro studies. So we hypothesized that IDH1 was involved in the regulation of neuronal apoptosis. The present research for the first time detected the expression and variation of IDH1 surrounding the hematoma, and all data proved the involvement of IDH1 in neuronal apoptosis following ICH.
Keywords: ICH, IDH1, Neuron, Apoptosis, Active caspase-3
Introduction
Intracranial hemorrhage (ICH) is the second most common subtype of stroke characterized by bleeding into the brain parenchyma (Feigin et al. 2009; van Asch et al. 2010; Wilson et al. 2014). Although ICH is a horrible disease with a high rate of morbidity and mortality, there still lacks effective medical and surgical strategies for ICH treatment (van Asch et al. 2010; Wilson et al. 2014). In the pathological processes of ICH contain both the primary and secondary damage. The primary damage is caused by the dynamic of hematoma expansion associated with mass effect; and the secondary damage consists of many parallel pathways including cytotoxicity of blood, inflammation, oxidative stress, and so on. Together, primary and secondary damages lead up to a neuronal injury, and ultimately cause disability or even the death (Aronowski and Zhao 2011). Neuronal apoptosis, astrocytic proliferation and oligodendrocytic death have been involved in these processes (Bradl and Lassmann 2010; Gong et al. 2001); and among them, neuronal apoptosis is counted as one of the most crucial events. Classical apoptosis can be divided into extrinsic pathway and intrinsic pathway (Bredesen et al. 2006). Caspase-3, a significant factor in the execution phase of cell apoptosis, can be continually activated during apoptosis via both pathways. Although multiple researches have focused on the mechanisms underlying ICH, we merely have understood a sketchy knowledge of the molecular and cellular mechanisms, and still more work should be done to realize the pathological progress and to improve in the further treatment of ICH.
Isocitrate dehydrogenase 1 (IDH1) is a member of the IDH family which comprises three isozymes (IDH1, IDH2, and IDH3). The IDH family convert isocitrate to α-ketoglutarate (α-KG) via oxidative decarboxylation (Dang et al. 2016). IDH1 and IDH2 mutations have been identified in multiple tumor types (Borger et al. 2012; Kosmider et al. 2010; Mardis et al. 2009). The mutations confer neomorphic activity in the mutant protein, resulting in the conversion of α-KG to the oncometabolite, D-2-hydroxyglutarate (2-HG). The subsequent accumulation of 2-HG results in epigenetic dysregulation via inhibiting α-KG-dependent histone and DNA demethylases (Kranendijk et al. 2012, 2010). And the glutamate levels are reduced in IDH mutant cells compared to wild-type (Izquierdo-Garcia et al. 2015; Ohka et al. 2014; Reitman et al. 2011). We have known that diffuse gliomas contain a high frequency of mutations in the IDH1 gene. However, the expression of IDH1 and its roles in ICH remain largely unknown. We observed increased expression of IDH1 in neurons after intracerebral hemorrhage. Up-regulation of IDH1 was found to be accompanied by the increased expression of active caspase-3 and pro-apoptotic bcl-2-associated X protein (bax) and decreased expression of anti-apoptotic protein B cell lymphoma-2 (bcl-2) in vivo and vitro studies. So we hypothesized that IDH1 was involved in the regulation of neuronal apoptosis. In this report, we show that synergy between the IDH1 pathway and the intrinsic mitochondrial apoptotic pathway results in increased pro-apoptotic proteins bax, Bim expression, and reduced anti-apoptotic proteins bcl-2, bcl-XL expression. Our research was conducted to gain greater insight into the functions of IDH1 in the CNS, further making appropriate suggestions for clinical trials.
Methods and Materials
Animals and the ICH Model
Male Sprague–Dawley rats (220–250 g) provided by the Department of Animal Center, Medical College of Nantong University were used in this study. They were kept in a temperature-controlled environment (21 °C) on a 12 h light–dark cycle. Rats were anesthetized intraperitoneally with sodium pentobarbital (50 mg/kg) positioned in a stereotaxic frame, and a cranial burr hole (1 mm in diameter) was drilled near the right coronal suture 3.5 mm lateral to the midline. Autologous whole blood (50 μL) was collected from its tail tip and was collected in a sterile syringe. The sterile syringe was inserted stereotactically into the right basal ganglia (coordinates: 0.2 mm anterior, 5.5 mm ventral, and 3.5 mm lateral to the bregma) (Li et al. 2013; Yang et al. 2008).The autologous blood was injected at the rate of 10 μL/min. 10 min later, the needle was removed, the skin incision closed, and the animals were allowed to recover. Sham-controlled rats received an equivalent volume of saline. Experimental animals (n = 8 per time point) were sacrificed to extract the protein for Western blot analysis at 6 and 12 h, 1, 2, 3, 5, 7, and 14 days after ICH, respectively. The sham-operated animals (n = 8 per time point) were sacrificed on the second day. Additional experimental animals at each time point were killed for pathologic studies. Experiments were carried out in accordance with the National Institutes of Health (NIH) guidelines for the care and use of laboratory and approved by the Chinese National Committee to use of experimental animals for medical purposes, Jiangsu branch. All efforts were made to minimize the number of animals used and suffering.
Behavioral Testing Procedures
Forelimb placing and corner turn tests were used to assess the neurological deficits induced by ICH.
Forelimb Placing Test
Forelimb placing test was performed as described (Karabiyikoglu et al. 2004). The rats were held by torsos, allowing the forelimb to hang free. Testing of each forelimb was performed by brushing the vibrissae on the corner edge of a countertop. Intact rats placed the forelimb quickly onto the countertop. According to the severity of injury, placing of the forelimb contralateral to the injury was impaired. During the experiments, every rat was tested ten times for each forelimb. The percentage of trials in which the rat placed its left forelimb was calculated.
Corner Turn Test
Corner turn test was performed as described (Li et al. 2013). Rats proceed into a corner, whose angle was 30°. In order to exit the corner, the rats should turn to the left or the right, only the turns involving full rearing along either wall were involved (a total of eight per animal). Injured rats would show a tendency to turn to the side of the injury. The percentage of right turns was used as the corner turn score. After each turn, the rats were not picked up immediately, so that they would not develop an aversion for their prepotent turning response.
Protein Extraction and Western Blot Analysis
After injected an overdose of chloral hydrate (10 % solution), rats were executed at different time points postoperatively, and the brain tissue around the hematoma (extending 3 mm to the incision) as well as an equal part of the normal, sham-controlled, and contralateral cortex were dissected out and stored at −80°C until use. To prepare the lysates, frozen samples were weighed and minced on ice. The samples were then homogenized in lysis buffer (1 % NP-40, 50 mmol/L Tris, pH = 7.5, 5 mmol/L EDTA, 1 % SDS, 1 % sodium deoxycholate, 1 % Triton X-100,1 mmol/L PMSF, 10 μg/mL aprotinin, and 1 μg/mL leupeptin) and centrifuged at 12,000 rpm and 4°C for 20 min to collect the supernatant (Liu et al. 2010). After ascertain of its protein concentration with the Brad-fordassay (Bio-Rad), protein samples were sustained SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene diflouride filter (PVDF) membrane by a transfer apparatus at 300 mA for 2 h. The membranes were blocked with 5 % non-fat milk for 2 h and incubated with primary antibody against IDH1 (1:500; Santa Cruz; Sigma-Aldrich), active caspase-3 (1:1000; Cell Signaling), β-actin (1:1000, Santa Cruz), GAPDH (1:1000, Santa Cruz), Bcl-2 (1:500; Santa Cruz), p53 (1:500; Santa Cruz), and Bax (1:500; Santa Cruz), at 4°C overnight. At last, the membrane was incubated with a second antibody for 2 h and visualized using an enhanced chemiluminescence system (Pierce Company, USA) (Hua et al. 2002).
Immunohistochemistry
As the survival times determined, rats were deeply anesthetized and perfused with saline and 4 % paraformaldehyde following through the ascending aorta. After perfusion, the brains were took away and post-fixed in the same fixative for 3 h and then replaced with 20 % sucrose for 2–3 days, followed by 30 % sucrose for 2–3 days. Tissues were then cut at 7 μm with a cryostat. All sections were stored at −20 °C before used. Slide-mounted sections were picked up from the freezer, kept in an oven at 37°C for 30 min, and rinsed twice in 0.01 M PBS for 5 min. The sections were handled with 10 mmol/L citrate buffer (pH = 6.0) and heated to 121 °C in an autoclave for 3 min to retrieve the antigen. The sections were taken from the pressure cooker and cooled to room temperature spontaneously. Then, we blocked the sections with confining liquid which including 10 % donkey serum, 1 % BSA, 0.3 % Triton X-100 and 0.15 % Tween-20 for 2 h at room temperature, then incubated with anti-IDH1 antibody (mouse, 1:100, Santa Cruz) overnight at 4 °C. Following incubation in the secondary antibody at 37 °C, the sections were color-reacted with 0.02 % diaminobenzidine tetrahydrochloride (DAB), 0.1 % phosphate buffer solution (PBS), and 3 % H2O2. At last, slides were counterstained with hematoxylin, dehydrated, and cover slipped. IDH1 staining was assessed under a Leica light microscope (Germany). Cells with strong or moderate brown staining were believed as positive; cells with no staining were rated as negative, while cells with weak staining were scored separately.
Double Immunofluorescent Labeling
Additional sets of sections were used for multiple fluorescence staining. After air-dried for 1 h, sections were first blocked with 10 % normal donkey serum blocking solution species the same as secondary antibody, containing 3 % (w/v) bovine serum albumin (BSA), 0.1 % Triton X-100 and 0.05 % Tween 20 for 2 h at room temperature in order to avoid unspecific staining. The sections were then incubated with primary antibodies against IDH1 (mouse, 1:100; Santa Cruz), NeuN (neuron marker, 1:100; Chemicon, Temecula, CA, USA), glial fibrillary acidic protein (GFAP; astrocytes marker, 1:100; Sigma, St. Louis, MO, USA), CD11b (microglia marker, 1:100; Abcam, Cambridge, MA, USA), active caspase-3 (rabbit, 1:100; Santa Cruz) overnight at 4 °C, followed by a mixture of FITC- and TRITC-conjugated secondary antibodies (Jacks on Immuno Research) for 2 h at 4 °C. In order to detect the morphology of apoptotic cells, sections were covered with DAPI (0.1 mg/mL in PBS; Sigma) for 1 h at 30 °C. The stained sections were examined with Leica confocal microscope or Leica fluorescence microscope (Germany).
Quantitative Analysis
Cell quantification was performed according to the principles described by Koep et al. (1970). Cells double labeled for IDH1 and phenotypic markers used in the experiment were counted. To identify the proportion of each phenotype-specific marker-positive cell expressing IDH1, a minimum of 200 phenotype-specific marker-positive cells were counted in adjacent to the wound in each section. Three adjacent sections per animal were sampled.
Cell Cultures and Stimulation
Rat pheochromocytoma (PC12) cells were obtained from the American Type Culture Collection (Rockville, MD) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % (v/v) fetal bovine serum and 1 % penicillin/streptomycin at 37 °C under 5 % CO2 and 95 % oxygen in humidified air. The medium was changed every 2 days. In order to study apoptosis, PC12 cells were seeded onto 60-mm dishes and incubated in a low concentration of serum (1 % horse serum) for 24 h prior to treatment with hemin (100 μmol/L) for different time points.
siRNAs and Transfection
The DNA target sequence for the IDH1 siRNA construct was as follows: 5′GAGATCCAAGACTAACAAA3′. For transient transfection, the siRNA vector and the nonspecific vector were carried out using lipofectamine 2000 (Invitrogen) and plus reagent basic DMEM (serum free) according to the manufacturer’s instructions. Transfected cells were used for the subsequent experiments 48 h after transfection.
Lactate Dehydrogenase (LDH) Release Assay
Apoptotic cells were quantitated by measurement of extracellular LDH activity using a LDH-Cytotoxicity Test (WAKO), as recommended by the manufacturer. Briefly, PC12 cells were seeded onto a 96-well plate, and then nonspecific siRNA or IDH1siRNA was transfected using lipofectamine 2000 (Invitrogen), and hemin in 100 L of SF-DMEM was used to treat the cells for 12 h. The control group was also cultured in SF-DMEM. Then, the SF-DMEM was recovered, and the LDH activity released was measured by adding reaction reagent comprising nitro blue tetrazolium (NBT), diaphorase, and nicotinamide adenine dinucleotide (NAD) to produce a colored diformazan. After 45 min, 100 L of 1 M HCl was added to each sample to stop the reaction. Then, the absorbance, which reflects cell damage, was detected with a colorimeter (Viento, Dainippon) at 560 nm.
Statistical Analysis
All data in this paper were analyzed with Stata 8.0 statistical software. All values are expressed as mean ± SEM. The statistical analysis was determined by One-way ANOVA followed by the Tukey’s post hoc multiple comparison tests. P < 0.05 was considered statistically significant. Each experiment consisted of at least three replicates per condition.
Results
Rat ICH Model was Established by Behavioral Tests
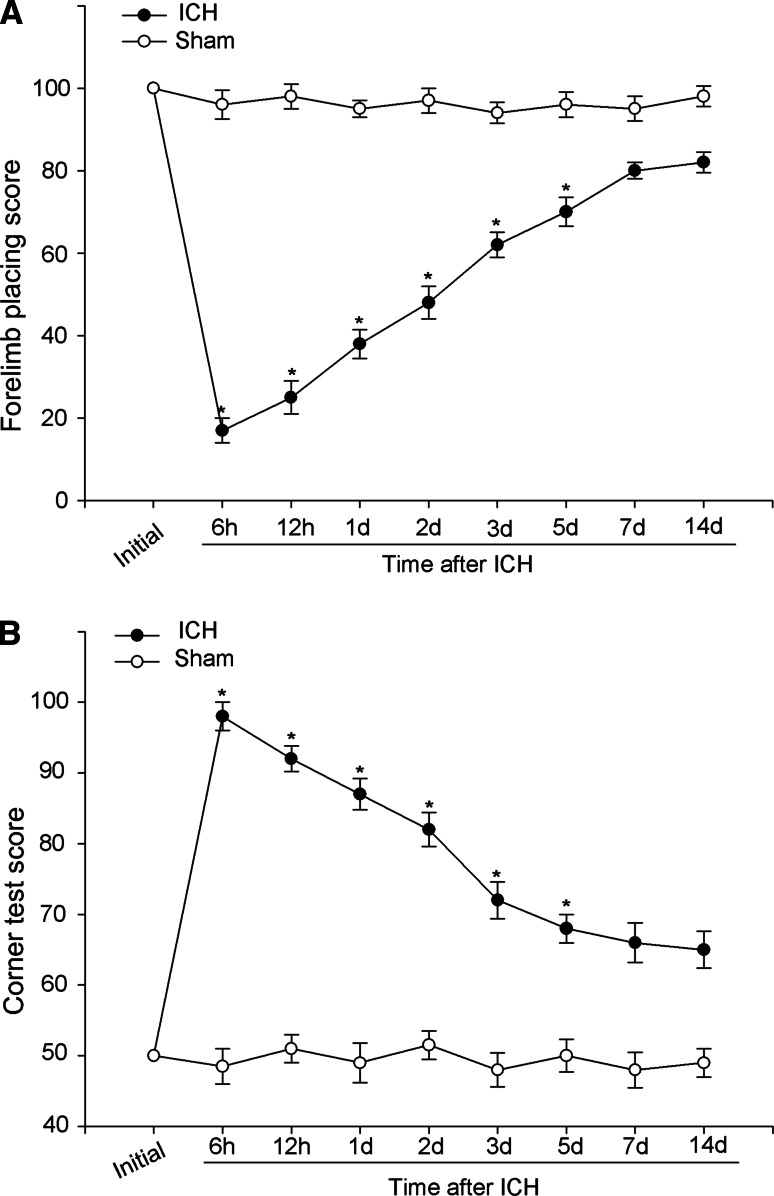
According to previous reports a series of behavior tests were applied to asses acute and chronic changes in sensorimotor function and plasticity in rat models of unilateral brain injury such as ICH (Hua et al. 2002). In this study, forelimb placing and corner turn tests were employed to evaluate the neurological deficits at different time points following ICH. As was shown in Fig. 1, the ICH group was obviously worse impaired when compared with the sham-operated group over the first 5 days (*P < 0.05). By 7 days and thereafter, neurological test scores of the rats went back to baseline.
Fig. 1.
Estimations and scores of behavioral tests on rats suffering from ICH. Behavioral tests were executed in rats after ICH or sham operation. Forelimb placing (a) and corner turn testing scores (b) showed that the ICH group exhibited remarkable deficits compared with the sham-operated group over the first 5 days (*P < 0.05, significantly different from the sham-operated group), with no significant difference at baseline or 5 days later
Changes in Protein Expression of IDH1 After ICH by Western Blot Analysis
To quantify IDH1 expression profile after ICH, protein extracts from rat brains were separated by SDS-PAGE and analyzed by western blot. As shown in Fig. 2, IDH1 level was low in sham-control group progressively increased from 6 h after ICH, reached the peak at day 2, and then gradually returned to baseline (Fig. 2a, b). By the way, we also detected IDH1 protein expression in the contralateral brain, but failed to find any significant fluctuations (data not shown).These findings revealed that the expression of IDH1 undergoes a substantial alteration after rat ICH.
Fig. 2.
Western blot analysis protein level change of Bex1 after ICH. Western blot was performed to study the protein level of IDH1 surrounding the hematoma at various survival times. a Time courses of IDH1 expression after ICH, peaked at day 2, and declined thereafter. b Quantification graphs (relative optical density) of the intensity of staining of IDH1 to β-actin at each time point. β-actin was used to confirm equal amount of protein was run on gel. The data are mean ± SEM. (*P < 0.05, significantly different from the sham-operated group)
Expression and Distribution of IDH1 by Immunohistochemistry
To determine the expression and distribution of IDH1 after ICH, we performed immunohistochemistry staining 2 days after ICH with anti- IDH1 antibody. The sham group showed a low level of IDH1 staining (Fig. 3a, b), similar to the profiles in the contralateral side of the experimental brains (Fig. 3c, d). The number of IDH1-positive cells was observably increased in the brain tissue surrounding the hematoma at day 2 after ICH (Fig. 3e, f, h) and these results were consistent with Western blot results. No staining was observed in the negative control (Fig. 3g).
Fig. 3.
Representative microphotographs for IDH1 immunohistochemistry surrounding the hematoma. Low level of IDH1 was detected in the sham group (a, b). At day 2 after ICH, the contralateral group showed no significant difference in IDH1 (c, d) compared with the sham ones, while the ipsilateral group (e, f) showed increased IDH1 expression. No positive signals were found in the negative control (g). The number of IDH1 positive cells was largely increased comparing the ipsilateral group with the sham and contralateral groups (h). Asterisk denotes P < 0.05. Scale bar left column, 50 μm; right columns, 20 μm
The Colocalizations of IDH1 with Different Cellular Markers by Double Immunofluorescent Staining
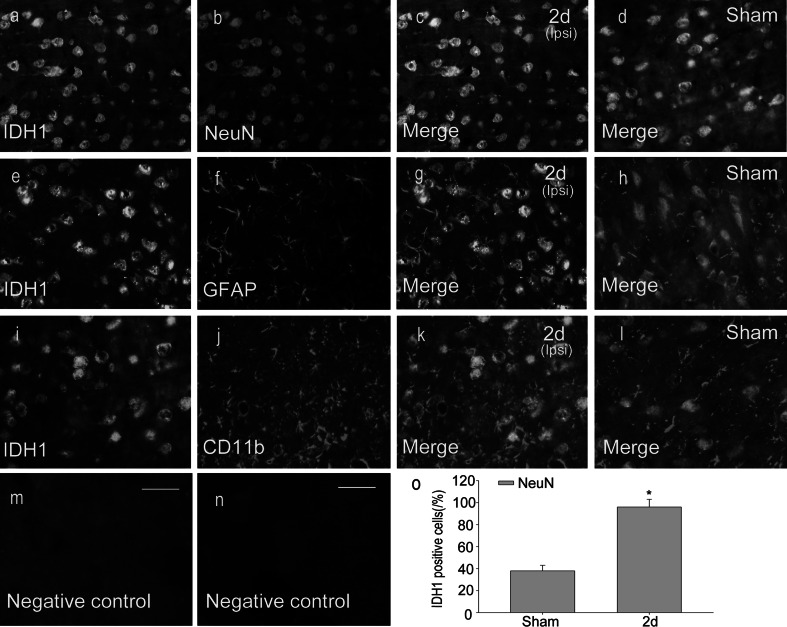
To further investigate the cell types expressing IDH1 following ICH, double Immunofluorescent staining was performed with different markers: NeuN, GFAP, CD11b. They are markers of neurons, astrocytes and oligodendrocytes respectively. As shown in Fig. 4, IDH1 was collocated with the neurons in the cytoplasm and nucleus (Fig. 4c, d). However, colocalization was not observed in microglias or astrocytes (Fig. 4e–l). To identify the proportion of neurons expressing IDH1, at least 200 NeuN-positive cells were counted between ICH 2 day group and the sham group (Fig. 4o). IDH1 expression was markedly increased in neurons (the NeuN-positive cells) after ICH compared with the sham group, which were consistent with the results of immunohistochemistry staining. The expression and distribution of IDH1 in NeuN positive cells indicated that IDH1 might be associated with the changes of biological function of neurons after ICH.
Fig. 4.
The colocalization of IDH1 with different cellular markers by double immunofluorescent staining. In the adult rat caudate within 3 mm distance from the hematoma at the second day after ICH, horizontal sections were labeled with IDH1 (green, a, e, i), different cell markers (red, b, f, j), such as neuronal marker (NeuN), astrocyte marker (GFAP) and microglia marker (CD11b). The yellow color visualized in the merged images represents the colocalization of IDH1 with different specific phenotype markers (c, g, k). The colocalizations of IDH1 with different specific phenotype markers are also shown in the sham-control group (d, h, l). No positive signals were found in the negative control (m, n). The number of NeuN-positive cells expressing IDH1 (%) was remarkably increased in the ICH group compared with the sham group (o). Asterisk means P < 0.05. Scale bars 20 μm (a–n) (Color figure online)
Association of IDH1 with Neuronal Apoptosis After ICH
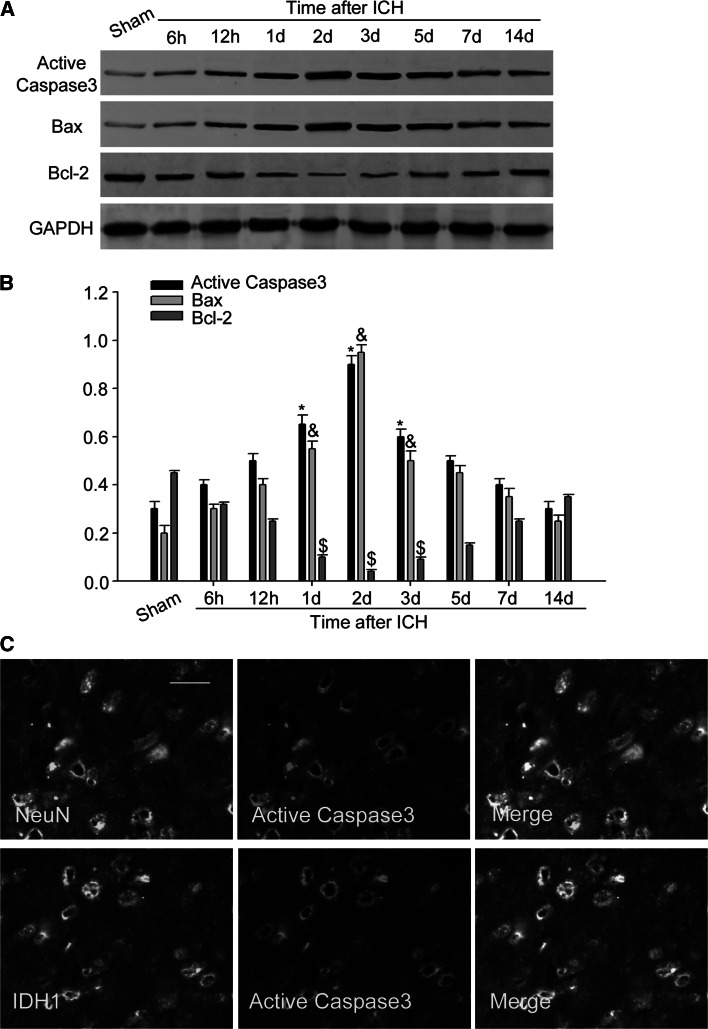
As reported that neuronal apoptosis is regarded as one of the most crucial events after ICH (Sehba and Bederson 2006; Sun et al. 2013). Our research above found that the increase and distribution of IDH1 after ICH appeared in neurons, hence it is reasonable for us to investigate whether IDH1 is correlated with neuronal apoptosis following ICH. Then, we examined the expression profiles of active caspase-3 (a marker of apoptosis). As shown in the results, the expression of active caspase-3 increased after ICH, peaked at day 2, which was relevant with the expression of IDH1 in a time-dependent manner (Fig. 5a, b). In addition, immunofluorescent labeling showed that IDH1 co-localized well with active caspase-3 in neurons (Fig. 5c).
Fig. 5.
Association of IDH1 with cell apoptosis after ICH. The expression of active caspase-3 and Bax increased, peaked at day 2 following ICH (a). The expression of Bcl-2 decreased after ICH and reached valley at 2 days (a). The bar graphs indicated the relative density of active caspase-3, Bax, and Bcl-2 versus GAPDH at each time point (b). Data are presented as mean ± SEM. (*, &, $ P < 0.05,significantly distinct from the sham group). Immunofluorescent staining showed co-staining of NeuN (green) and IDH1 (green) with active caspase-3 (red) in rat brain around hematoma (c). Scale bar 20 μm (c)
Detection Change of IDH1 and Cellular Apoptosis In Vitro
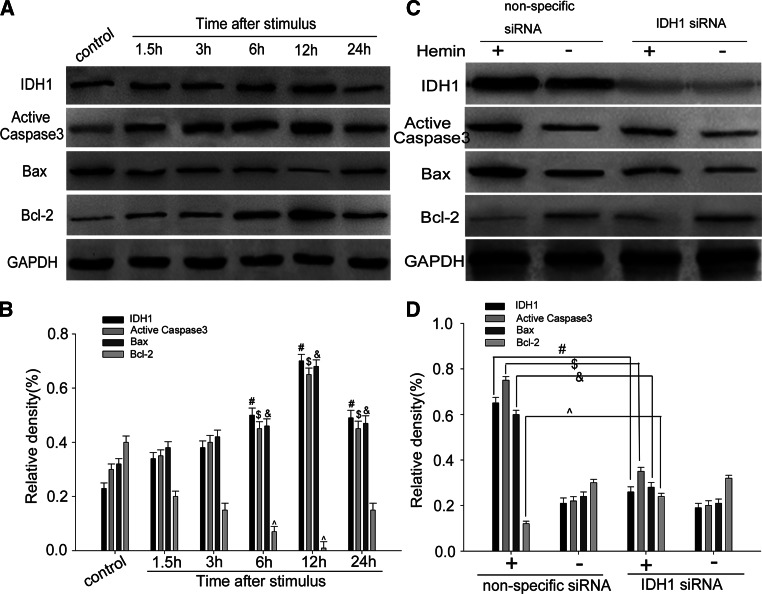
To further investigate the role of IDH1 on neuronal apoptosis after ICH, hemin-induced apoptosis model in PC12 cells was built as described. We incubated PC12 cells with 100 mol/L hemin at different time points and found the consistency up-regulation of IDH1, active caspase-3, and Bax and down-regulation of Bcl-2 in a time-dependent way (Fig. 6a, b). Besides, RNAi-specific to IDH1 was employed to knock down IDH1 in PC12 cells. Western blot analysis showed that knocking down of IDH1 reduced the expression of active caspase-3 and Bax and up-regulated the expressing of Bcl-2 (Fig. 6c, d). Furthermore, cell immunofluorescent staining displayed the co-localization of IDH1 and active caspase-3 in PC12 cells and also revealed that IDH1 silencing reduced the expression of active caspase-3 and nuclear condensation of these cells after hemin treatment (Fig. 7a). Besides, LDH release assay revealed that IDH1 silencing reduced the cytotoxicity (or cell death) after the stimulation of hemin (Fig. 7b).These results suggested that IDH1 could contribute to neuronal pro-apoptosis function following ICH.
Fig. 6.
Modulation of IDH1 on cell apoptosis in vitro. PC12 cells were incubated with hemin at 100 μmol/L for different times. IDH1, active caspase-3, and Bax were up-regulated, peaked at 12 h, while Bcl-2 had the opposite regulation (a). The bar graph indicated the relative density of IDH1, active caspase-3, Bax, and Bcl-2 versus GAPDH at each time point (b). Western blot showed that Knocking IDH1 down induced decreasing levels of active caspase-3 and Bax and had up-regulation of Bcl-2 expression (c). The bar chart indicated the density of IDH1, active caspase-3, Bax, and Bcl-2 versus GAPDH (d). The data are mean ± SEM(#, $, &, ^ P < 0.05, significantly different from the control group)
Fig. 7.
The relationship of IDH1 and PC12 cell apoptosis. Compared with the control group, active caspase-3 and nuclei condensation were observed in PC12 cells at 12 h after the stimulation of hemin, but when IDH1 siRNA was used, these changes were reduced. The yellow color in the merged images represented co-localization of IDH1 (green) with active caspase-3 (red). Scale bars 50 μm (a). Cytotoxicity were estimated after the addition of reagents by means of LDH release assay as described under ‘‘Materials and Methods’’ section (b). The data are mean ± SEM (*P < 0.05, significantly different from the nonspecific siRNA-treated group) (Color figure online)
Discussion
In this manuscript, we analyzed the role of IDH1 in neuronal apoptosis following ICH. The rats suffering from ICH exhibited significantly functional damages assessed by behavioral tests. In this study, IDH1 was up-regulated adjacent to the hematoma of ICH and the temporal changes were striking in neurons, but not in astrocytes or microglia; meanwhile, there was a concomitant increase of active caspase-3 and Bax and down-regulation of Bcl-2 in vivo and in vitro studies. In addition, silencing of IDH1 by siRNA in PC12 cells reduced the expression of active caspase-3 and Bax and increased the expression of Bcl-2. Based on the data, we speculated that IDH1 might exert pro-apoptotic function in neurons after ICH.
IDH1, one member of the IDH family can convert isocitrate toα-ketoglutarate (α-KG) via oxidative decarboxylation (Dang et al. 2016).We have known that the IDH1 mutation is thought to be a “driver” mutation for tumorigenesis (Suzuki et al. 2015). From a metabolic perspective, mutations in IDH1 lead not only to the loss of wild-type enzyme activity [interconversion of isocitrate to α-ketoglutarate (α-KG)] but also to a gain-of-function that results in the conversion of α-KG to the“oncometabolite” 2-HG (Dang et al. 2009). 2-HG is a competitive inhibitor of multiple α-KG-dependent dioxygenases, such as the prolyl hydroxylases, the Jumonji C family of histone demethylases, and the TET family of DNA hydroxylases (Xu et al. 2011). As a result, IDH1/2 mutant cells undergo extensive epigenetic modifications that ultimately result in tumorigenesis (Duncan et al. 2012; Lu et al. 2012; Sasaki et al. 2012; Turcan et al. 2012). Since IDH1 plays an important role in the formation of tumors, how does it play a role in nerve injury. Based on the metabolic perspective, wild-type IDH1 can interconvert isocitrate to α-ketoglutarate (α-KG) and produce glutamate. Previous researches have shown that a large number of glutamate accumulation have neurotoxicity effects (Finlay and Duty 2014; Picconi and Calabresi 2014). On the basis of previous studies of IDH1, we wonder whether IDH1 can induce neuronal/cellular activities through some other ways, such as promoting neuronal apoptosis after ICH.
In addition to the primary damage, ICH leads to series of unfavorable events, causing following secondary damage, and finally gives rise to serious neurological deficits or even death. Neuronal apoptosis, astrocyte proliferation, microglia activation, and oligodendrocyte death all have been implicated in the above. Among them, neuronal apoptosis is the most severe consequence after ICH (Matsushita et al. 2000; Wu et al. 2008). Apoptosis, also known as programmed cell death, can be divided into extrinsic pathway and intrinsic pathway. The former is triggered by the combine of death receptors and recruitment and activation of caspase-8. While the intrinsic one depends on mitochondria-mediated apoptosis pathway. Up-regulation of pro-apoptosis protein Bax levels and meanwhile downregulation of anti-apoptotic protein Bcl-2 facilitate release of cytochrome c and activation of caspases and eventually give rise to cell apoptosis (Cregan et al. 1999; Plesnila et al. 2007). As the key factor of execution phase of apoptosis, caspase-3 converges signals from both the intrinsic and extrinsic pathways (Porter and Janicke 1999).
In conclusion, the present research for the first time detected the expression and variation of IDH1 surrounding the hematoma, and all data proved the involvement of IDH1 in neuronal apoptosis following ICH. Nevertheless, molecular mechanism that IDH1 regulates apoptosis remains to be explored and further studies remain to be done to seek therapeutic potentials of IDH1 for ICH. Thus, we can achieve better prognosis for patients suffering from ICH.
Compliance with Ethical Standards
Conflict of Interests
The authors have declared that no conflict of interest exists.
Footnotes
Xing Chen and Hongmei Wang have contributed equally to this work.
References
- Aronowski J, Zhao X (2011) Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 42:1781–1786. doi:10.1161/STROKEAHA.110.596718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borger DR et al (2012) Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 17:72–79. doi:10.1634/theoncologist.2011-0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M, Lassmann H (2010) Oligodendrocytes: biology and pathology. Acta Neuropathol 119:37–53. doi:10.1007/s00401-009-0601-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredesen DE, Rao RV, Mehlen P (2006) Cell death in the nervous system. Nature 443:796–802. doi:10.1038/nature05293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregan SP, MacLaurin JG, Craig CG, Robertson GS, Nicholson DW, Park DS, Slack RS (1999) Bax-dependent caspase-3 activation is a key determinant in p53-induced apoptosis in neurons. J Neurosci 19:7860–7869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L et al (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462:739–744. doi:10.1038/nature08617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L, Yen K, Attar EC (2016) IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol 27:599–608. doi:10.1093/annonc/mdw013 [DOI] [PubMed] [Google Scholar]
- Duncan CG et al (2012) A heterozygous IDH1R132H/WT mutation induces genome-wide alterations in DNA methylation. Genome Res 22:2339–2355. doi:10.1101/gr.132738.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V (2009) Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 8:355–369. doi:10.1016/S1474-4422(09)70025-0 [DOI] [PubMed] [Google Scholar]
- Finlay C, Duty S (2014) Therapeutic potential of targeting glutamate receptors in Parkinson’s disease. J Neural Transm (Vienna) 121:861–880. doi:10.1007/s00702-014-1176-4 [DOI] [PubMed] [Google Scholar]
- Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF (2001) Intracerebral hemorrhage-induced neuronal death. Neurosurgery 48:875–882 discussion 882–873 [DOI] [PubMed] [Google Scholar]
- Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G (2002) Behavioral tests after intracerebral hemorrhage in the rat. Stroke 33:2478–2484 [DOI] [PubMed] [Google Scholar]
- Izquierdo-Garcia JL, Viswanath P, Eriksson P, Chaumeil MM, Pieper RO, Phillips JJ, Ronen SM (2015) Metabolic reprogramming in mutant IDH1 glioma cells. PLoS One 10:e0118781. doi:10.1371/journal.pone.0118781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabiyikoglu M, Hua Y, Keep RF, Ennis SR, Xi G (2004) Intracerebral hirudin injection attenuates ischemic damage and neurologic deficits without altering local cerebral blood flow. J Cereb Blood Flow Metab 24:159–166. doi:10.1097/01.WCB.0000100062.36077.84 [DOI] [PubMed] [Google Scholar]
- Koep LJ, Konigsmark BW, Sperber EE (1970) Cellular changes in the human supraoptic and paraventricular hypothalamic nuclei in dehydration. J Neuropathol Exp Neurol 29:254–265 [PubMed] [Google Scholar]
- Kosmider O et al (2010) Mutations of IDH1 and IDH2 genes in early and accelerated phases of myelodysplastic syndromes and MDS/myeloproliferative neoplasms. Leukemia 24:1094–1096. doi:10.1038/leu.2010.52 [DOI] [PubMed] [Google Scholar]
- Kranendijk M et al (2010) IDH2 mutations in patients with D-2-hydroxyglutaric aciduria. Science 330:336. doi:10.1126/science.1192632 [DOI] [PubMed] [Google Scholar]
- Kranendijk M, Struys EA, Salomons GS, Van der Knaap MS, Jakobs C (2012) Progress in understanding 2-hydroxyglutaric acidurias. J Inherit Metab Dis 35:571–587. doi:10.1007/s10545-012-9462-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L et al (2013) Up-regulation of NFATc4 involves in neuronal apoptosis following intracerebral hemorrhage. Cell Mol Neurobiol 33:893–905. doi:10.1007/s10571-013-9955-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y et al (2010) A relationship between p27(kip1) and Skp2 after adult brain injury: implications for glial proliferation. J Neurotrauma 27:361–371. doi:10.1089/neu.2008.0581 [DOI] [PubMed] [Google Scholar]
- Lu C et al (2012) IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483:474–478. doi:10.1038/nature10860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardis ER et al (2009) Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 361:1058–1066. doi:10.1056/NEJMoa0903840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Meng W, Wang X, Asahi M, Asahi K, Moskowitz MA, Lo EH (2000) Evidence for apoptosis after intercerebral hemorrhage in rat striatum. J Cereb Blood Flow Metab 20:396–404. doi:10.1097/00004647-200002000-00022 [DOI] [PubMed] [Google Scholar]
- Ohka F et al (2014) Quantitative metabolome analysis profiles activation of glutaminolysis in glioma with IDH1 mutation. Tumour Biol 35:5911–5920. doi:10.1007/s13277-014-1784-5 [DOI] [PubMed] [Google Scholar]
- Picconi B, Calabresi P (2014) Targeting metabotropic glutamate receptors as a new strategy against levodopa-induced dyskinesia in Parkinson’s disease? Mov Disord 29:715–719. doi:10.1002/mds.25851 [DOI] [PubMed] [Google Scholar]
- Plesnila N et al (2007) Delayed neuronal death after brain trauma involves p53-dependent inhibition of NF-kappaB transcriptional activity. Cell Death Differ 14:1529–1541. doi:10.1038/sj.cdd.4402159 [DOI] [PubMed] [Google Scholar]
- Porter AG, Janicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104. doi:10.1038/sj.cdd.4400476 [DOI] [PubMed] [Google Scholar]
- Reitman ZJ et al (2011) Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci USA 108:3270–3275. doi:10.1073/pnas.1019393108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M et al (2012) IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 488:656–659. doi:10.1038/nature11323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehba FA, Bederson JB (2006) Mechanisms of acute brain injury after subarachnoid hemorrhage. Neurol Res 28:381–398. doi:10.1179/016164106X114991 [DOI] [PubMed] [Google Scholar]
- Sun H et al (2013) The member of high temperature requirement family HtrA2 participates in neuronal apoptosis after intracerebral hemorrhage in adult rats. J Mol Histol 44:369–379. doi:10.1007/s10735-013-9489-4 [DOI] [PubMed] [Google Scholar]
- Suzuki H et al (2015) Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet 47:458–468. doi:10.1038/ng.3273 [DOI] [PubMed] [Google Scholar]
- Turcan S et al (2012) IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483:479–483. doi:10.1038/nature10866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ (2010) Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 9:167–176. doi:10.1016/S1474-4422(09)70340-0 [DOI] [PubMed] [Google Scholar]
- Wilson D, Charidimou A, Werring DJ (2014) Advances in understanding spontaneous intracerebral hemorrhage: insights from neuroimaging. Expert Rev Neurother 14:661–678. doi:10.1586/14737175.2014.918506 [DOI] [PubMed] [Google Scholar]
- Wu J, Yang S, Xi G, Song S, Fu G, Keep RF, Hua Y (2008) Microglial activation and brain injury after intracerebral hemorrhage. Acta Neurochir Suppl 105:59–65 [DOI] [PubMed] [Google Scholar]
- Xu W et al (2011) Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 19:17–30. doi:10.1016/j.ccr.2010.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Song S, Hua Y, Nakamura T, Keep RF, Xi G (2008) Effects of thrombin on neurogenesis after intracerebral hemorrhage. Stroke 39:2079–2084. doi:10.1161/STROKEAHA.107.508911 [DOI] [PubMed] [Google Scholar]