Abstract
Inflammation within the central nervous system (CNS) is a major component of many neurodegenerative diseases. The underlying mechanisms of neuronal loss are not fully understood, but the activation of CNS resident phagocytic microglia seems to be a significant element contributing to neurodegeneration. At the onset of inflammation, high levels of microglial phagocytosis may serve as an essential prerequisite for creating a favorable environment for neuronal regeneration. However, the excessive and long-lasting activation of microglia and the augmented engulfment of neurons have been suggested to eventually govern widespread neurodegeneration. Here, we investigated in a functional assay of acute inflammation how the small GTPase RhoA and its main target the Rho kinase (ROCK) influence microglial phagocytosis of neuronal debris. Using BV-2 microglia and human NT2 model neurons, we demonstrate that the pain reliever Ibuprofen decreases RhoA activation and microglial phagocytosis of neuronal cell fragments. Inhibition of the downstream effector ROCK with the small-molecule agents Y-27632 and Fasudil reduces the engulfment of neuronal debris and attenuates the production of the inflammatory mediator nitric oxide during stimulation with lipopolysaccharide. Our results support a therapeutic potential for RhoA/ROCK-inhibiting agents as an effective treatment of excessive inflammation and the resulting progression of microglia-mediated neurodegeneration in the CNS.
Keywords: RhoA, Y-27632, Fasudil, BV-2 microglia, Human NT2 neurons
Introduction
Inflammation within the central nervous system (CNS) has been implicated as a common factor contributing to the progression of various neurodegenerative diseases (Block and Hong 2005; Block et al. 2007). The mechanisms participating in inflammation-mediated neurodegeneration have not been fully elucidated, but the activation of microglia seems to be an essential component. As resident immune effector cells of the CNS, microglia constantly surveil their neuronal microenvironment with the capacity to respond to even minimal homeostatic changes (Kreutzberg 1996; Nimmerjahn et al. 2005). In response to pathological and physiological threats, microglia undergo dramatic alterations changing from resting ramified to reactive ameboid cells with a high level of cellular adaptation and the ability to phagocytize damaged or apoptotic neuronal material (Kreutzberg 1996; Streit et al. 1999; Hanisch and Kettenmann 2007). At the onset of inflammation, the increased phagocytic capacity of microglia may serve to clear the damaged tissue initiating repair mechanisms for regeneration. However, there is increasing evidence that the uncontrolled phagocytic activity of excessive activated microglia govern the neuronal loss under inflammatory conditions (Block and Hong 2005), while the molecular mechanisms are largely unknown.
There is growing evidence that the small GTPase RhoA and its main effector protein, the Rho-associated coiled-coil-containing protein kinase (ROCK), are associated with various disorders of the CNS (Mueller et al. 2005). As a member of the Ras superfamily, RhoA functions as a molecular switch for multiple signaling pathways by cycling between an inactive GDP-bound and an active GTP-bound state (Bishop and Hall 2000). In the active state, RhoA is able to interact with downstream effector proteins such as ROCK, thereby leading to the phosphorylation of various target proteins regulating cytoskeletal dynamics (Mueller et al. 2005). In general, RhoA/ROCK signaling is involved in numerous neuronal functions including cytoskeletal dynamics, neurite outgrowth regulation, and cellular motility (Kopp et al. 2012; Roloff et al. 2015). However, the abnormal activation of RhoA and the dysregulation of ROCK have been implicated in the pathogenesis of neuronal disorders including spinal cord injury, Alzheimer’s disease, stroke, and neuroinflammatory diseases such as multiple sclerosis (Mueller et al. 2005; Kubo et al. 2008; Tönges et al. 2011; Hensel et al. 2015). Since several inhibitors of ROCK have been confirmed to exert beneficial effects in processes relevant to neurodegenerative disease (Kubo et al. 2008), the RhoA/ROCK pathway has become an attractive target for the treatment of neurological disorders (Raad et al. 2012; Hensel et al. 2015). However, except for a recent study on the modulation of ROCK expression and phagocytosis of latex beads (Cui et al. 2013), very little is known about the relationship between RhoA/ROCK signaling and microglial phagocytosis.
Here, we quantified the phagocytic capacity of microglia by co-culturing fluorescent-labeled apoptotic human NT2 model neurons with microglia from the cell line BV-2 (Scheiblich and Bicker 2015a, b) under RhoA- and ROCK-inhibiting conditions. We used the non-steroid anti-inflammatory drug (NSAID) Ibuprofen as an inhibitor of RhoA (Kopp et al. 2012) and determined the level of RhoA activity in a pull-down assay. Inhibition of RhoA/ROCK signaling significantly reduced the engulfment of neuronal debris by microglia. In addition, application of the ROCK inhibitors Fasudil and Y-27632 down-regulated the inflammatory response of microglia as determined by quantifying the generation of the inflammatory mediator nitric oxide (NO).
Methods
Materials
All substances were obtained from Sigma (Taufkirchen, Germany) unless otherwise noted.
Cell Culture
Microglial cells from the murine microglial cell line BV-2 (Blasi et al. 1990) were grown in T175 culture flasks (Nunc, Thermo Fisher Scientific; Langenselbold, Germany) in Dulbecco’s Modified Eagle Medium (DMEM; Gibco-Invitrogen, Karlsruhe, Germany) supplemented with 10 % fetal calf serum (FCS) and 1 % penicillin/streptomycin (P/S; Gibco-Invitrogen). Semi-adherent cells were passaged using a cell scraper (Greiner Bio-One, Frickenhausen, Germany) for a maximum of 30 passages.
Human Ntera2/D1 precursor cells (NT2) were purchased from the American Type Culture Collection (ATCC; Manassas, Virginia, USA). Precursor cells were cultivated in T175 culture flasks in DMEM/F-12 (Gibco-Invitrogen) supplemented with 10 % FCS and 1 % P/S until confluence. Confluent NT2 precursor cells were trypsinized (Trypsin–EDTA, Gibco-Invitrogen) and seeded in 95-mm bacteriological-grade Petri dishes (Greiner, Hamburg, Germany) at a density of 5 × 106 cells/dish. After 24 h, medium of free-floating spherical aggregates was changed to DMEM/F-12 containing retinoic acid (RA) at a final concentration of 10 µM to induce neuronal differentiation (Paquet-Durand et al. 2003; Podrygajlo et al. 2009). After 7–10 days, cells were transferred to T75 culture flasks (Nunc) and cultured for another 7–10 days in DMEM/F-12 containing RA. Cells were trypsinized again and 1 × 108 cells/flask were seeded into T175 culture flasks in DMEM/F-12 for two days. Cells were trypsinized and 4 × 107 cells/flask were cultured in T75 culture flasks in DMEM/F-12 containing mitotic inhibitors (1 μM 1-6-D-arabinofuranosylcytosine, 10 μM 2′-deoxy-5-fluorouridine, and 10 μM 1-β-D-ribofuranosyluracil). Neurons became visible after 7–10 days in inhibitor medium. Differentiated neurons (hNT2) were selectively trypsinized to remove undifferentiated cells, counted, and 1 × 106 neurons/well were cultured in poly-d-lysine (PDL, 10 µg/ml)- and laminin (100 µg/ml)-coated 6-well plates (Costar) in DMEM/F-12.
Phagocytosis Assay
Phagocytosis assay was performed as previously described (Scheiblich and Bicker 2015a, b). In brief, microglia from the cell line BV-2 (passage 7–24) were seeded at a density of 2.5 × 104 cells/well into poly-d-lysine-coated 96-well plates (Corning Costar). To induce different activation states of microglia, cells were either allowed to adhere 24 h prior to experiments in DMEM supplemented with 2 % FCS and 1 % P/S or pre-incubated for 24 h with DMEM containing LPS at a final concentration of 1 µg/ml. Simultaneously, hNT2 neurons (passage 27–35) were labeled with CellTracker™ Red CMTPX (5 µM, Gibco-Invitrogen) for 30 min, washed with PBS (137 mM NaCl, 2.6 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4), and incubated for 24 h in DMEM/F-12 containing staurosporine (100 nM) to induce apoptosis. Apoptotic neurons were collected, washed twice with PBS, and added to BV-2 cells at a ratio of 2:1 in 200 µl DMEM/F-12 supplemented with 10 % FCS and 1 % P/S containing chemical compounds interfering with the RhoA/ROCK signaling pathway. The RhoA stimulator 1-oleoyl lysophosphatidic acid (LPA, Cayman Chemicals, Ann Arbor, Michigan, USA) was dissolved in dimethylsulfoxide (DMSO) as a 20-mM stock. The RhoA inhibitor Ibuprofen and the ROCK inhibitor Fasudil (HA-1077, AbcamBiochemicals®, Cambridge, UK) were dissolved in the medium as 10 mM Stock. The ROCK inhibitor Y–27632 ((1R,4r)-4-((R)-1-aminoethyl)-N-(pyridin-4-yl)cyclohexanecarboxamide) was dissolved in H2O as a 10-mM stock solution. BV-2 microglia and apoptotic hNT2 neurons were co-cultured for 24 h followed by a fixation with paraformaldehyde (PFA, 4 %) for immunofluorescence analysis. Images were taken using a ×20 objective. Phagocytosis was calculated by dividing the number of BV-2 cells that had engulfed red-labeled neuronal fragments by the total number of BV-2 cells. Phagocytic activity was calculated as percentage of control. Experiments were performed at least in four wells and data were collected from three independent experiments, in each of which a minimum of 250 cells were counted per group.
Cell Viability/Cytotoxicity Assay
Cell viability of BV-2 microglia was quantified using the Alamar Blue® assay (Trek Diagnostic Systems, East Grinstead, UK). Cells were treated for 24 h with chemical agents, washed once with BV-2 Ringer (130 mM NaCl, 3 mM KCl, 2 mM CaCl2,1 mM MgCl2, 10 mM HEPES, 4.5 g d-Glucose, pH 7.4), and incubated for 2 h in DMEM containing 5 % Alamar Blue. Fluorescence intensity of Alamar Blue was measured with a microplate reader (Infinite M200, Tecan, Männedorf, Switzerland).
Lectin Staining
To determine microglial phagocytosis, co-cultures were fixed with 4 % PFA and washed three times with PBS. 5 % nonfat-dry milk was used as a blocking solution. BV-2 cells were labeled using biotinylated Lectin from Lycopersicon esculentum (tomato lectin, 1:250 in PBS) for at least 2 h and streptavidin Alexa Fluor® 488 conjugates (1:200 in PBS, Invitrogen) for detection. 4′,6-diamidino-2′-phenylindole dihydrochloride (DAPI) was used as a nuclear counter stain at 0.1 µg/ml for 10 min in PBS.
Nitric Oxide Determination
To provide a quantitative estimate of microglial NO production, the nitrite concentration in the cell culture supernatant was used. BV-2 cells were seeded at a density of 3.5 × 104 cells/well into poly-d-lysine-coated 96-well plates. Cells were incubated for 24 h with 85 µl DMEM supplemented with 2 % FCS and 1 % P/S containing chemical agents. Using 50 µl of the supernatants, a Griess Reagent System was applied according to the manufacturer’s protocol (Promega Corporation, Madison, Wisconsin, USA). Nitrite concentration was determined by interpolation in a nitrite standard curve.
RhoA Pull-Down Assay
For estimation of activated RhoA levels, an RhoA pull-down assay was performed according to the manufacturer’s instructions (Cytoskeleton, Denver, Colorado, USA). BV-2 cells were allowed to adhere to PDL-coated T25 culture flasks at a density of 3.5 × 106 cells overnight in DMEM supplemented with 2 % FCS and 1 % P/S. For lysate collection, BV-2 cells were cultured under control conditions or in medium containing Ibuprofen (100 µM), or LPA (100 µM) before cells were washed with ice-cold BV-2 ringer and lysed in ice-cold lysis puffer for 1 min. Lysates were centrifuged for 1 min at 4 °C and 10,000 g. Supernatants were snap frozen and kept in a −80 °C freezer until use in the pull-down assay. Total protein quantification was performed with the Pierce BCA Protein Assay Kit (Thermo Scientific) and a microplate reader (Infinite M200, Tecan). Cell lysates (600 µg) were incubated with Rhotekin-RBD beads for 60 min at 4 °C on a rocker followed by centrifugation for 1 min at 4 °C and 5000 g. Supernatants were nearly completely removed, and beads were washed in ice-cold washing buffer and centrifuged again to pellet the beads. 10 µl of 2× Laemmli buffer supplemented with 5 % β-mercaptoethanol was added before samples were denatured at 95 °C for 2 min. Samples were then subjected to Western blot analysis according to Roloff et al. (2015).
Western Blotting
Samples from the RhoA pull-down assay were separated by a 10 % Mini-PROTEAN® TGX™ Precast Gel (Bio-Rad Laboratories, Inc., California, USA), and transferred to a PVDF membrane (Bio-Rad Laboratories, Inc.). Membranes were washed with Tris-buffered saline (TBS, 10 mM Tris–HCl, 150 mM NaCl, pH 8.0) for 5 min. Membrane surface was blocked with 5 % nonfat-dry milk in TBST (TBS + 0.05 % Tween-20) for 2 h. For RhoA detection, membranes were incubated with the mouse monoclonal anti-RhoA antibody (1:500 in TBST, provided with RhoA pull-down assay kit) for 2 h. A biotinylated horse anti-mouse antibody (1:2000 in TBST, Vector) was applied for 2 h. Proteins were visualized using the Vectastain ABC Kit (Vector) and the DAB peroxidase substrate kit (Vector) as recommended by the manufacturer.
Microscopy and Data Analysis
All experiments were examined with a Zeiss Axiovert 200 fluorescence microscope (Zeiss, Göttingen, Germany) equipped with a Cool SNAP digital camera (Visitron Systems GmbH, Puchheim, Germany). Acquired images were processed using ImageJ (Wayne Rusband, National Institute of Health, USA). Data were evaluated using GraphPad Prism and presented as mean ± SEM of at least three independent experiments with four replicates. Data were analyzed for Gaussian distribution. When data passed the normality test, statistical comparisons of controls versus treatment were performed with one-way ANOVA in conjunction with a Tukey’s test. Otherwise, data were analyzed with the Kruskal–Wallis test and a Dunn’s post hoc test for non-parametric data. Levels of significance are indicated as *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
RhoA Inhibition Down-Regulates Microglial Phagocytosis
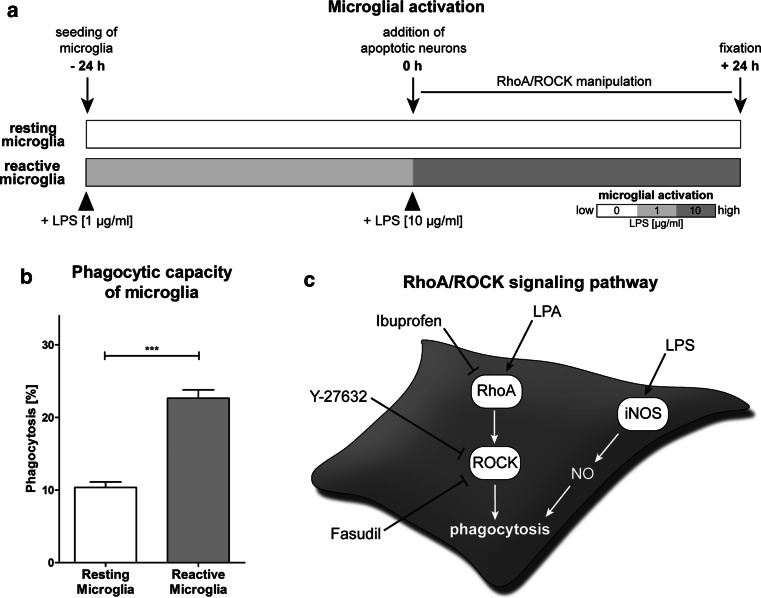
To test for a potential role of RhoA/ROCK signaling as a modulator of microglial phagocytosis, we applied small-molecule agents in an in vitro phagocytosis assay. For quantification of cellular responses at different activation states, we cultured murine microglial BV-2 cells without and with pre-exposure to the inflammogen LPS (1 µg/ml for 24 h) and termed them as “resting microglia” and “reactive microglia,” respectively (Fig. 1a). Incubation with LPS caused microglial cell activation as has been measured by the endogenous NO production (Scheiblich and Bicker 2015a, b). LPS treatment markedly increased the phagocytic activity of reactive microglia (22.64 ± 1.16 %) compared to resting microglia (10.35 ± 0.75 %) (Fig. 1b), confirming our previous results about the engulfment of apoptosis-induced postmitotic human NT2 model neurons (Scheiblich and Bicker 2015a, b). Figure 1c provides a schematic of chemical agents used to manipulate the RhoA/ROCK signaling pathway in microglia.
Fig. 1.
Microglial activation levels and dual regulation of phagocytosis by RhoA/ROCK and NO signaling. a The schematic indicates different activation levels of microglia upon stimulation with LPS and incorporates results from previous studies determining the effects of microglial activation on their phagocytic capacity (Scheiblich and Bicker 2015a, b). Resting microglia were cultured in LPS-free medium prior to and during the phagocytosis experiments, whereas reactive microglia were stimulated with low concentrations (1 µg/ml) of LPS 24 h before and high concentrations (10 µg/ml) of LPS during neuronal co-culture. b The conversion from resting to reactive microglia results in a more than twofold increased phagocytic capacity of neuronal debris. c The image shows chemical agents for the manipulation of RhoA/ROCK signal transduction and incorporates results of the effects of NO in the regulation of microglial phagocytosis from a previous publication on BV-2 cells (Scheiblich and Bicker 2015b). Data are mean ± SEM of at least three independent experiments (***p < 0.001)
At the outset, we asked whether blocking of RhoA activation by Ibuprofen (Fig. 1c) could affect the phagocytic capacity of microglia. For reasons of comparability among the large number of different experiments, phagocytosis is plotted as percentage of control in the following parts of this study. Application of the RhoA inhibitor Ibuprofen did not elicit any striking changes of the phagocytic activity of resting microglia at concentrations between 10 and 100 µM in comparison to the untreated control (Fig. 2a, b). In contrast, treatment of LPS-induced reactive microglia with Ibuprofen dose-dependently decreased the engulfment of red-labeled neuronal fragments (Fig. 2a) down to 63.2 ± 6.8 % at 50 µM and 65.2 ± 7.3 % at 100 µM compared to the LPS control (100.0 ± 10.5 %) (Fig. 2a, c). Since microglia generate high levels of NO upon stimulation with LPS, quantification of microglial-derived NO can give some indication of the activation level of the cells (Zielasek and Hartung 1996; Minghetti and Levi 1998; Vicente et al. 2001). Using the Griess assay as an indicator of NO release, we did not find any influence of Ibuprofen on the LPS-induced NO generation of microglia (Fig. 2d). None of the used concentrations of Ibuprofen affected the viability of the cells (Fig. 2e).
Fig. 2.
Effects of RhoA inhibition via Ibuprofen on microglia at different activation levels. a Response of resting and reactive microglia to Ibuprofen was tested during neuronal co-culture. b Treating resting microglia with different concentrations of Ibuprofen showed no significant changes in their phagocytic capacity compared to the untreated control. c In reactive microglia, Ibuprofen application results in a dose-dependent reduction of the phagocytic activity down to ~65.2 % at 100 µM. d In the Griess assay, the NO production of LPS-treated microglia was measured. Ibuprofen did not change the LPS-induced NO production at any applied concentration. e All used concentrations of Ibuprofen did not affect the viability of microglia. Data are mean ± SEM of at least three independent experiments (*p < 0.05). Magenta cell tracker red for NT2 neuronal staining; green tomato lectin for BV-2 microglial staining; cyan DAPI nuclear staining. Scale bar 50 µm (Color figure online)
Next, we investigated whether the activation of RhoA could modulate the phagocytic activity of microglia. Co-culturing microglia and apoptotic neurons with different concentrations (10–100 µM) of the RhoA activator LPA did not result in significant changes of the phagocytic capacity of resting microglia (Fig. 3a, b) and reactive microglia (Fig. 3a, c), respectively. In addition, LPA did not change the NO generation of LPS-treated microglia (Fig. 3d). None of the used LPA concentrations affected the viability of the cells (Fig. 3e).
Fig. 3.
Effects of RhoA activation via LPA on microglia at different activation levels. a Phagocytic response of microglia during neuronal co-culture was tested by chemical inhibition of RhoA. b Treating resting microglia with different concentrations of the RhoA inhibitor LPA had no effect on their phagocytic capacity. c Additionally, LPA treatment did not affect the capacity of reactive microglia to engulf neuronal debris. d LPA did not change the LPS-induced NO production of microglia. e The viability of microglia was not influenced by LPA treatment. Data are mean ± SEM of at least three independent experiments. Magenta cell tracker red for NT2 neuronal staining; green tomato lectin for BV-2 microglial staining; cyan DAPI nuclear staining. Scale bar 50 µm (Color figure online)
Assay for RhoA Regulation
To estimate levels of activated RhoA in microglia after Ibuprofen and LPA treatment, we used a RhoA pull-down assay (Fig. 4). For quantification of blotted protein bands, gray values were normalized using the “sum of all data points in a replicate” (Degasperi et al. 2014). Culturing microglia for 15 min with the RhoA inhibitor Ibuprofen (100 µM) did not cause any changes of the level of activated RhoA compared to the untreated control. In contrast, the RhoA stimulator LPA caused an almost threefold increased level of activated RhoA within 15 min of exposure at 100 µM. This LPA-induced RhoA level could be down-regulated by the co-application of 100 µM Ibuprofen.
Fig. 4.
Pull-down assay of activated RhoA. An RhoA pull-down assay was used to determine the level of activated RhoA in microglia upon treatment with the RhoA inhibitor Ibuprofen and the RhoA activator LPA for 15 min. Treatment with Ibuprofen did not cause any changes of the level of activated RhoA compared to the untreated cells. In contrast, LPA treatment results in an almost threefold increased level of activated RhoA. The LPA-induced RhoA activation level was down-regulated by the co-application of Ibuprofen. Cells lysates were analyzed by western blotting. Blots were scanned and mean signal intensities were normalized in comparison to untreated control cell homogenates. Molecular weight of stained protein bands corresponds to about 24 kDa as is indicative for RhoA. Relative gray values represent mean of five independent experiments
Inhibition of ROCK Prevents NO Production and Down-Regulates Phagocytosis
To uncover the effects of the downstream target of RhoA, the Rho Kinase ROCK, we applied two specific enzyme inhibitors. Treating co-cultures of microglia and apoptotic neuronal remnants with the ROCK inhibitor Y-27632 resulted in no significant reduction in phagocytic activity of resting microglia in comparison to the control (Fig. 5a, b). However, Y-27632 treatment of reactive microglia markedly reduced the engulfment of red-labeled neuronal fragments (Fig. 5a) in a dose-dependent manner down to 63.7 ± 7.1 % of control at 50 µM and 58.5 ± 7.4 % of control at 100 µM (Fig. 5a, c). When BV-2 microglia were treated with different concentrations of Y–27632 (10-100 µM), the LPS-induced endogenous NO production of the cells was markedly reduced in a dose-dependent manner (Fig. 5d). Even, the lowest used concentration of 10 µM Y-27632 significantly reduced the NO generation in LPS-treated microglia (Fig. 5d). Y-27632 did not affect the viability of microglia at any used concentration (Fig. 5e).
Fig. 5.
ROCK inhibition via Y-27632 down-regulates phagocytosis and NO production in microglia. a Treatment of resting and reactive microglia with Y-27632 during neuronal co-culture. b Application of Y-27632 did not cause any conspicuous changes of the phagocytic activity of resting microglia. c In contrast, treatment of reactive microglia with Y-27632 dose-dependently decreased the LPS-induced engulfment of neuronal fragments to ~58.5 % at 100 µM. d Treatment of microglia with Y-27632 largely suppresses LPS-induced NO production in a dose-dependent manner. e Viability of microglia was not affected at any used Y-27632 concentration. Data are mean ± SEM of at least three independent experiments (*p < 0.05, **p < 0.01, ***p < 0.001). Magenta cell tracker red for NT2 neuronal staining; green tomato lectin for BV-2 microglial staining; cyan DAPI nuclear staining. Scale bar 50 µm (Color figure online)
To obtain independent support for a strategy of attenuating microglial phagocytosis by RhoA/ROCK inhibition, we used Fasudil as a second ROCK blocker. Treatment of phagocytic microglia with Fasudil showed similar results to Y-27632. While treatment of resting microglia with the ROCK inhibitor had no effect on their phagocytic activity (Fig. 6a, b), Fasudil reduced the phagocytic capacity of reactive microglia dose-dependently (Fig. 6a, c). Culturing reactive microglia with 10 µM Fasudil reduced their phagocytic activity down to 72.0 ± 8.7 %, while the application of 50 µM Fasudil resulted in a reduction of about 65.1 ± 4.3 % of its control (100.0 ± 6.3 %) (Fig. 6c). Treating LPS-stimulated microglia with Fasudil reduced the endogenous NO production in a dose-dependent manner, as measured in the Griess assay (Fig. 6d), while all used concentrations had no effect on the viability of the cells (Fig. 6e).
Fig. 6.
ROCK inhibition via Fasudil suppresses phagocytosis and NO production in microglia. a Phagocytosis of resting and reactive microglia upon treatment with the ROCK inhibitor Fasudil. b Treating resting microglia with increasing concentrations of Fasudil did not affect the phagocytic capacity of the cells. c In contrast, Fasudil significantly down-regulates phagocytic activity of reactive microglia at concentrations between 10 and 50 µM to ~65.1 %. d The LPS-induced NO production of microglia was dose-dependently reduced by the application of Fasudil. e All used concentrations of Fasudil did not affect the viability of the cells. Data are mean ± SEM of at least three independent experiments (*p < 0.05, **p < 0.01, ***p < 0.001). Magenta cell tracker red for NT2 neuronal staining; green tomato lectin for BV-2 microglial staining; cyan DAPI nuclear staining. Scale bar 50 µm (Color figure online)
Discussion
Many studies have concluded that the neuronal loss upon inflammation is mediated by the excessive activation of microglia which has been closely associated with neurodegenerative processes (Lucin and Wyss-Coray 2009; Glass et al. 2010). The molecular mechanisms contributing to the inflammation-mediated neuronal loss implicating the abnormal activation of the RhoA/ROCK cascade (Mueller et al. 2005; Kubo et al. 2008) are of considerable interest for the development of therapeutic treatments. However, so far it is largely unknown whether the manipulation of RhoA/ROCK signaling could modulate the phagocytic capacity of microglia (Fig. 1). Using BV-2 microglia in an in vitro phagocytosis assay, we have already investigated the phagocytic capacity at different activation levels to engulf apoptosis-induced postmitotic neurons (Scheiblich and Bicker 2015a, b). We used LPS as an adequate in vitro activation agent to cause alterations from resting to reactive microglia (Hanisch and Kettenmann 2007; Block et al. 2007; Neher et al. 2011). These investigations unraveled an antagonistic contribution of the cell endogenous gaseous messengers NO and CO to the modulation of glial phagocytosis (Scheiblich and Bicker 2015a, b). In the present study, we analyzed the regulatory role of RhoA/ROCK signaling during microglial phagocytosis in an in vitro approach containing microglia from the cell line BV-2 (Blasi et al. 1990) and human model neurons derived from the cell line Ntera2/D1 (Andrews 1984; Paquet-Durand and Bicker 2007; Tegenge et al. 2011).
Ibuprofen Prevents Phagocytosis of Neuronal Debris Via RhoA Inhibition
RhoA is an Ras-related GTP-binding protein that interacts with multiple downstream targets to regulate various cellular functions including cytoskeletal rearrangement and cellular dynamics (Hall 1998; Bishop and Hall 2000). Since Rho family proteins including RhoA have been reported to play a central role in cytoskeletal changes during phagocytosis in macrophages (Caron and Hall 1998; Chimini and Chavrier 2000), we were now wondering about its impact on the regulation of microglial phagocytic capacity.
Using the NSAID Ibuprofen as an inhibitor of RhoA (Kopp et al. 2012), we showed that the phagocytic capacity of resting microglia was not affected (Fig. 2a, b), while Ibuprofen significantly decreased the engulfment of neuronal debris by reactive microglia by about ~40 % of control (Fig. 2a, c). In contrast, activation of RhoA by the stimulator of the Rho pathway LPA did not affect the engulfment of neuronal fragments in both resting and reactive microglia, respectively (Fig. 3a–c). Our data are in line with studies on macrophages showing that the presence of RhoA allows for the engulfment of apoptotic remnants, while the blockage of RhoA signals inhibited the phagocytic activity of these cells (Hackam et al. 1997; Caron and Hall 1998; Chimini and Chavrier 2000). RhoA has been reported to be involved in the phagocytosis that is mediated by the complement receptor 3 (CR3), thereby leading to the reorganization of the F-actin cytoskeleton which is required for the uptake of the particles into the phagocyte (Caron and Hall 1998). In addition, recognition of apoptotic neuronal fragments by microglial CR3 acts as an “eat-me” signal resulting in the engulfment of the detected fragments (Brown and Neher 2014).
We used the Griess assay as an endpoint to determine the activation level of microglia via the intracellular release of the inflammatory mediator NO (Scheiblich and Bicker 2015b). However, neither Ibuprofen (Fig. 2d) nor LPA (Fig. 3d) did affect the activation state of our LPS-treated cells, suggesting that Ibuprofen modulates the phagocytic activity of microglia via its inhibitory action on RhoA. We examined the activation level of RhoA following application of Ibuprofen and LPA in the pull-down assay (Fig. 4). Treatment of BV-2 microglia with LPA almost tripled the level of activated RhoA, while Ibuprofen alone showed no effect. Co-application of Ibuprofen and LPA down-regulated the LPA-induced activation of RhoA. However, for a straightforward comparison of RhoA activation and inhibition, the serum concentrations in the cell culture medium should be considered. To avoid serum starvation of the co-cultured neurons, phagocytosis experiments were performed in medium containing 10 % FCS, whereas microglia in the pull-down assay were cultured in medium supplemented with 2 % FCS. These conditions were essential to prevent the serum-mediated activation of the cells and to allow for substrate adhesion in the assay (Laurenzi et al. 2001). Since serum has been reported to greatly enhance RhoA activation, while serum deprivation down-regulated the activation level of RhoA (Ren et al. 1999), it is quite likely that serum starvation of microglia occludes the inhibitory effects of Ibuprofen. Likewise, the serum-enriched environment of the phagocytosis assay may mask the stimulatory effects on RhoA activation (Ren et al. 1999).
ROCK Inhibition Down-Regulates Phagocytosis
The intracellular signaling mechanisms of RhoA activation implicate downstream the stimulation of the Rho kinase ROCK as the principle mediator (Schmandke et al. 2007). Using the two specific ROCK inhibitors Y-27632 and Fasudil (Kubo et al. 2008), we determined the effects of ROCK on the engulfment of neuronal fragments by microglia. We found that inhibition of ROCK via Y-27632 (Fig. 5a–c) and Fasudil (Fig. 6a–c) down-regulated the phagocytic response of reactive microglia by about ~40 % of control, while both ROCK inhibitors did not affect the engulfment of neuronal debris by resting microglia. These data are consistent with previous reports demonstrating that ROCK inhibition prevents the phagocytosis by microglia (Cui et al. 2013) and monocytes (Orlando et al. 2006). Similar to RhoA, ROCK is required for the CR3-mediated phagocytosis of apoptotic cells (Olazabal et al. 2002). Thus, ROCK inhibition presumably acts by blocking the actin cytoskeletal remodeling which is necessary for the process of engulfment (Chimini and Chavrier 2000). Previous studies have revealed ROCK as a major mediator of neuroinflammatory processes (Mueller et al. 2005) showing that ROCK is up-regulated in response to inflammatory stimuli including LPS (Hiroki et al. 2004; Wang et al. 2015). Interestingly, disruption of ROCK signaling prevented the release of LPS-induced pro-inflammatory cytokines in a model of hyperalgesia in the mouse spinal cord (Wang et al. 2015).
Here, we showed that both ROCK inhibitors Y-27632 (Fig. 5d) and Fasudil (Fig. 6d) significantly down-regulated the LPS-induced NO production. We have already demonstrated that the generation of NO by activated BV-2 cells results in the exposure of the “eat-me” signal phosphatidylserine to the outer membrane leaflet of NT2 neurons, which causes an increased phagocytic activity of microglia (Scheiblich and Bicker 2015b). Blocking of the microglial-derived NO generation attenuates the phagocytic capacity of reactive microglia (Scheiblich and Bicker 2015a; b). Thus, it is quite likely that application of the ROCK inhibitors suppresses the phagocytic activity of microglia not only via its actions on the actin cytoskeleton, but also via inhibition of NO generation.
Functional Implications
Inhibition of the RhoA/ROCK pathway has been suggested as a promising strategy for stimulating axonal recovery after spinal cord injury and as a therapeutic avenue for the treatment of major neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and multiple sclerosis (Mueller et al. 2005; Tönges et al. 2011; Hensel et al. 2015). Although these neurodegenerative disorders have different causes, chronic neuroinflammation contributes as a common mechanism to disease progression. As resident immune effector cells of the CNS, microglia are key players in the inflammation-mediated neurodegeneration (Block and Hong 2005; Block et al. 2007; Neher et al. 2011) and might serve as a potential pharmacological target. Using a rather simple in vitro experimental system, we showed that chemical manipulation of the RhoA/ROCK pathway affected a microglial cell line engaged in phagocytosis of fragmented human model neurons. There is now common consensus that microglia exhibit cellular plasticity and exist in multiple phenotypes with different functional profiles. In the healthy CNS, a resting state performs homeostatic tissue surveillance functions, while contact with harmful stimuli during disease causes microglia to enter into activated states with the capacity to engulf neuronal tissue (Kreutzberg 1996; Streit et al. 1999; Nimmerjahn et al. 2005; Hanisch and Kettenmann 2007). Especially, the inflammatory conditions in neurodegenerative diseases are accompanied by sustained microglial activation. The release of neurotoxic mediators, such as cytokines, free radicals, and NO, by inflamed microglia contributes to the excessive neuronal loss of the various neurodegenerative diseases.
Inhibition of microglial activation has been tested as a therapeutic strategy to prevent inflammation-mediated neuronal loss (Takeuchi 2010). However, under conditions in which both neurotoxic and neuroprotective properties of activated microglia are affected, this strategy will fail. Therapeutic approaches require therefore strategies that specifically target the neurotoxic properties of activated microglia without causing adverse effects. Remarkably, in our cell culture model the drugs Ibuprofen (Fig. 2), Y-27632 (Fig. 5), and Fasudil (Fig. 6) specifically attenuate the phagocytic capacity of LPS-activated microglia without affecting the basal response of the cells. These encouraging in vitro results require now confirmation by drug treatments in animal studies of neuroinflammation. Since the anti-inflammatory pain reliever Ibuprofen reduces RhoA activation (Kopp et al. 2012; Roloff et al. 2015) independent of the effect on cyclooxygenases, the application of this drug into the CNS should be further explored as a possible therapeutic strategy. Ongoing studies with the two small-molecule ROCK inhibitors Fasudil and Y-27623 have revealed beneficial effects in rodent models of neurodegenerative diseases (Mueller et al. 2005; Hensel et al. 2015). Fasudil is an approved drug in Japan for the treatment of cardiovascular diseases (Mueller et al. 2005) that has been extensively characterized in its safety profile and pharmacokinetic properties. In comparison, in vivo administration and characterization of Y-27632 has been much less investigated so far (Mueller et al. 2005). Even though Fasudil and Y-27632 are active after oral application, both ROCK inhibitors are rapidly metabolized and penetrate poorly via the blood–brain barrier (Mueller et al. 2005). To attain therapeutic efficiency in human neurodegenerative disorders, it will be essential to design pharmaceutical formulations that increase the drug concentration within the CNS. A further technical challenge will be the development of additional small-molecule blockers of the RhoA/ROCK pathway by methods of chemical biology.
Conclusions
In summary, the present results show that LPS-induced microglial phagocytosis can be regulated by the Rho/ROCK pathway whose inhibition suppresses the engulfment of co-cultured neurons. Application of ROCK inhibitors prevented the LPS-induced intracellular release of the inflammatory mediator NO which we have previously shown to amplify the inflammatory response and the engulfment of neurons by microglia. It is intriguing that attenuation of phagocytosis by Ibuprofen, Y-27632, and Fasudil is restricted to activated and not to resting microglia. This opens the prospect for therapeutic manipulations of activated microglia under conditions of excessive tissue inflammation while leaving basal phagocytic activity in healthy parts of the nervous system intact. Our present results on cultures BV-2 microglia and human NT2 model neurons represent an important initial step for designing novel strategies that selectively target the RhoA/ROCK pathway during neuroinflammatory processes.
Acknowledgments
This work was supported by a grant of the German Research Foundation: BI 262/16-2. The authors would like to thank Saime Tan for generating the human NT2 neurons and our colleagues from the Forschergruppe 1103 for their contributions regarding glial culture. We are grateful to Frank Roloff and Michael Stern for helpful discussions.
References
- Andrews PW (1984) Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol 103:285–293 [DOI] [PubMed] [Google Scholar]
- Bishop A, Hall A (2000) Rho GTPases and their effector proteins. Biochem J 348:241–255 [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F (1990) Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol 27:229–237 [DOI] [PubMed] [Google Scholar]
- Block ML, Hong J-S (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76:77–98 [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong J-S (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69 [DOI] [PubMed] [Google Scholar]
- Brown GC, Neher JJ (2014) Microglial phagocytosis of live neurons. Nat Rev Neurosci 15:209–216 [DOI] [PubMed] [Google Scholar]
- Caron E, Hall A (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717–1721 [DOI] [PubMed] [Google Scholar]
- Chimini G, Chavrier P (2000) Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat Cell Biol 2:E191–E196 [DOI] [PubMed] [Google Scholar]
- Cui G, Zuo T, Zhao Q, Hu J, Jin P, Zhao H, Jing J, Zhu J, Chen H, Liu B (2013) ROCK mediates the inflammatory response in thrombin induced microglia. Neurosci Lett 554:82–87 [DOI] [PubMed] [Google Scholar]
- Degasperi A, Birtwistle MR, Volinsky N, Rauch J, Kolch W, Kholodenko BN (2014) Evaluating strategies to normalise biological replicates of western blot data. PLoS ONE 9(1):e87293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam DJ, Rotstein OD, Schreiber A, Zhang W, Grinstein S (1997) Rho is required for the initiation of calcium signaling and phagocytosis by Fcγ receptors in macrophages. J Exp Med 186:955–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A (1998) Rho GTPases and the actin cytoskeleton. Science 279:509–514 [DOI] [PubMed] [Google Scholar]
- Hanisch U-K, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394 [DOI] [PubMed] [Google Scholar]
- Hensel N, Rademacher S, Claus P (2015) Chatting with the neighbors: crosstalk between Rho-kinase (ROCK) and other signaling pathways for treatment of neurological disorders. Front Neurosci 9:198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroki J, Shimokawa H, Higashi M, Morikawa K, Kandabashi T, Kawamura N, Kubota T, Ichiki T, Amano M, Kaibuchi K (2004) Inflammatory stimuli upregulate Rho-kinase in human coronary vascular smooth muscle cells. J Mol Cell Cardiol 37:537–546 [DOI] [PubMed] [Google Scholar]
- Kopp M, Liebscher T, Niedeggen A, Laufer S, Brommer B, Jungehulsing G, Strittmatter S, Dirnagl U, Schwab J (2012) Small-molecule-induced Rho-inhibition: NSAIDs after spinal cord injury. Cell Tissue Res 349:119–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318 [DOI] [PubMed] [Google Scholar]
- Kubo T, Yamaguchi A, Iwata N, Yamashita T (2008) The therapeutic effects of Rho-ROCK inhibitors on CNS disorders. Ther Clin Risk Manag 4:605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenzi MA, Arcuri C, Rossi R, Marconi P, Bocchini V (2001) Effects of microenvironment on morphology and function of the microglial cell line BV-2. Neurochem Res 26:1209–1216 [DOI] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T (2009) Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64:110–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L, Levi G (1998) Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog Neurobiol 54:99–125 [DOI] [PubMed] [Google Scholar]
- Mueller BK, Mack H, Teusch N (2005) Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov 4:387–398 [DOI] [PubMed] [Google Scholar]
- Neher JJ, Neniskyte U, Zhao J-W, Bal-Price A, Tolkovsky AM, Brown GC (2011) Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol 186:4973–4983 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318 [DOI] [PubMed] [Google Scholar]
- Olazabal IM, Caron E, May RC, Schilling K, Knecht DA, Machesky LM (2002) Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcγR, phagocytosis. Curr Biol 12:1413–1418 [DOI] [PubMed] [Google Scholar]
- Orlando KA, Stone NL, Pittman RN (2006) Rho kinase regulates fragmentation and phagocytosis of apoptotic cells. Exp Cell Res 312:5–15 [DOI] [PubMed] [Google Scholar]
- Paquet-Durand F, Bicker G (2007) Human model neurons in studies of brain cell damage and neural repair. Curr Mol Med 7:541–554 [DOI] [PubMed] [Google Scholar]
- Paquet-Durand F, Tan S, Bicker G (2003) Turning teratocarcinoma cells into neurons: rapid differentiation of NT-2 cells in floating spheres. Dev Brain Res 142:161–167 [DOI] [PubMed] [Google Scholar]
- Podrygajlo G, Tegenge MA, Gierse A, Paquet-Durand F, Tan S, Bicker G, Stern M (2009) Cellular phenotypes of human model neurons (NT2) after differentiation in aggregate culture. Cell Tissue Res 336:439–452 [DOI] [PubMed] [Google Scholar]
- Raad M, El Tal T, Gul R, Mondello S, Zhang Z, Boustany R, Guingab J, Wang KK, Kobeissy F (2012) Neuroproteomics approach and neurosystems biology analysis: ROCK inhibitors as promising therapeutic targets in neurodegeneration and neurotrauma. Electrophoresis 33:3659–3668 [DOI] [PubMed] [Google Scholar]
- Ren X, Kiosses WB, Schwartz MA (1999) Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J 18:578–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roloff F, Scheiblich H, Dewitz C, Dempewolf S, Stern M, Bicker G (2015) Enhanced neurite outgrowth of human model (NT2) neurons by small-molecule inhibitors of Rho/ROCK signaling. PLoS ONE 10:e0118536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiblich H, Bicker G (2015a) Regulation of microglial migration, phagocytosis, and neurite outgrowth by HO-1/CO signaling. Dev Neurobiol 75:854–876 [DOI] [PubMed] [Google Scholar]
- Scheiblich H, Bicker G (2015b) Nitric oxide regulates antagonistically phagocytic and neurite outgrowth inhibiting capacities of microglia. Dev Neurobiol. doi:10.1002/dneu.22333in press [DOI] [PubMed] [Google Scholar]
- Schmandke A, Schmandke A, Strittmatter SM (2007) ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist 13:454–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA (1999) Reactive microgliosis. Prog Neurobiol 57:563–581 [DOI] [PubMed] [Google Scholar]
- Takeuchi H (2010) Neurotoxicity by microglia: mechanisms and potential therapeutic strategy. Clin Exp Neuroimmunol 1:12–21 [Google Scholar]
- Tegenge M, Roloff F, Bicker G (2011) Rapid differentiation of human embryonal carcinoma stem cells (NT2) into neurons for neurite outgrowth analysis. Cell Mol Neurobiol 31:635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tönges L, Koch J-C, Bähr M, Lingor P (2011) ROCKing regeneration: Rho kinase inhibition as molecular target for neurorestoration. Front Mol Neurosci 4:39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente AM, Guillén MI, Alcaraz MJ (2001) Modulation of haem oxygenase-1 expression by nitric oxide and leukotrienes in zymosan-activated macrophages. Br J Pharmacol 133:920–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Song S, Zhang Y, Ge Y, Fang X, Huang T, Du J, Gao J (2015) Inhibition of the Rho/Rho kinase pathway prevents lipopolysaccharide-induced hyperalgesia and the release of TNF-α and IL-1β in the mouse spinal cord. Sci Rep. 5:14553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielasek J, Hartung H-P (1996) Molecular mechanisms of microglial activation. Adv Neuroimmunol 6:191–222 [DOI] [PubMed] [Google Scholar]