Abstract
Transplantation of bone marrow stromal cells (BMSCs) is a promising therapy for ischemic stroke, but the poor oxygen environment in brain lesions limits the efficacy of cell-based therapies. Here, we tested whether hypoxic preconditioning (HP) could augment the efficacy of BMSC transplantation in a rat ischemic stroke model and investigated the underlying mechanism of the effect of HP. In vitro, BMSCs were divided into five passage (P0, P1, P2, P3, and P4) groups, and HP was applied to the groups by incubating the cells with 1% oxygen for 0, 4, 8, 12, and 24 h, respectively. We demonstrated that the expression of hypoxia-inducible factor-1α (HIF-1α) was increased in the HP-treated BMSCs, while their viability was unchanged. We also found that HP decreased the apoptosis of BMSCs during subsequent simulated ischemia–reperfusion (I/R) injury, especially in the 8-h HP group. In vivo, a rat transient focal cerebral ischemia model was established. These rats were administered normal cultured BMSCs (N-BMSCs), HP-treated BMSCs (H-BMSCs), or DMEM cell culture medium (control) at 24 h after the ischemic insult. Compared with the DMEM control group, the two BMSC-transplanted groups exhibited significantly improved functional recovery and reduced infarct volume, especially the H-BMSC group. Moreover, HP decreased neuronal apoptosis and enhanced the expression of BDNF and VEGF in the ischemic brain. Survival and differentiation of transplanted BMSCs were also increased by HP, and the quantity of engrafted BMSCs was significantly correlated with neurological function improvement. These results suggest that HP may enhance the therapeutic efficacy of BMSCs in an ischemic stroke model. The underlying mechanism likely involves the inhibition of caspase-3 activation and an increasing expression of HIF-1α, which promotes angiogenesis and neurogenesis and thereby reduces neuronal death and improves neurological function.
Electronic supplementary material
The online version of this article (doi:10.1007/s10571-016-0445-1) contains supplementary material, which is available to authorized users.
Keywords: Bone marrow stromal cells (BMSCs), Ischemic stroke, Hypoxic preconditioning (HP), Neurogenesis, Angiogenesis
Introduction
Ischemic stroke is one of the leading causes of death and disability worldwide; however, there are currently few effective clinical therapeutic approaches for ischemic stroke (Nishijima et al. 2015). Thrombolytic treatment is beneficial for acute ischemic stroke, but the short-time window limits its application (Singh et al. 2013). Alternatively, cell-based therapies have displayed promise as means to promote functional recovery and tissue restoration after stroke (Malgieri et al. 2010; Momin et al. 2010). Bone marrow stromal cells (BMSCs) can be easily taken from patients themselves without ethical or immunological problems and can proliferate in large numbers in vitro. As transplanted, BMSCs promote neurogenesis and angiogenesis in the ischemic brain. BMSCs have been demonstrated immunomodulatory, anti-inflammatory, anti-apoptotic, and tissue repair properties in rodent studies and in preliminary clinical trials (Rowart et al. 2015; Souidi et al. 2013).
However, such cell-based therapies are restricted by the poor survival of engrafted cells within the lesion site (Wang et al. 2015). Recent studies have shown that a large amount of transplanted cells in the ischemic brain die due to hypoxia, inflammation, oxidative stress, and deficits in neurotrophic factor (Otero et al. 2012). Thus, it is very necessary to identify treatment that can increase the viability and differentiation of implanted stem cells in hazardous ischemic tissue (Guo et al. 2012; van Velthoven et al. 2009). Stem cell proliferation and differentiation are often analyzed under conditions of 20% O2, which is much greater than the concentration found in vivo. Arterial oxygen concentration is approximately 12%, whereas the concentration of oxygen experienced by BMSCs in bone marrow under physiological conditions is thought to range from 2 to 8% (Mohyeldin et al. 2010). Nevertheless, hypoxic preconditioning (HP) can increase tolerance to hypoxic-ischemic injury and decrease apoptosis and is associated with changes in the expression of genes such as hypoxia-inducible factor-1α (HIF-1α) (Feng and Bhatt 2015; Huang et al. 2013). Previous reports have shown that HP of transplanted cells promotes their regenerative capability and therapeutic potential for the treatment of both ischemic stroke and hemorrhagic stroke (Wei et al. 2012; Sun et al. 2015). However, many questions remain regarding the efficacy of HP in the treatment of stroke, and more foundational animal experiments are needed to elucidate its underlying neuroprotective mechanisms. Importantly, understanding of the mechanisms and genes involved in HP-induced BMSCs may provide new promising strategy for treating ischemic stroke and promote neurorecovery. The aim of the present research was to evaluate whether HP might decrease the apoptosis of BMSCs due to simulated ischemia–reperfusion (I/R) injury in vitro by measuring cell viability and the activation of caspase-3 and to identify a more suitable time course for HP. Furthermore, we treated focal ischemic rats with normal-cultured BMSCs (N-BMSCs), HP-treated BMSCs (H-BMSCs), or DMEM cell culture medium to evaluate the survival and differentiation of donor BMSCs in the ischemic brain and to assess whether HP could increase the functional improvements induced by the cell therapy after stroke.
Materials and Methods
Animals and Experimental Groups
The male Sprague–Dawley rats used in the present study were obtained from the Experimental Animal Centre of Nantong University (Nantong, China). The experimental design is shown in Table 1. Stem cell treatment did not significantly affect the mortality rate of the rats. All behavioral measurements (neurological severity scores, adhesive-removal test, and foot-fault test) and biochemical (Western blot and ELISA) and histological (immunofluorescence and cell counting) assessments were operated by investigators who were blinded to experimental design. All procedures were strictly followed by the institutional guidelines of Nantong University, which comply with international rules and policies. Ethics guidelines in accordance with the Animal Research: Reporting In Vivo Experiments (ARRIVE) were followed in the in vivo experiments, which were approved by the Animal Care and Use Committee of Nantong University, Nantong, China (Permit Number: 20130304-01). All surgical procedures were performed under anesthesia and sterilize conditions, and no infected wounds were identified.
Table 1.
Experimental design
| Group | No. of rats for | Total | |
|---|---|---|---|
| In vitro | Isolation and culture of BMSCs | ||
| Test | Treatment | ||
| 3 | 9 | 12 | |
| Group | No. of rats for | Total | |||
|---|---|---|---|---|---|
| In vivo | Neurological evaluation | Infarct volume | Western blot/ ELISA |
Immunostaining | |
| Sham | 6 | 6 | |||
| Control | (10) | 6 | 6 | 6 | 18 |
| N-BMSCs | (10) | 6 | 6 | 6 | 18 |
| H-BMSCs | (10) | 6 | 6 | 6 | 18 |
| Excluded animalsa | 10 | ||||
| Total number of rats used for all groups | 82 | ||||
aAnimals were excluded from further experimentation due to anesthetic accident, failure to achieve ischemic model or post-ischemic death. Among these rats, four were from the control group, three were from N-BMSCs group, and the remaining three were from H-BMSCs
Isolation and Culture of BMSCs
Bone marrow stromal cells were isolated according to previous method (Deng et al. 2004). Briefly, bone marrow was prepared from the tibias and femurs of male Sprague–Dawley rats (3- to 4-week old). Cells were centrifuged at 1000 rpm for 5 min and suspended in low-glucose Dulbecco’s modified Eagle medium (L-DMEM, Invitrogen, Carlsbad, USA) added with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, USA). Cells were cultured at 37 °C and 95% air/5% CO2 in a standard humidified incubator. Culture medium was changed at 3 days after the cells were seeded. Cells were harvested for experiments at passage 4 (P4). Some BMSCs were marked with 30 μg/mL bromodeoxyuridine (5-bromo-2-deoxyuridine, BrdU; Sigma, St Louis, USA) for 3 days before they were intravenously administered to the animals to assess cell migration in the ischemic brain area (Cui and Almazan 2007). After digestion and centrifugation, the cells were resuspended in 1 mL L-DMEM containing 2 × 106 cells for transplantation. The remaining unlabeled cells were incubated in flasks at 37 °C to investigate the protective effects of HP against simulated I/R BMSCs.
HP and Simulated I/R Model in Cultured BMSCs
Cultured BMSCs were divided into six groups (n = 8/group) as follows. (1) Normal control group: BMSCs cultured under normal conditions (21% O2 and normal culture medium) throughout the whole experiment; (2) I/R group: BMSCs (P4) were subjected to oxygen-serum deprivation injury; the culture medium was transferred to serum-free medium, and the cells were placed in a chamber with hypoxic conditions (<0.5% O2) for 2 h, and then, the BMSCs were switched to normal culture conditions (HP treatment did not precede I/R injury); (3) 4-h HP group: 4 h of HP treatment at every passage before I/R injury; (4) 8-h HP group: 8 h of HP treatment at every passage before I/R injury; (5) 12-h HP group: 12 h of HP treatment at every passage before I/R injury; and (6) 24-h HP group: 24 h of HP treatment at every passage before I/R injury. The BMSCs were exposed to HP by inducing the cells to hypoxia (1% O2). The cells were then allowed 12 h of reoxygenation before they were subjected to 2 h of simulated ischemia. Some assays were carried out immediately after HP, and others were conducted after the cells were exposed to the 24-h reperfusion attack.
Cell Viability Assay
Cells were seeded in octuplicate at a density of 5 × 104 cells/well in 96-well plates at P4. After the HP and simulated I/R procedures, the medium was replaced with 3-(4, 5-methylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT; Sigma-Aldrich Corporation, Saint Louis, USA)-containing DMEM. The cells were then cultured at 37 °C for 4 h, and an equal volume of solubilization solution (10% sodium dodecyl sulfate (SDS), 0.01 M HCl) was added. The plate was then incubated at 37 °C overnight to solubilize the formazan crystals. After the purple formazan producer was dissolved in SDS, the absorbance was tested at 490 and 630 nm using a universal microplate reader (Elx800, BioTek Instruments, Winooski, USA).
Western Blot Analysis In Vivo and In Vitro
Western blotting was performed to evaluate the expression of BDNF and VEGF at 14 days after occlusion, as described previously (Wang et al. 2007). Briefly, the ischemic border zone (IBZ), which is defined as the area surrounding the lesion, was harvested under a coronal Rat Brain Matrices (Stoelting Co.) for assay. The brain samples were then homogenized in ice-cold buffer (50 mM tris-(hydroxymethyl) aminomethane, pH 7.4, 150 mM NaCl, 0.5% Triton X-100, 1 mM edetic acid, 1 M phenylmethylsulfonyl fluoride, and 5 mg/L aprotinin) and centrifuged at 14,000×g at 4 °C for 30 min. The supernatants were then gathered as the total protein. Proteins were electrophoresed through 15% SDS polyacrylamide gels and electrically transferred to nitrocellulose membranes. The membranes were incubated at 4 °C overnight in tris-(hydroxymethyl)-aminomethane-buffered saline (TBS) containing 5% milk before the rabbit polyclonal primary antibodies against rat BDNF and VEGF were applied (1:1000 dilutions, Abcam, Cambridge, USA). After the membranes were washed with TBS, they were incubated with secondary antibodies (horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin, Pierce Biotechnology Inc., Rockford, USA) at room temperature for 1 h. In vitro, whole cell lysates were collected immediately after the hypoxic treatment at P4, and 40 μg of protein per sample was run on 10% gradient gels. Then, the proteins were transferred to PVDF membranes (BioRad, Hercules, USA). After blocked with buffer (tris-buffered saline containing 0.1% Tween-20 (TBST), pH 7.6, 5% milk) at room temperature for 2 h, the membranes were incubated overnight at 4 °C with a rabbit polyclonal anti-HIF-1α primary antibody (1:200, Santa Cruz Biotechnology, USA). The mouse monoclonal anti-β-actin antibody (1:100, Sigma-Aldrich, USA) was used as a control. The membranes were washed in TBST and incubated with alkaline phosphatase-conjugated goat anti-rabbit and anti-mouse secondary antibodies (1:1000, Sigma-Aldrich, USA) for 2 h at room temperature. Signals were finally detected by the addition of BeyoECL Plus solution (Beyotime, China). Protein expression was quantified using ImageJ analysis software (National Institutes of Health, Bethesda, USA). The levels of the analyzed proteins were normalized to the level of β-actin, which was set to 1.
In Vitro Enzyme-Linked Immunosorbent Assay (ELISA)
Activated caspase-3 protein was detected in BMSCs using an ELISA kit (BlueGene Biotech Co. Ltd., Shanghai, China) in accordance with the manufacturer’s instructions. Goat anti-active caspase-3 polyclonal antibodies (10 μL) were added to each well of the 96-well ELISA plate, and the plate was then incubated for 36 h at 4 °C. Then, 100 μL of a BMSC suspension was added to each of the wells, and the plate was incubated for 1 h at 37 °C. Subsequently, 100 μL of rabbit anti-active caspase-3 polyclonal antibodies (diluted 1:200) was added to each of the wells, and the plate was incubated for 1 h at 37 °C. HRP-conjugated goat anti-rabbit immunoglobulin (diluted 1:200; 100 μL) was added, and the plate was incubated for 1 h at 37 °C. Binding was developed with orthophenylene diamine, and absorbance was measured at 450 nm on a microplate reader.
Rat Model of Transient Focal Cerebral Ischemia and Experimental Groups
Adult male Sprague–Dawley rats weighing 240–270 g were randomly divided into different groups. Transient focal cerebral ischemia was induced to rats as previously described with slight modifications (Ren et al. 2013; Wang et al. 2007; Zhang et al. 2009). Briefly, the animals were quickly anesthetized with 3% isoflurane in a mixture of 30% oxygen and 70% nitrogen until they were irresponsive to the tail pinch test. The animals were then fitted with a facemask blowing 1–1.5% isoflurane to maintain the level of anesthesia during surgery. A 0.24 mm diameter silicone tip-coated 4–0 monofilament nylon suture was induced into the cut-off right external carotid artery and advanced into the internal carotid artery until slight resistance was felt (approximately 18–19 mm), resulting in the block of the blood flow in the right middle cerebral artery. Successful occlusion was confirmed with laser Doppler flowmetry (PeriFlux 5010; Perimed Stockholm, Sweden) or 3.0-Tesla magnetic resonance imaging (MRI, Signa Excite HD 3.0 T, GE Healthcare, Chalfont St. Giles, UK) after the operation. A reduction of regional cerebral blood flow (rCBF) to below 20% of baseline following insertion of the filament and a hyper-intense area on the diffusion-weighted imaging (DWI) were required. At 2 h after the operation, reperfusion was initiated by removing the nylon filament to the lumen of the external carotid artery. Sham-operated rats were subjected to all surgical procedures except filament insertion. Throughout the operation, the rats’ body temperature was maintained at a constant 37.0 ± 0.5 °C using a heating pad. After revival from anesthesia, animals were housed back with room temperature 24 ± 1 °C. To maintain a high-survival rate, the post-ischemic animals were closely observed for changes in behavior, activity, posture, malocclusion and general well-being at least daily for 2 weeks. The animals were also weighed at least three times per week.
The rats in the vehicle-treated group, the BMSC-treated group, and the H-BMSC-treated group received an injection of DMEM cell culture medium (1 mL), N-BMSCs (2 × 106) and H-BMSCs (2 × 106), respectively, via the tail vein at 24 h after occlusion. All transplantation procedures were performed under aseptic conditions. Animals of the control group, the N-BMSC-treated group and the H-BMSC-treated group were anesthetized with an overdose of chloral hydrate (500 mg/kg, i.p.) and sacrificed at 7 or 14 days after the operation to identify engrafted BMSCs, respectively.
In Vivo Enzyme-Linked Immunosorbent Assay (ELISA)
An ELISA kit (BlueGene Biotech Co. LTD, Shanghai, China) was used to detect the levels of VEGF and BDNF in the IBZ according to the protocol booklet. Goat anti-VEGF or anti-BDNF polyclonal antibodies (100 μL) were added to a 96-well ELISA plate in each well, which was then incubated at 4 °C for 36 h. Then, 100 μL of rat brain suspension was added to each well, and the plate was then incubated at 37 °C for 1 h. Subsequently, 100 μL of rabbit anti-BDNF or anti-VEGF polyclonal antibodies (diluted 1:200) was added to each of the wells, and the plate was incubated for 1 h at 37 °C. HRP-conjugated goat anti-rabbit immunoglobulin (diluted 1:200; 100 μL) was added, and the plate was incubated for 1 h at 37 °C. The absorbance was read at 450 nm by Microplate reader (BioTek ELx808, USA).
Immunofluorescence Staining
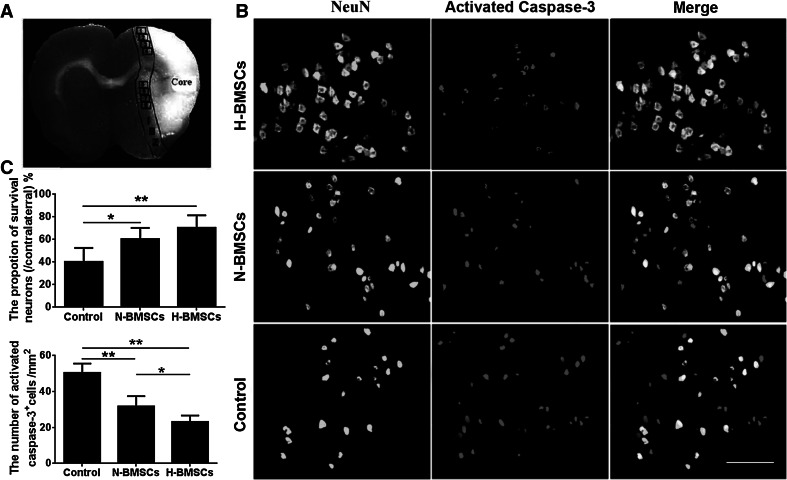
A series of 5-μm-thick sections were cut from a standard paraffin-embedded tissue block obtained from the center of the lesion after TTC staining, which corresponded to Bregma −1 to +1 mm. Double immunofluorescence staining was performed to visualize the cellular colocalization of BrdU, Tuj-1, and GFAP to identify engrafted BMSCs, neurons, and astrocytes. The primary antibodies were used as flow: mouse anti-BrdU (5-bromo-2′-deoxyuridine, 1:800, Sigma-Aldrich), rabbit anti-Nestin (1:100, Sigma-Aldrich), rabbit anti-β-Tubulin (1:100, Sigma-Aldrich), rabbit anti-GFAP (glial fibrillary acidic protein, 1:100, Santa Cruz Biotechnology, USA), rabbit anti-vWF (von Willebrand factor, 1:100, Sigma-Aldrich), mouse anti-caspase-3 (1:100, Sigma-Aldrich), and rabbit anti-NeuN (1:100, Sigma-Aldrich). The sections were incubated with primary antibodies at room temperature for 2 h. The sections were then washed in PBS and incubated with secondary antibodies (Alexa Fluor 594 and Alexa Fluor 488, 1:1000, Jackson, USA) for 2 h. All Sections were photographed using the fluorescent microscopes (DM4000B, Leica, Bensheim, Germany) or confocal microscopy (TCS SP8, Leica, Bensheim, Germany) and analyzed with ImageJ software by researchers blinded to the groups. IBZ was identified as previous method (Yan et al. 2014). Briefly, three slides from each brain (Bregma −1 to +1 mm), with each slide containing eight fields from cortex and striatum of IBZ (Fig. 5a), were digitized under a 20× objective using a Leica DM4000B fluorescence microscope, a CCD (charge-coupled device) camera, and Leica Qwin software.
Fig. 5.
HP facilitated the survival of neurons in the ischemic brain. a Eight fields were defined as standard regions for cell counting, and cell counts were averaged for each animal and expressed as cells/mm2. b Immunofluorescence staining for NeuN (green) and activated caspase-3 (red) at day 7. c The proportion of surviving neurons and the number of activated caspase-3-positive cells in the three groups. n = 6 animals per group; *p < 0.05, **p < 0.01. Scale bar 100 μm (Color figure online)
Measurements of Infarct Volume
Two methods were used to calculate the infarct size of the rats after focal ischemia. 2,3,5-triphenyltetrazolium chloride (TTC) (Merck, Darmstadt, German) staining method was performed as described previously (Wang et al. 2007). Briefly, the animals were sacrificed at 7 days after the end of 2-h ischemia. The brains were removed and sectioned into 2-mm-thick consecutive coronal slices using a vibratome (Leica VT1000 S, Wetzlar, Germany). After washed in phosphate-buffered saline (PBS) for 5 min, stained slices were then fixed for 24 h in 4% buffered formaldehyde solution. TTC color images of these slices were finally captured by a video camera (PowerShot SX710 HS, Canon, Tokyo, Japan).
Before MRI examination, rats were anesthetized using 2 ml of enflurane in an ether jar and maintained with 10% chloral hydrate (400 mg/kg, i.p.). The anesthetized animals of the ischemia control, N-BMSC-treated, and H-BMSC-treated groups (n = 10/group) were examined by 3.0-Tesla MRI (Signa Excite HD 3.0 T, GE Healthcare, Chalfont St. Giles, USA) at 14-day post stroke. Infarct area was determined as described by Wang et al. (2007). T2WI was performed for each animal using a FRFSE sequence and the slice thickness was 1.5 mm. The ischemic area was defined as the hyper-intense areas on the T2WI (TE = 96 ms).
All image data were analyzed and quantified by Image-Pro Plus 6.0 software. The corrected infarct area was calculated by the following formula: the total contralateral hemisphere area minus the area of ipsilateral normal brain. The corrected total infarct volume was calculated by multiplying the respective corrected infarct areas by slice thickness. The infarct volume percentage was calculated as follows: [(VC−VI)/VC] × 100, where VI indicated the volume of the non-infarcted tissue in the ipsilateral hemisphere, and VC indicated the volume of the contralateral hemisphere.
Neurological Function Tests
All animals were randomly divided into experimental groups using a lottery drawing, and there were no differences in functional test results between the groups before surgery. The modified neurological severity score (mNSS) test, adhesive-removal test, and foot-fault test (Shehadah et al. 2014) were performed blindly at 1, 3, 7, and 14 days after occlusion.
Statistical Analysis
Statistical analysis was performed using SPSS 17.0 software (IBM, USA). The homogeneity of variance were examined before applying parametric test and made sure that the data do not depart too much from normality. All values were expressed as the mean ± standard deviation (SD). Significant differences between groups were evaluated with one-way analysis of variance (ANOVA) and Tukey’s post hoc tests for multiple comparisons. Data of behavior tests were analyzed with two-way repeated measures ANOVA and Tukey’s post hoc tests. Pearson correlation coefficients were used to assess the correlations between each pair of the following variables: functional outcome, number of engrafted BMSCs, and neuronal density. A value of p < 0.05 was considered statistically significant.
Results
Immunofluorescence Staining of BMSCs
Hoechst 33,342 dye, which stains nuclei, was used to label all cells. Because BrdU is used for birth dating and to monitor cell proliferation, BMSCs were identified by double immunofluorescence staining for BrdU and Hoechst 33,342; between 98 and 100% of cells were positive for both labels (Fig. 1a).
Fig. 1.
Hypoxic preconditioning (HP) protected cultured BMSCs from simulated I/R injury. a Double immunofluorescence staining for BrdU and the nuclear marker Hoechst 33,342 was used to label BMSCs, with almost 100% overlap. b The viability of BMSCs after 0, 4, 8, 12, and 24 h of HP conditions without I/R injury. c The viability of BMSCs after simulated I/R injury before 0, 4, 8, 12, and 24 h of HP. The data are expressed as fold changes versus the normal control and are presented as the mean ± standard deviation. n = 12 per group; **p < 0.01. Scale bar 100 μm
HP Protected Cultured BMSCs Against Simulated I/R Injury
BMSCs were exposed to hypoxic conditions (1.0% O2) for 0, 4, 8, 12, or 24 h at every passage. Based on the results of MTT assay, HP treatment from 4 to 24 h did not affect cell viability (Fig. 1b). As expected, simulated I/R injury induced significant cell death in the vehicle group, as shown by a decrease in cell viability. However, HP significantly enhanced cell viability in a time-dependent manner. Maximum protective effect from HP treatment was observed in the 8-h group at 24-h post-injury (all p < 0.01, Fig. 1d).
HP Up-Regulated the Expression of HIF-1α in Cultured BMSCs
Western blot was used to assess the expression of HIF-1α in BMSCs at P4. HIF-1α was expressed at a low level in the control group, but HIF-1α expression was markedly increased by HP treatment, especially in the 8-h HP group (Fig. 2a).
Fig. 2.
HP up-regulated the expression of HIF-1α and down-regulated the level of activated caspase-3 in BMSCs. a Western blotting analysis of the level of HIF-1α in BMSCs after 0, 4, 8, 12, and 24 h of HP conditions. b ELISA-based analysis of the level of activated caspase-3 in BMSCs under 0, 4, 8, 12, and 24 h of HP conditions. n = 6 per group; *p < 0.05, **p < 0.01
HP Down-Regulated the Expression of Activated Caspase-3 in BMSCs
ELISA showed that I/R injury induced the expression of activated caspase-3 (Fig. 2b). However, this increase was reversed by HP (*p < 0.05, **p < 0.01), particularly in the 8-h HP group.
Cell viability and HIF-1α levels in BMSCs were positive correlated (r = 0.86, p < 0.01). In contrast, a negative correlation was found between cell viability and activated caspase-3 levels (r = −0.83, p < 0.01).
HP Significantly Improved Neurological Outcomes and Decreased Infarct Volume
Physiological parameters such as blood pressure, heart rate, and tcPO2 were monitored as previously described (Ren et al. 2013); however, no notable differences were observed between the treatment groups (data were not shown).
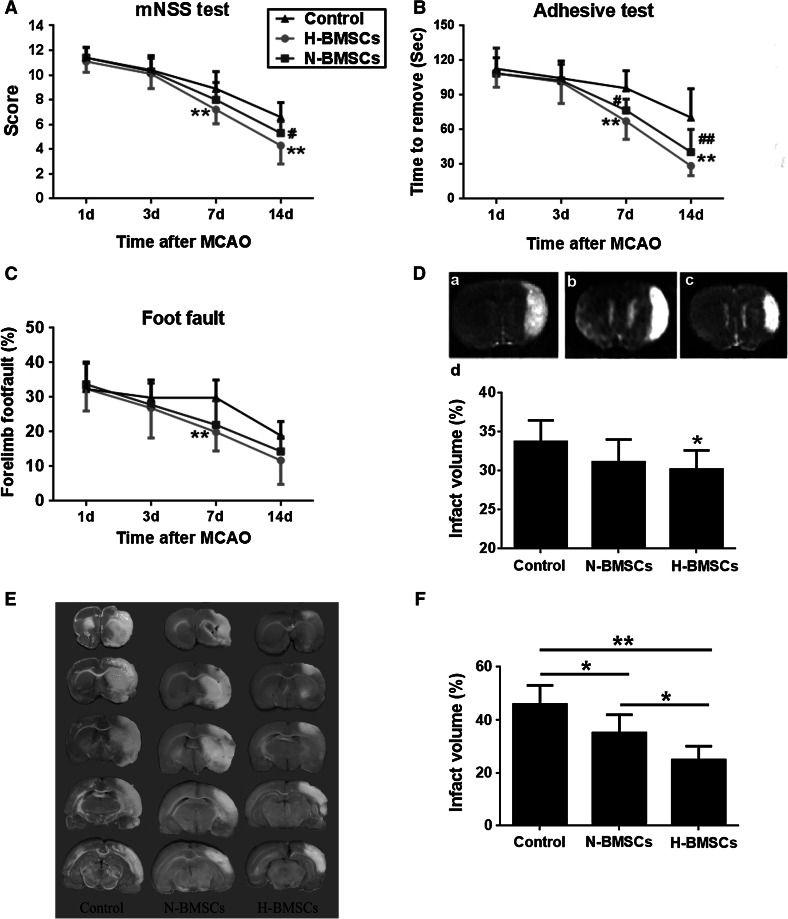
As shown in Fig. 3, both H-BMSC and N-BMSC transplantation attenuated functional deficits, as demonstrated by performance on the mNSS test (Fig. 3a), adhesive-removal test (Fig. 3b), and foot-fault test (Fig. 3c) at 7 and 14 days after focal ischemia (** p < 0.01).
Fig. 3.
Transplantation of H-BMSCs or N-BMSCs improved functional outcomes in focal ischemia rats. Performance on the a mNSS test, b adhesive-removal test, and c foot-fault test was evaluated at 1, 3, 7, and 14 days after occlusion. ** p < 0.01 versus the control group (DMEM treated);# p < 0.05,## p < 0.01 versus control group. d HP markedly decreased infarct volume, as evaluated by T2WI, at 14 days after focal ischemia. a The control group, b the N-BMSC group, c the H-BMSC group, and d the percentage of infarct volume in the three groups. n = 10 per group; *p < 0.05 versus control group. e An example of TTC staining of each treatment at 7 days after focal ischemia. f TTC measurements of the infarction volume at 7 days after occlusion (n = 6). *p < 0.05, **p < 0.01
Although N-BMSCs therapy did not reduce infarct volume compared with the control group at 14 d after focal ischemia, as quantified using T2-weighted images (T2WI), and this effect was enhanced for the HP-induced BMSCs (Fig. 3d, *p < 0.05). To further investigate whether H-BMSCs convey additional benefit relative to N-BMSCs on infarct volume, TTC staining was performed at 7 days after focal ischemia. In this separate observation, the damage percentage of both N-BMSCs and H-BMSC-treated rats exhibited a significant reduction (Fig. 3e, f; *p < 0.05, **p < 0.01). As expected, this effect of the H-BMSCs group was more prominent than that of the N-BMSCs (Fig. 3f, *p < 0.05).
HP Up-Regulated BDNF and VEGF Levels in the Ischemic Brain
BDNF and VEGF levels were examined by Western blotting and ELISA. Compared with the control treatment, transplantation of BMSCs increased the expression of both BDNF and VEGF in the IBZ. ELISA was also performed on brain tissue extracts to determine the levels of these two proteins at day 14 after occlusion. Consistent with the Western blotting result, HP was found to augment the expression of BDNF and VEGF in the IBZ (Fig. 4, **p < 0.01).
Fig. 4.
HP increased the expression of BDNF and VEGF in the ischemic brain. a The expression of BDNF and VEGF in the IBZ was increased in the N-BMSC and H-BMSC groups at 14 days after occlusion, as shown by Western blot analysis. β-actin was used as the loading control. b ELISA was used to analyze the level of BDNF and VEGF protein in the control, N-BMSC, and H-BMSC groups at day 14. n = 6 per group; **p < 0.01
HP Prevented Neuronal Loss and Caspase-3 Activity in the Ischemic Brain
Double immunofluorescence staining for NeuN and caspase-3 was used to identify apoptotic neurons within the ischemic area of the brain. Notable differences in neuronal density were found between the three groups (Fig. 5b, c; **p < 0.01). When rats were treated with N-BMSCs or H-BMSCs, the density of activated caspase-3-positive cells was significantly reduced only in the H-BMSC-treated group (**p < 0.01). These data suggest that HP might prevent neurons from undergoing ischemia-induced apoptosis.
HP Enhanced the Survival and Differentiation of BMSCs After Transplantation
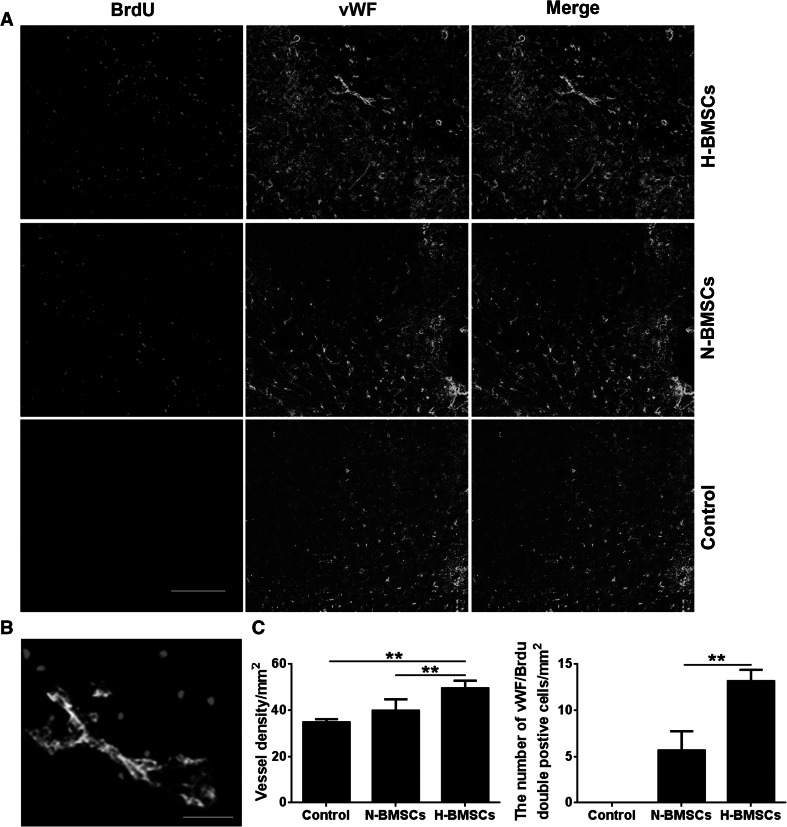
BrdU-positive cells were interpreted as corresponding to engrafted BMSCs. Nestin, Tuj-1, GFAP, and vWF staining were performed to evaluate neural stem cells, neurons, glial cells, and vascular endothelial cells, respectively, in the ischemic boundary. Double immunostaining for BrdU/Nestin, BrdU/Tuj-1, BrdU/GFAP, and BrdU/vWF was used to assess the differentiation of engrafted BMSCs (Figs. 6, 7 and S1), and cells of each type were observed, indicating that advanced neurogenesis and angiogenesis had occurred in the ischemic brain after the transplantation. Furthermore, even greater regenerative capability was observed in the H-BMSC-treated group compared with the N-BMSC-treated group (Fig. 7, **p < 0.01). As expected, neurological restoration was significantly correlated with the number of engrafted BMSCs in the ischemic brain (r = 0.74, p < 0.01).
Fig. 6.
HP improved the survival and differentiation of BMSCs in the ischemic brain. a Double immunofluorescence staining for engrafted BMSCs (BrdU-positive cells, red) and Nestin (green)-positive cells in the ischemic brain at day 7 after occlusion. b BrdU-positive cells (red) and Tuj-1-positive cells (green) at day 14 and c BrdU-positive cells (red) and GFAP-positive cells (green) at day 14 after focal ischemia. n = 6 animals per group; **p < 0.01. Scale bar 100 μm (Color figure online)
Fig. 7.
The effect of HP on angiogenesis in focal ischemia rats. a and c Endothelial cell differentiation in the ischemic brain at day 14 after occlusion. BrdU (red) was used as an indicator of BMSCs, and vWF (green) was used as an indicator of vascular endothelial cells. Co-labeling for these two markers was interpreted as a marker of endothelial cell differentiation. In the H-BMSC group, more vessels and double-positive cells were detected in the IBZ. Scale bar 200 μm. b A higher magnification photograph showing that the BrdU-positive cells expressed the vWF marker. n = 6 animals per group; **p < 0.01. Scale bar 50 μm (Color figure online)
Discussion
Functional recovery is highly correlated with the number of surviving neurons in brain lesions; however, many neurons die following an ischemic attack (Shen et al. 2012). Studies have confirmed that BMSCs might replace the injured neurons and promote structural recovery after stroke (Chacko et al. 2010; Du et al. 2014; Francis and Wei 2010; Yu et al. 2013a, b; Yan et al. 2012). Stem cells can differentiate into a neural lineage to replace lost neurons (Oliveira and Barreto-Filho 2015), provide trophic support to at-risk neurons near the infarct area, and promote the survival and differentiation of endogenous precursor cells after stroke (Guo et al. 2012; van Velthoven et al. 2009), and all of these functions help repair the injured brain by enhancing neuronal survival. However, a large number of transplanted cells die in the ischemic brain due to the low supply of oxygen (0.4–2.3% O2) (Andres et al. 2008; Haider and Ashraf 2008). Standard in vitro culture conditions include 21% O2, while the physiological oxygen tension experienced by BMSCs that are located in bone marrow and not exposed to the atmosphere is thought to range from 2 to 8% (Mohyeldin et al. 2010). The optimal culture conditions for BMSCs have been debated for many years. Thus, it is imperative to identify therapies that can improve the viability of stem cells in hazardous tissues. Previous studies have shown that HP (0.5% O2) might attenuate cerebral ischemic injury, and rats administered with HP-treated BMSCs showed better locomotion recovery compared with those administered normal BMSCs (Wei et al. 2012). However, many questions remain concerning the use of HP-treated BMSCs as a therapy for stroke, such as the most effective hypoxic time window for the application of BMSCs, the safety of hypoxic O2 concentration, and the underlying mechanisms. In the present study, we investigated the time window and the therapeutic effects of HP treatment for I/R injury in vivo and in vitro. We used a safe hypoxic O2 concentration (2% O2) to treat the BMSCs and evaluated the protective effects of HP on stem cell viability. Cell viability was not influenced by the HP treatment itself, but HP inhibited the subsequent serious consequences of oxygen deprivation, and the 8-h HP treatment exhibited the most protective effect against injury.
To elucidate the potential mechanism, we assessed the levels of HIF-1α and activated caspase-3 in the cultured BMSCs. HIF-1α is a transcription factor that is specifically activated by hypoxia, especially at low-oxygen pressures. The level of HIF-1α is highly regulated through multiple mechanisms at the level of mRNA expression, protein stability, nuclear translocation, and transactivation activity (Chang et al. 2014; Taie et al. 2009). The contributions of HIF-1α to post-hypoxia adaptive responses might be related to its cytoprotective and anti-apoptotic effects. In addition, HIF-1α can promote the migration of stem cells to the damaged tissue via the activation of its receptor CXCR4 (Wang et al. 2008b; Yu et al. 2015). Caspase-3, a member of the caspase family, is downstream of Bax and is commonly involved in the execution of the apoptosis that is induced by many stimuli, including the cleavage of DNA repair molecules, the disruption of anti-apoptosis proteins, and the cleavage of extracellular matrix proteins and other related molecules (Ahmad et al. 2014; Zuliani et al. 2008). Here, we also found a correlation between cell viability and the level of HIF-1α in BMSCs, whereas a negative correlation was found between cell viability and the level of activated caspase-3. Wang et al. (2008a) reported that HP-mediated apoptotic suppression was correlated with the prevention of mitochondrial dysfunction and the promotion of ERK and Akt phosphorylation in hypoxia and reoxygenation injury, and the Akt signaling pathway was also found to play a critical role in the prevention of apoptotic cell death by inhibiting caspase release (Uchiyama et al. 2004). Moreover, the overexpression of Bcl-2 and the maintenance of MMP in HP-treated cells were hypothesized to be involved in the protective effects (Cai et al. 2015). The up-regulated expression of HIF-1α and decreased release of caspase-3 protected the BMSCs from subsequent I/R injury, as demonstrated by the increase in cell viability in the HP groups. According to these findings, 8 h of HP treatment is most suitable for transplantation.
Therefore, we used 8-h H-BMSCs for cell therapy after focal ischemia. HP enhanced the expression of BDNF and VEGF in the IBZ as well. In the brain, BDNF can activate ERK and counter-regulate the expression of apoptotic factors such as caspase-3 and Bax to promote neuronal survival. VEGF is also a peptide cytokine that is involved in vasculogenesis and angiogenesis (Liu et al. 2015; Schmidt et al. 2009; Horne et al. 2010). Thus, BDNF and VEGF may promote brain plasticity (Chen et al. 2005) and improve regional blood flow (Schanzer et al. 2004). Although BDNF and VEGF were up-regulated in the ischemic brain following focal ischemia, the beneficial effects of this upregulation were too weak to overcome the damage. Here, we showed that HP treatment significantly increased neurotrophin levels and reduced caspase-3 activity and neuronal loss in the ischemic tissue. Consistent with this effect, HP decreased the infarct volume, as demonstrated using TTC and T2WI staining, and facilitated the survival of neurons in the ischemic brain. Basically, TTC and T2WI results have been shown to be in a good agreement and complementary for detecting the subtle post-ischemic pathology (Sicard et al. 2006). Previous study has shown that the result of TTC was highly consistent with the NeuN staining to determine the size of the damaged area until 28 days after focal ischemia (Lapi et al. 2015). Therefore, hypoxic preconditioning of BMSCs may provide strong neuroprotection in brain tissue that is subjected to I/R.
Previous research has reported that hypoxia also prolongs the half-life of mRNAs such as VEGF and EPO, which have been demonstrated to be neuroprotective and angiogenic factors in the post-ischemic stroke brain (Keogh et al. 2007; Liu et al. 2008). This effect could strongly enhance neurogenesis and angiogenesis after transplantation. Additionally, HP pretreatment could further improve the anti-inflammatory effects of BMSCs, and this effect could contribute to the enhanced neuroprotection observed after H-BMSC transplantation (Wei et al. 2012). On the other hand, HP could probably increase the release of the peptide cytokines from BMSCs, such as BDNF and VEGF (Chacko et al. 2010). The conditional media from HP-pretreated BMSCs is enriched in various metabolites and solutes, and will be examined the direct neuroprotective effect on neuron and endothelial cells in our future works.
Double immunostaining for BrdU and neuronal markers was performed to identify newly generated cells and to assess the regenerative capability. Nestin expression is frequently detected in regeneration areas, and Nestin-positive cells may serve as a reservoir of stem cells capable of proliferation and differentiation. In the central nervous system, Tuj-1-positive neurons play a key role in providing the trophic and growth factors that are associated with tissue repair and functional recovery after injury. GFAP-positive astrocytes provide supportive functions that are essential for neuronal functioning (Sofroniew 2005). These cells interact with all types of neurons and increase the expression of various trophic and growth factors following BMSC administration. vWF+ cells can be used as an indicator of vasculogenesis, and the number of new vessels that surround the injured tissue correlates with longer survival and better outcomes (Jung and Roh 2008; Kaneko et al. 2012; Liman and Endres 2012; Parra-Cordero et al. 2013). In the present study, more cells that were double-positive for BrdU/Nestin, BrdU/Tuj-1, BrdU/GFAP, and BrdU/vWF were found in the ischemic brains of the H-BMSC-treated group, suggesting that the exogenous H-BMSCs had differentiated into neurons, vascular endothelial cells, and other functional cells.
In conclusion, HP provides an effective strategy for improving cell-based therapies after ischemic stroke. HP may help the transplanted BMSCs adapt to the environment of the ischemic brain and promote functional recovery by increasing neurogenesis and angiogenesis. Moreover, we identified 8 h as a more suitable HP treatment time for cell-based therapies; this finding should improve the efficacy of HP treatment of transplanted cells for the treatment of ischemic stroke.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1. Confocal microscopy detected that HP promote the increase of BrdU-positive cells expressing nestin. Double immunofluorescence staining for engrafted BMSCs (BrdU-positive cells, red) and Nestin (green)-positive cells in the ischemic brain at day 7 after occlusion. Scale bar 250 μm. (TIFF 3697 kb)
Acknowledgments
Funding
This work was supported by the Chinese Natural Science Foundation (Grants 81000497 and 81471257) and Natural Science Foundation of Jiangsu Province of China (Grant BK20161283), and sponsored by Qing Lan Project (to G.W). The funders had no role in the study design, the data collection and analysis, the decision to publish, or the preparation of the manuscript.
Compliance with Ethical Standards
Competing interests
The authors declare the absence of competing interests.
Footnotes
Jin Chen and Yuanyuan Yang have contributed equally to this work.
Contributor Information
Lihua Shen, Email: lihuashen139@yeah.net.
Guohua Wang, Email: wgh036@hotmail.com.
References
- Ahmad S, Elsherbiny NM, Haque R, Khan MB, Ishrat T, Shah ZA, Khan MM, Ali M, Jamal A, Katare DP, Liou GI, Bhatia K (2014) Sesamin attenuates neurotoxicity in mouse model of ischemic brain stroke. Neurotoxicology 45:100–110. doi:10.1016/j.neuro.2014.10.002 [DOI] [PubMed] [Google Scholar]
- Andres RH, Choi R, Steinberg GK, Guzman R (2008) Potential of adult neural stem cells in stroke therapy. Regen Med 3(6):893–905. doi:10.2217/17460751.3.6.893 [DOI] [PubMed] [Google Scholar]
- Cai XY, Xia Y, Yang SH, Liu XZ, Shao ZW, Liu YL, Yang W, Xiong LM (2015) Ropivacaine- and bupivacaine-induced death of rabbit annulus fibrosus cells in vitro: involvement of the mitochondrial apoptotic pathway. Osteoarthr Cartil. doi:10.1016/j.joca.2015.05.013 [DOI] [PubMed] [Google Scholar]
- Chacko SM, Ahmed S, Selvendiran K, Kuppusamy ML, Khan M, Kuppusamy P (2010) Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol 299(6):C1562–C1570. doi:10.1152/ajpcell.00221.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Liu HS, Lai MD, Tsai YS, Tzai TS, Cheng HL, Chow NH (2014) Hypoxia promotes nuclear translocation and transcriptional function in the oncogenic tyrosine kinase RON. Cancer Res 74(16):4549–4562. doi:10.1158/0008-5472.CAN-13-3730 [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M (2005) Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab 25(2):281–290. doi:10.1038/sj.jcbfm.9600034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui QL, Almazan G (2007) IGF-I-induced oligodendrocyte progenitor proliferation requires PI3 K/Akt, MEK/ERK, and Src-like tyrosine kinases. J Neurochem 100(6):1480–1493. doi:10.1111/j.1471-4159.2006.04329.x [DOI] [PubMed] [Google Scholar]
- Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z, You S, Deng H, Zhao RC (2004) Allogeneic bone marrow-derived flk-1 + Sca-1-mesenchymal stem cells leads to stable mixed chimerism and donor-specific tolerance. Exp Hematol 32(9):861–867. doi:10.1016/j.exphem.2004.06.009 [DOI] [PubMed] [Google Scholar]
- Du S, Guan J, Mao G, Liu Y, Ma S, Bao X, Gao J, Feng M, Li G, Ma W, Yang Y, Zhao RC, Wang R (2014) Intra-arterial delivery of human bone marrow mesenchymal stem cells is a safe and effective way to treat cerebral ischemia in rats. Cell Transpl 23(Suppl 1):S73–S82. doi:10.3727/096368914X685023 [DOI] [PubMed] [Google Scholar]
- Feng Y, Bhatt AJ (2015) Corticosteroid responses following hypoxic preconditioning provide neuroprotection against subsequent hypoxic-ischemic brain injury in the newborn rats. Int J Dev Neurosci 44:6–13. doi:10.1016/j.ijdevneu.2015.04.010 [DOI] [PubMed] [Google Scholar]
- Francis KR, Wei L (2010) Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis 1:e22. doi:10.1038/cddis.2009.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Lv S, Lou Y, Tu W, Liao W, Wang Y, Deng Z (2012) Bone marrow stromal cells enhance the angiogenesis in ischaemic cortex after stroke: involvement of notch signalling. Cell Biol Int 36(11):997–1004. doi:10.1042/CBI20110596 [DOI] [PubMed] [Google Scholar]
- Haider H, Ashraf M (2008) Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol 45(4):554–566. doi:10.1016/j.yjmcc.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne MK, Nisbet DR, Forsythe JS, Parish CL (2010) Three-dimensional nanofibrous scaffolds incorporating immobilized BDNF promote proliferation and differentiation of cortical neural stem cells. Stem Cells Dev 19(6):843–852. doi:10.1089/scd.2009.0158 [DOI] [PubMed] [Google Scholar]
- Huang W, Mo X, Qin C, Zheng J, Liang Z, Zhang C (2013) Transplantation of differentiated bone marrow stromal cells promotes motor functional recovery in rats with stroke. Neurol Res 35(3):320–328. doi:10.1179/1743132812Y.0000000151 [DOI] [PubMed] [Google Scholar]
- Jung KH, Roh JK (2008) Circulating endothelial progenitor cells in cerebrovascular disease. J Clin Neurol 4(4):139–147. doi:10.3988/jcn.2008.4.4.139 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kaneko Y, Tajiri N, Shinozuka K, Glover LE, Weinbren NL, Cortes L, Borlongan CV (2012) Cell therapy for stroke: emphasis on optimizing safety and efficacy profile of endothelial progenitor cells. Curr Pharm Des 18(25):3731–3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh CL, Yu SP, Wei L (2007) The effect of recombinant human erythropoietin on neurovasculature repair after focal ischemic stroke in neonatal rats. J Pharmacol Exp Ther 322(2):521–528. doi:10.1124/jpet.107.121392 [DOI] [PubMed] [Google Scholar]
- Lapi D, Vagnani S, Sapio D, Mastantuono T, Boscia F, Pignataro G, Penna C, Pagliaro P, Colantuoni A (2015) Effects of bone marrow mesenchymal stem cells (BM-MSCs) on rat pial microvascular remodeling after transient middle cerebral artery occlusion. Front Cell Neurosci 9:329. doi:10.3389/fncel.2015.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman TG, Endres M (2012) New vessels after stroke: postischemic neovascularization and regeneration. Cerebrovasc Dis 33(5):492–499. doi:10.1159/000337155 [DOI] [PubMed] [Google Scholar]
- Liu L, Ning X, Han S, Zhang H, Sun L, Shi Y, Sun S, Guo C, Yin F, Qiao T, Wu K, Fan D (2008) Hypoxia induced HIF-1 accumulation and VEGF expression in gastric epithelial mucosa cell: involvement of ERK1/2 and PI3 K/Akt. Mol Biol (Mosk) 42(3):459–469 [PubMed] [Google Scholar]
- Liu J, Hao H, Xia L, Ti D, Huang H, Dong L, Tong C, Hou Q, Zhao Y, Liu H, Fu X, Han W (2015) Hypoxia pretreatment of bone marrow mesenchymal stem cells facilitates angiogenesis by improving the function of endothelial cells in diabetic rats with lower ischemia. PLoS ONE 10(5):e0126715. doi:10.1371/journal.pone.0126715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgieri A, Kantzari E, Patrizi MP, Gambardella S (2010) Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med 3(4):248–269 [PMC free article] [PubMed] [Google Scholar]
- Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A (2010) Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7(2):150–161. doi:10.1016/j.stem.2010.07.007 [DOI] [PubMed] [Google Scholar]
- Momin EN, Mohyeldin A, Zaidi HA, Vela G, Quinones-Hinojosa A (2010) Mesenchymal stem cells: new approaches for the treatment of neurological diseases. Curr Stem Cell Res Ther 5(4):326–344 [DOI] [PubMed] [Google Scholar]
- Nishijima Y, Akamatsu Y, Weinstein PR, Liu J (2015) Collaterals: implications in cerebral ischemic diseases and therapeutic interventions. Brain Res 1623:18–29. doi:10.1016/j.brainres.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MS, Barreto-Filho JB (2015) Placental-derived stem cells: culture, differentiation and challenges. World J Stem Cells 7(4):769–775. doi:10.4252/wjsc.v7.i4.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero L, Zurita M, Bonilla C, Aguayo C, Rico MA, Rodriguez A, Vaquero J (2012) Allogeneic bone marrow stromal cell transplantation after cerebral hemorrhage achieves cell transdifferentiation and modulates endogenous neurogenesis. Cytotherapy 14(1):34–44. doi:10.3109/14653249.2011.608349 [DOI] [PubMed] [Google Scholar]
- Parra-Cordero M, Rodrigo R, Barja P, Bosco C, Rencoret G, Sepulveda-Martinez A, Quezada S (2013) Prediction of early and late pre-eclampsia from maternal characteristics, uterine artery Doppler and markers of vasculogenesis during first trimester of pregnancy. Ultrasound Obstet Gynecol 41(5):538–544. doi:10.1002/uog.12264 [DOI] [PubMed] [Google Scholar]
- Ren TJ, Qiang R, Jiang ZL, Wang GH, Sun L, Jiang R, Zhao GW, Han LY (2013) Improvement in regional CBF by l-serine contributes to its neuroprotective effect in rats after focal cerebral ischemia. PLoS ONE 8(6):e67044. doi:10.1371/journal.pone.0067044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowart P, Erpicum P, Detry O, Weekers L, Gregoire C, Lechanteur C, Briquet A, Beguin Y, Krzesinski JM, Jouret F (2015) Mesenchymal stromal cell therapy in ischemia/reperfusion injury. J Immunol Res 2015:602597. doi:10.1155/2015/602597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG (2004) Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol 14(3):237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NO, Koeder D, Messing M, Mueller FJ, Aboody KS, Kim SU, Black PM, Carroll RS, Westphal M, Lamszus K (2009) Vascular endothelial growth factor-stimulated cerebral microvascular endothelial cells mediate the recruitment of neural stem cells to the neurovascular niche. Brain Res 1268:24–37. doi:10.1016/j.brainres.2009.02.065 [DOI] [PubMed] [Google Scholar]
- Shehadah A, Chen J, Pal A, He S, Zeitlin A, Cui X, Zacharek A, Cui Y, Roberts C, Lu M, Hariri R, Chopp M (2014) Human placenta-derived adherent cell treatment of experimental stroke promotes functional recovery after stroke in young adult and older rats. PLoS ONE 9(1):e86621. doi:10.1371/journal.pone.0086621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LH, Ye M, Ding XS, Han Q, Zhang C, Liu XF, Huang H, Wu EB, Huang HF, Gu XS (2012) Protective effects of MCI-186 on transplantation of bone marrow stromal cells in rat ischemic stroke model. Neuroscience 223:315–324. doi:10.1016/j.neuroscience.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Sicard KM, Henninger N, Fisher M, Duong TQ, Ferris CF (2006) Differential recovery of multimodal MRI and behavior after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metabol 26(11):1451–1462. doi:10.1038/sj.jcbfm.9600299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Kaur R, Kaur A (2013) Endovascular treatment of acute ischemic stroke. J Neurosci Rural Pract 4(3):298–303. doi:10.4103/0976-3147.118787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2005) Reactive astrocytes in neural repair and protection. Neuroscientist 11(5):400–407. doi:10.1177/1073858405278321 [DOI] [PubMed] [Google Scholar]
- Souidi N, Stolk M, Seifert M (2013) Ischemia-reperfusion injury: beneficial effects of mesenchymal stromal cells. Curr Opin Organ Transpl 18(1):34–43. doi:10.1097/MOT.0b013e32835c2a05 [DOI] [PubMed] [Google Scholar]
- Sun J, Wei ZZ, Gu X, Zhang JY, Zhang Y, Li J, Wei L (2015) Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Exp Neurol 272:78–87. doi:10.1016/j.expneurol.2015.03.011 [DOI] [PubMed] [Google Scholar]
- Taie S, Ono J, Iwanaga Y, Tomita S, Asaga T, Chujo K, Ueki M (2009) Hypoxia-inducible factor-1 alpha has a key role in hypoxic preconditioning. J Clin Neurosci 16(8):1056–1060. doi:10.1016/j.jocn.2008.09.024 [DOI] [PubMed] [Google Scholar]
- Uchiyama T, Engelman RM, Maulik N, Das DK (2004) Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation 109(24):3042–3049. doi:10.1161/01.CIR.0000130647.29030.90 [DOI] [PubMed] [Google Scholar]
- van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ (2009) Regeneration of the ischemic brain by engineered stem cells: fuelling endogenous repair processes. Brain Res Rev 61(1):1–13. doi:10.1016/j.brainresrev.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Wang GH, Jiang ZL, Fan XJ, Zhang L, Li X, Ke KF (2007) Neuroprotective effect of taurine against focal cerebral ischemia in rats possibly mediated by activation of both GABAA and glycine receptors. Neuropharmacology 52(5):1199–1209. doi:10.1016/j.neuropharm.2006.10.022 [DOI] [PubMed] [Google Scholar]
- Wang JA, Chen TL, Jiang J, Shi H, Gui C, Luo RH, Xie XJ, Xiang MX, Zhang X (2008a) Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin 29(1):74–82. doi:10.1111/j.1745-7254.2008.00716.x [DOI] [PubMed] [Google Scholar]
- Wang Y, Deng Y, Zhou GQ (2008b) SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res 1195:104–112. doi:10.1016/j.brainres.2007.11.068 [DOI] [PubMed] [Google Scholar]
- Wang F, Zhou H, Du Z, Chen X, Zhu F, Wang Z, Zhang Y, Lin L, Qian M, Zhang X, Li X, Hao A (2015) Cytoprotective effect of melatonin against hypoxia/serum deprivation-induced cell death of bone marrow mesenchymal stem cells in vitro. Eur J Pharmacol 748:157–165. doi:10.1016/j.ejphar.2014.09.033 [DOI] [PubMed] [Google Scholar]
- Wei L, Fraser JL, Lu ZY, Hu X, Yu SP (2012) Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis 46(3):635–645. doi:10.1016/j.nbd.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Yao Y, Chen L, Li Y, Sheng Z, Ma G (2012) Hypoxic preconditioning improves survival of cardiac progenitor cells: role of stromal cell derived factor-1alpha-CXCR4 axis. PLoS ONE 7(7):e37948. doi:10.1371/journal.pone.0037948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Venkat P, Ye X, Chopp M, Zacharek A, Ning R, Cui Y, Roberts C, Kuzmin-Nichols N, Sanberg CD, Chen J (2014) HUCBCs increase angiopoietin 1 and induce neurorestorative effects after stroke in T1DM rats. CNS Neurosci Ther 20(10):935–944. doi:10.1111/cns.12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SP, Wei Z, Wei L (2013a) Preconditioning strategy in stem cell transplantation therapy. Transl Stroke Res 4(1):76–88. doi:10.1007/s12975-012-0251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Lu C, Liu H, Rao S, Cai J, Liu S, Kriegel AJ, Greene AS, Liang M, Ding X (2013b) Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS ONE 8(5):e62703. doi:10.1371/journal.pone.0062703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Liu L, Lin J, Wang Y, Xuan X, Guo Y, Hu S (2015) SDF-1alpha/CXCR4 Axis mediates the migration of mesenchymal stem cells to the hypoxic-ischemic brain lesion in a rat model. Cell J 16(4):440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang S, Luo Y, Ji X, Nemoto EM, Chen J (2009) When hypothermia meets hypotension and hyperglycemia: the diverse effects of adenosine 5′-monophosphate on cerebral ischemia in rats. J Cereb Blood Flow Metabol 29(5):1022–1034. doi:10.1038/jcbfm.2009.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani T, Obriot H, Tual M, Lachman-Weber N, Dumas M, Formstecher P, Polakowska R, Ratinaud MH (2008) Variable Bax antigenicity is linked to keratinocyte position within epidermal strata and UV-induced apoptosis. Exp Dermatol 17(2):125–132. doi:10.1111/j.1600-0625.2007.00660.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Confocal microscopy detected that HP promote the increase of BrdU-positive cells expressing nestin. Double immunofluorescence staining for engrafted BMSCs (BrdU-positive cells, red) and Nestin (green)-positive cells in the ischemic brain at day 7 after occlusion. Scale bar 250 μm. (TIFF 3697 kb)