Abstract
Hemorrhagic stroke which consists of subarachnoid hemorrhage and intracerebral hemorrhage is a dominant cause of death and disability worldwide. Although great efforts have been made, the physiological mechanisms of these diseases are not fully understood and effective pharmacological interventions are still lacking. Melatonin (N-acetyl-5-methoxytryptamine), a neurohormone produced by the pineal gland, is a broad-spectrum antioxidant and potent free radical scavenger. More importantly, there is extensive evidence demonstrating that melatonin confers neuroprotective effects in experimental models of hemorrhagic stroke. Multiple molecular mechanisms such as antioxidant, anti-apoptosis, and anti-inflammation, contribute to melatonin-mediated neuroprotection against brain injury after hemorrhagic stroke. This review article aims to summarize current knowledge regarding the beneficial effects of melatonin in experimental models of hemorrhagic stroke and explores the underlying mechanisms. We propose that melatonin is a promising neuroprotective candidate that is worthy of further evaluation for its potential therapeutic applications in hemorrhagic stroke.
Keywords: Melatonin, Oxidative stress, Inflammation, Apoptosis, Neuroprotection, Hemorrhagic stroke
Introduction
Hemorrhagic stroke which consists of subarachnoid hemorrhage (SAH) and intracerebral hemorrhage (ICH) is a devastating disease that causes significant disability or even death (Ikram et al. 2012; Rinkel and Algra 2011; van Gijn et al. 2007). There are multiple molecular and cellular events such as inflammation, apoptosis, and oxidative stress that are involved in the pathogenesis of hemorrhagic stroke (Wasserman and Schlichter 2007; Zhou et al. 2004; Tang et al. 2005). These cellular events have the potential to eventually lead to cell death and brain injury. However, the pathological mechanisms of hemorrhagic stroke are not completely understood, and effective pharmacological approaches for this disease are lacking (Ayer et al. 2012; Sehba et al. 2012). Thus, it is critically important to investigate into the pathophysiology of hemorrhagic stroke and develop effective medications to treat this disease.
Melatonin (N-acetyl-5-methoxytryptamine) is a neurohormone that was first found to be a potent free radical scavenger and broad-spectrum antioxidant (Tan et al. 2003; Tan et al. 1993a). It has been reported to be mainly synthesized and secreted from the pineal gland during the dark phase of the light–dark cycle (Rodriguez et al. 2004). A wealth of experimental evidence has demonstrated that melatonin acts as a neuroprotective agent in both acute brain injuries and chronic neurodegenerative diseases (Samantaray et al. 2009; Cervantes et al. 2008; Li et al. 2014; Mesenge et al. 1998; Ali et al. 2015; Wang et al. 2013a; Regrigny et al. 1998, 2001). For example, pretreatment with melatonin diminished the increased expression of Nox2 and Nox4, reduced reactive oxygen species (ROS) generation, and inhibited apoptotic cell death in a rat model of cerebral ischemia/reperfusion (Li et al. 2014). Also, intraperitoneal administration of melatonin with a cumulative dose of 10 mg/kg prevented lipid peroxidation, suppressed microglial activation, and attenuated kainic acid-induced hippocampal neurodegeneration in rats (Chung and Han 2003). In particular, there has been accumulation of evidence suggesting that melatonin produces a number of neuroprotective benefits in experimental models of hemorrhagic stroke which includes SAH and ICH (Lekic et al. 2010; Ayer et al. 2008; Dong et al. 2016; Chen et al. 2015a; Ueda et al. 2014; Wang et al. 2012). Thus, in this article, we aim to survey the neuroprotective properties of melatonin and their underlying molecular mechanisms in the setting of experimental hemorrhagic stroke. We propose that melatonin is a promising neuroprotective candidate that is worthy of further evaluation for its potential therapeutic application in this devastating disease.
Melatonin-Mediated Neuroprotection in Central Nervous System Diseases
Melatonin is an evolutionarily conserved hormone and it is predominantly produced in the pineal gland. Also, it is produced in numerous extrapineal tissues such as the retina, gastrointestinal tract, and bone marrow (Reiter et al. 2013). The synthesis and secretion of this molecule is regulated by the central circadian pacemaker and is synchronized by the diurnal light–dark cycle which has a nocturnal maximum (≈ 200 pg/ml plasma in young subjects) and low diurnal baseline level (≈ 10 pg/ml plasma) (Pandi-Perumal et al. 2006). Melatonin is also a pleiotropic molecule with diverse physiological functions (Macchi and Bruce 2004). In addition to its time-keeping function, melatonin has potent antioxidant properties (Turjanski et al. 1998; Kleszczynski et al. 2016; Mukherjee et al. 2015; Leibowitz et al. 2016). As a powerful free radical scavenger, it directly detoxifies free radicals (e.g., hydroxyl radical) via electron donation and protects the nuclear DNA, membrane lipids, and possibly cytosolic proteins from oxidative damage (Tan et al. 1993b; Poeggeler et al. 1994; Bandyopadhyay et al. 2000; Reiter et al. 2016). Melatonin works at the level of the respiratory chain complexes I and IV to increase the efficiency of electron transfer and promote mitochondrial oxidative phosphorylation. It also curtails electron leakage and prevents the free radical-induced oxidative damage to mitochondria (Martin et al. 2000; Okatani et al. 2002a, b; Reiter et al. 2010). As a strong antioxidant, melatonin can stimulate gene expression of several antioxidative enzymes to reinforce endogenous antioxidant defenses such as catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) (Kotler et al. 1998; Fischer et al. 2013). Furthermore, differs from other conventional antioxidants, not only melatonin but also its metabolites (i.e., N 1-acetyl-N 2-formyl-5-methoxykynuramine (AFMK) and N 1-acetyl-5-methoxykynuramine (AMK)) possess potent antioxidant activity, and part of its therapeutic applications or preventive uses are based on this property (Galano et al. 2013). Moreover, this versatile hormonal compound displays anti-excitatory, anti-apoptotic, and anti-inflammatory activities (Skaper et al. 1998; Radogna et al. 2008; Negi et al. 2011; Zhang et al. 2016; Zhao et al. 2015; Ali et al. 2015). Because of its highly lipophilic nature, melatonin can easily pass through the blood–brain barrier (BBB) and functions within the membrane, the cytosol, the mitochondria, and the nucleus of cells in the brain (Jou et al. 2007). It protects brain and nerve cells from different kinds of damage (Lin and Ho 2000; Li et al. 2009; Wu et al. 2016; Carloni et al. 2016). For example, melatonin significantly reduces neurological functional deficits in a murine model of traumatic brain injury (TBI), which may be attributed to its antioxidant properties (Mesenge et al. 1998; Ding et al. 2014). Melatonin suppresses the upregulation of suppressor of cytokine signaling-3 (SOCS 3), interleukin-6 (IL-6), and inducible nitric oxide synthetase (iNOS), restores the DNA-binding activity of signal transducers and activators of transcription 1 (STAT 1), and attenuates oxidative stress-induced neuronal cell death following TBI (Tsai et al. 2011). Intraperitoneal injection of melatonin inhibits nitric oxide (NO) production, reduces protein nitrosylation, lipid peroxidation, and alleviates brain edema in a Mongolian gerbil model of transient global cerebral ischemia (Cuzzocrea et al. 2000). In a rat ischemic stroke model, pretreatment with melatonin significantly decreased cells with positive immunoreactivity of neuronal nitric oxide synthase (nNOS), cyclooxygenase-2 (COX-2), and myeloperoxidase (MPO) in the ischemic hemisphere of the brain, which suggests its anti-inflammatory effects (Pei and Cheung 2004). In experimental models of Alzheimer’s disease (AD), melatonin intervenes at a number of sites to abate the development of this disease which includes preventing the formation of neurotoxic Aβ aggregates, inhibiting the overactivation of microglia and neuroinflammatory response, limiting oxidative stress, alleviating mitochondrial dysfunction, and attenuating mitochondria-dependent apoptosis (Rosales-Corral et al. 2012). In experimental models of Parkinson’s disease (PD), melatonin suppressed neuroinflammation, reduced oxidative stress, restored mitochondrial function, and protected dopaminergic neurons from neurodegeneration (Singhal et al. 2012). In an animal model of Huntington’s disease (HD), melatonin reversed 3-nitropropionic acid (3-NP)-induced oxidative stress and mitochondrial electron transport interruption in striatal and cortical synaptosomes of rats (Tunez et al. 2004). Also, melatonin administration partially reduced 3-NP-induced loss of dendritic spines in the striatum and the cortex, restored cerebellar granular cell arborization, and corrected 3-NP-induced gait abnormalities (Chakraborty et al. 2014). Altogether, these findings suggest that melatonin is a multi-functioning molecule that produces multiple benefits in experimental models of central nervous system (CNS) disorders. It is interesting to note that the CNS, in addition to the pineal gland, produces melatonin at several sites, including nucleus gracilis, pons, medulla, cerebellum, and cerebral cortex (Jimenez-Jorge et al. 2007). Experimental evidences demonstrated that both neurons and astrocytes can synthesize melatonin, which is critical for maintaining cellular homeostasis and survival following brain injury (Acuna-Castroviejo et al. 2014; Liu et al. 2007, 2012).
The Neuroprotective Actions of Melatonin in Hemorrhagic Stroke
In experimental models of hemorrhagic stroke, consisting of SAH and ICH, melatonin has been proven to be neuroprotective (Table 1). The following sections will survey these neuroprotective properties of melatonin in both in vitro and in vivo models of hemorrhagic stroke and delve into the underlying molecular mechanisms.
Table 1.
The main findings of melatonin-mediated neuroprotection in hemorrhagic stroke
| Model | Therapeutic paradigm | Main findings | References |
|---|---|---|---|
| Cisterna magna model of SAH, rat | 10 mg/kg, i.p., repeated daily for 2 days after SAH | Inhibits lipid peroxidation, restores glutathione levels | Ersahin et al. (2009) |
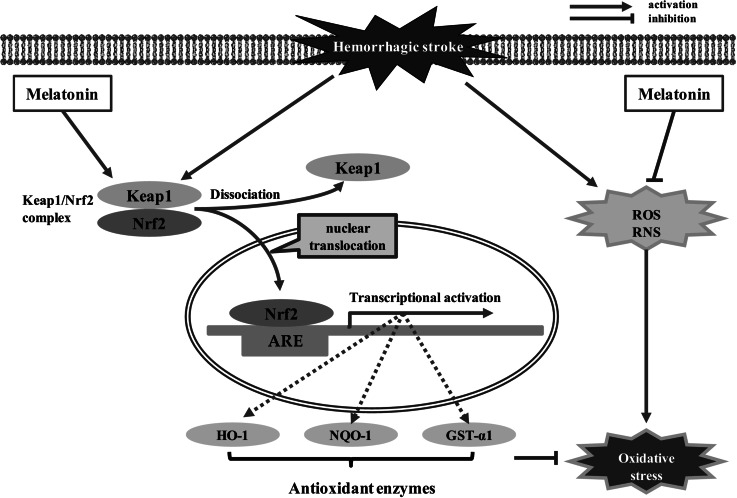
| Prechiasmatic cistern model of SAH, rat | 150 mg/kg, i.p., 2 and 24 h after SAH | Activates Nrf2-ARE pathway, increases the expression of antioxidant and detoxifying enzymes | Wang et al. (2012) |
| Endovascular filament model of SAH, rat | 150 mg/kg, i.p., 2 and 24 h after SAH | Reduces brain water content, decreases mortality | Ayer et al. (2008) |
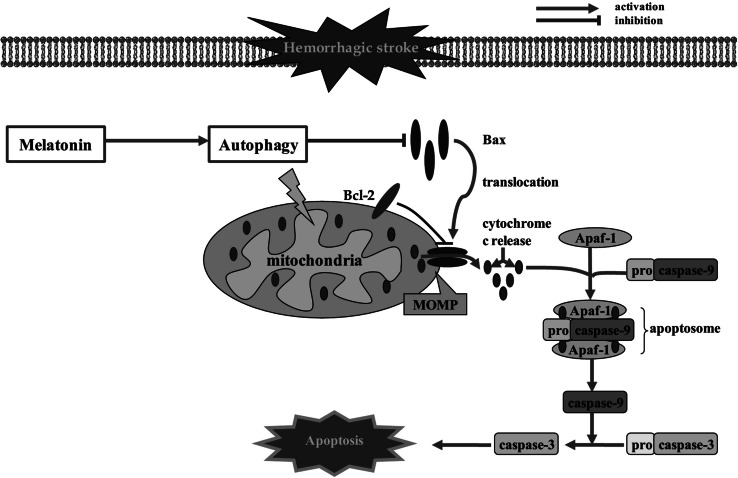
| Endovascular filament model of SAH, rat | 150 mg/kg, i.p., 2 h after SAH | Enhances autophagy, inhibits mitochondrial- dependent apoptosis | Chen et al. (2014b) |
| Endovascular filament model of SAH, rat | 150 mg/kg, i.p., 2 h after SAH | Inhibits cortical expressions of proinflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α), maintains tight junction proteins and BBB integrity | Chen et al. (2014a) |
| Prechiasmatic cistern model of SAH, rat | 150 mg/kg, i.p., 2 and 24 h after SAH | Lessens the protein expressions of TLR4 pathway-related mediators | (Wang et al. 2013b) |
| Cisterna magna model of SAH, rabbit | 5 mg/kg, i.p., every 12 h after SAH for 5 days | Reduces arterial NF-κB binding activity, decreases vascular levels of proinflammatory cytokines (IL-1β, IL-6, and TNF-α), enhances the antioxidant defense system, reduces lipid peroxidation | Fang et al. (2009) |
| Collagenase -induced ICH model, rat | 15 mg/kg, oral, every 24 h after ICH for 7 days | Reduces oxidative stress, enhances electrical responsiveness | Ueda et al. (2014) |
| Collagenase -induced ICH model, rat | 15 or 150 mg/kg, i.p., 15 min or 3 h after ICH | Decreases lipid peroxidation | Rojas et al. (2008) |
| Collagenase -induced ICH model, rat | 15 mg/kg, i.p., 1, 24, 48, and 72 h after ICH | Do not improve short-term outcomes | Hartman et al. (2008) |
| Collagenase -induced ICH model, rat | 5 mg/kg, i.p., 1 h and every 24 h after ICH for 3 days | Alleviates long-term brain atrophy and reverses striatal and cognitive functional deficits | Lekic et al. (2010) |
| 60-min transient MCAO model, mice | 5 mg/kg, i.p., 60 min after the initiation of ischemia | Attenuates the postischemic increase in BBB permeability and decreases HT of t-PA therapy following ischemic stroke | Chen et al. (2006b) |
| 60-min transient MCAO model, mice | 5 mg/kg, i.p., 60 min after the initiation of ischemia | Mitigates excessive production of ROS and RNS, improves BBB integrity, and decreases HT of t-PA therapy following ischemic stroke | Chen et al. (2006a) |
| 90-min transient MCAO model, rat | 5 mg/kg, i.v., 90 min after the initiation of ischemia | Negatively regulates postischemic expression and activation of MMP-9, decreases HT of t-PA therapy following ischemic stroke | Hung et al. (2008) |
| 60-min transient MCAO model, mice | 5 mg/kg, i.v., 60 min after the initiation of ischemia | Increases the expression of TIMP-1, enhances the activity of PAI-1, reduces the activity of u-PA, inhibits the enzyme activity and protein expression of MMP-9, and decreases HT of t-PA therapy following ischemic stroke | Tai et al. (2010) |
Melatonin and Subarachnoid Hemorrhage
SAH is a common and life-threatening type of hemorrhagic stroke. It accounts for approximately 5% of all strokes and affects up to 30,000 individuals per year in the United States (Zacharia et al. 2010; Kapadia et al. 2014). SAH is clearly a multifaceted disorder with complex pathophysiological mechanisms (Chen et al. 2015b). A series of ongoing pathological processes are involved in the pathogenesis of brain injury after SAH. The term “early brain injury” (EBI) and “delayed brain injury” (DBI) are coined to explain the temporal progression of SAH-induced brain injury (Chen et al. 2014c). EBI refers to the acute pathological events that occur within the first 72 h of SAH. When SAH occur, there are initial pathophysiological changes which include a sharp increase of intracranial pressure (ICP), a subsequent fall in the cerebral perfusion pressure (CPP), a significant decrease in the cerebral blood flow (CBF), and a disturbance of cerebral autoregulation (Fujii et al. 2013). These pathological cascades propagate and induce excitotoxicity, inflammation, oxidative stress, and cell death, which result in BBB disruption, cerebral edema, and EBI after SAH (Caner et al. 2012). In contrast, DBI describes a host of critical, interrelated pathological processes that arise during the late phase of SAH. Cerebral vasospasm (CVS), microcirculatory dysfunction, microthrombosis, and cortical spreading ischemia are all possible causes of delayed cerebral ischemia (DCI) and delayed ischemic neurological deficit (DIND) after SAH (Macdonald 2014). Advancements in the pathophysiology of SAH have lead to the understanding that the mechanisms leading to EBI and DBI are not exactly mutually exclusive. A host of critical pathologies can arise in the subacute phase of SAH as a result of EBI and can cause the pathogenesis of DBI. Therefore, therapeutic interventions have to combat the molecular cascades of both EBI and DBI, which may lead to long-term deficits after SAH.
It is important to note that melatonin provides powerful protection in experimental models of SAH by reducing EBI, averting CVS, alleviating neurological deficits, and decreasing mortalities (Ersahin et al. 2009; Ayer et al. 2008). For instance, subarachnoid injection of melatonin (5 or 10 mg/kg), either simultaneously or 60 min before injecting lysed blood cells, attenuates cerebellum oxidative stress in a rat model of SAH (Martinez-Cruz et al. 2002). In a rat cisterna magna single-injection model, melatonin (10 mg/kg, i.p.) significantly inhibits lipid peroxidation and efficiently prevents the depletion of antioxidant glutathione (GSH) stores in the brain, thereby ameliorating oxidative brain injury and improving the neurological state of the rats at 48 h after SAH (Ersahin et al. 2009). In a prechiasmatic rat model of SAH, Wang et al. reported that administration of melatonin (150 mg/kg, i.p.) activates Nrf2-ARE pathway in the cortex during the early stage of SAH, leading to increased expressions of antioxidant and detoxifying enzymes, such as Nrf2, heme oxygenase-1 (HO-1), NAD(P)H: quinone oxidoreductase 1 (NQO1), and glutathione S-transferase α-1 (GST-α1) (Wang et al. 2012). As a result, the degree of EBI, such as apoptotic cell death, BBB permeability, and brain edema is diminished by melatonin after SAH (Wang et al. 2012). Of note, the mechanisms by which melatonin promoted the upregulation of Nrf2 and HO-1 after SAH are not investigated in this work, and the whole-signaling pathway in the brain remains unclarified. Ayer et al. demonstrated in an endovascular filament model of SAH that administration of melatonin at a high dose (150 mg/kg, i.p.) significantly reduces brain water content and decreases mortality at 24 h after SAH; However, the lower dose (15 mg/kg, i.p.) was ineffective (Ayer et al. 2008). Neither dose of melatonin had an effect on the lipid peroxidation of brain samples, suggesting that the mechanisms are unrelated to antioxidant properties and that contributions to neuroprotection were observed in the experimental SAH model (Ayer et al. 2008). In fact, numerous experimental studies have proved that melatonin provides other protective effects (e.g., anti-apoptotic and anti-inflammatory effects) on the SAH brain in addition to its antioxidant properties. For an example, Chen et al. found that injection of melatonin (150 mg/kg, i.p.) 2 h after SAH induction inhibits neural apoptosis, reduces brain edema, decreases mortality, and improves neurological outcomes of rats subjected to SAH (Chen et al. 2014b). Melatonin-enhanced autophagy reduces Bax translocation to the mitochondria and decreases the release of cytochrome c into the cytosol, thereby protecting neural cells from mitochondria-dependent apoptosis after SAH (Chen et al. 2014b). It is important to note that the molecular mechanisms by which melatonin induced autophagy are not explained in this study and need for further research. By using a filament perforation model of SAH, Chen and colleagues found that post-SAH treatment with melatonin (150 mg/kg, i.p.) inhibits cortical expressions of proinflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α), which may lead to the downregulation of matrix metallopeptidase 9 (MMP-9) and vascular endothelial growth factor (VEGF) (Chen et al. 2014a). These changes contribute to the maintenance of tight junction proteins, BBB integrity, and attenuation of brain edema secondary to BBB dysfunction in EBI after SAH (Chen et al. 2014a). It is noteworthy that the mechanisms by which melatonin affected the expressions of inflammatory cytokines are still unknown and require further investigation. In addition, administration of melatonin (150 mg/kg, i.p.) markedly lessens the protein expressions of TLR4 pathway-related mediators, including upstream factors such as TLR4, high mobility group box 1 (HMGB1), myeloid differentiation factor 88 (MyD88), and nuclear factor-κB (NF-κB) and downstream factors such as IL-1β, TNF-α, IL-6, and iNOS have also been shown to be activated and help prevent inflammatory brain damage, spatial learning, and memory deficits after SAH in a prechiasmatic blood injection model (Wang et al. 2013b).
In an experimental rabbit model of SAH, treatment with melatonin (5 mg/kg, i.p.) ameliorates apoptosis of endothelial cells and mitigates CVS after experimental SAH by inhibiting excessive production of free radicals and lipid peroxidation (Aydin et al. 2005). In a rabbit cisterna magna double-injection SAH model, intraperitoneal administration of melatonin at a dose of 5 mg/kg for 5 days downregulated the binding activity of arterial NF-κB, decreased vascular levels of proinflammatory cytokines (IL-1β, IL-6, and TNF-α), and attenuated the inflammatory response in the basilar arteries (Fang et al. 2009). Also, treatment with melatonin (5 mg/kg, i.p.) enhanced SOD and GSH-Px which lead to a reduction in lipid peroxidation in the basilar artery (Fang et al. 2009). All these beneficial effects of melatonin contributed to the amelioration of CVS after SAH. Taken together, preclinical evidences have demonstrated the beneficial effects of melatonin administration in reducing brain tissue damage in experimental models of SAH. Melatonin confers neuroprotection against EBI and CVS through multiple molecular actions, including antioxidant, anti-apoptotic, and anti-inflammatory activities. These beneficial effects of melatonin may be attributed to its direct free radical scavenging activities and interaction with specific molecules in apoptotic pathways. In addition, these protective effects of melatonin may be associated with its indirect actions in stimulating antioxidant enzymes and downregulating gene expressions of proinflammatory mediators. However, the detail mechanisms underlying melatonin-mediated neuroprotection in SAH are far from being clarified and remain to be established.
Melatonin and Intracerebral Hemorrhage
ICH is another common devastating clinical entity of hemorrhagic stroke. It accounts for about 10–15% of all strokes and affects approximately 120,000 people in the United States each year (Adeoye and Broderick 2010). In all cases of ICH, extravagated blood accumulates and compresses the surrounding brain tissue leading to primary brain injury (Keep et al. 2012). More importantly, secondary brain injury evolves over a period of hours to days after the primary brain injury, which is an important determinant of ICH outcomes (Xi et al. 2006). Multiple pathological events, such as inflammation, apoptosis, and oxidative stress, are activated and implicated in ICH pathology, resulting in BBB disruption, brain edema formation, and brain injury after ICH (Zhou et al. 2014; Wang and Dore 2007; Qureshi et al. 2003). As a consequence, they are regarded as therapeutic targets for the treatment of ICH.
Of interest, by using a modified collagenase-induced ICH model in rats, Ueda et al. found that oral administration (15 mg/kg) for 7 days protects oligodendrocytes and astrocytes in the vicinity of the lesion in the corticospinal tract from oxidative stress (Ueda et al. 2014). Ueda et al. also found that melatonin reduces corticospinal tract damage, enhances electrical responsiveness in the ipsilateral cerebral cortex remote to the ICH pathology, and eventually improves motor abilities after ICH (Ueda et al. 2014). Rojas et al. reported that intraperitoneal injection of melatonin (15 or 150 mg/kg) at either 15 min or 3 h after ICH induction dramatically decreases lipid peroxidation at 24 h post-ICH (Rojas et al. 2008). However, no significant differences of neurological scoring and brain water content in the right basal ganglia at any treatment regimens or time points of drug administration were observed in this rat model of ICH (Rojas et al. 2008). Also, Hartman et al. demonstrated that repeated intraperitoneal injections of melatonin (15 mg/kg) post-ICH did not improve neurological performances in a rat collagenase-induced ICH model (Hartman et al. 2008). On the other side, it is important to mention that although short-term outcomes are evaluated by brain edema and neurological deficits are unchanged, repeated intraperitoneal administration with lower doses of melatonin is beneficial over time (Lekic et al. 2010). Low dosages of melatonin (5 mg/kg) given at 1 h and every 24 h thereafter for 3 days after ICH alleviated long-term brain atrophy and reverse striatal and cognitive functional deficits back to near-normal levels over the course of 8 weeks (Lekic et al. 2010). Additionally, melatonin treatment at daily doses of 5 mg/kg was able to reduce neuronal death after injections of iron into the parietal cortex of rats (Hayter et al. 2004). The hippocampal co-infusion of melatonin (10 mg/kg) with iron was beneficial for the prevention of neurotoxicity and free radical formation (Maharaj et al. 2006). Overall, these experimental evidences suggested that melatonin offers protective effects against ICH-induced brain injury by scavenging free radicals and reducing oxidative stress. It is noteworthy that the neuroprotective mechanisms of melatonin in ICH are not absolutely elucidated and require further investigation.
Melatonin and Hemorrhagic Transformation
Hemorrhagic transformation (HT) refers to a spectrum of ischemia-related hemorrhage in which ischemic brain tissue converts into a hemorrhagic lesion with blood vessel leakage, extravasation, leading to an exacerbation of brain injury (Wang and Lo 2003). It has been estimated that up to 30–40% of all patients with ischemic strokes undergo spontaneous HT (Terruso et al. 2009; Beslow et al. 2011), and this phenomenon becomes even more prevalent after thrombolytic therapy (Tissue plasminogen activator for acute ischemic stroke, The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group 1995; Lees et al. 2010). Fundamentally, post-stroke HT occurs when cerebral blood flow is restored to the damaged vasculature, and disruption of the BBB is central to HT formation after ischemic stroke (Jickling et al. 2014). Diverse molecular mechanisms such as reperfusion injury, oxidative stress, leukocyte infiltration, vascular activation, and dysregulation of extracellular proteolysis all play important roles in BBB breakdown and are potential triggers of HT in the ischemic brain (Wang et al. 2015; Benchenane et al. 2005). It is interesting to know that cerebral ischemia elicits biphasic elevations in the BBB permeability, occurring at 3–6 h and 24–48 h after the onset of cerebral ischemia, respectively (Belayev et al. 1996; Albayrak et al. 1997; Huang et al. 1999). This time-dependent increase in the BBB permeability is correlated with reversible and irreversible damage to the neurovascular unit, which may subsequently result in brain edema and even HT after stroke (Dijkhuizen et al. 2002; Han and Suk 2005; Gursoy-Ozdemir et al. 2004). Molecules such as MMPs are crucial mediators that contribute to BBB disruption and HT pathogenesis after ischemic stroke (Jickling et al. 2014). Thus, therapeutic interventions should be designed to target these and other pathogenic factors to prevent and treat HT.
In a 60-min transient MCAO model in mice, treatment with melatonin (5 mg/kg, i.p.) at the initiation of reperfusion inhibits postischemic increase in BBB permeability and reduces the risk of HT following the therapy of tissue plasminogen activator (t-PA) thrombolysis at 24 h after transient focal cerebral ischemia (Chen et al. 2006b). Chen et al. demonstrated that a single intraperitoneal treatment with melatonin (5 mg/kg) at the initiation time of reperfusion mitigates excessive production of ROS and reactive nitrogen species (RNS) in the neurovascular unit of penumbral areas (Chen et al. 2006a). In this study, melatonin was also shown to reduce both oxidative and nitrosative damage to the ischemic neurovascular unit and improve the anatomical, biochemical, and functional integrity of the BBB at the early phase after transient focal cerebral ischemia in C57/B6 mice (Chen et al. 2006a). As a result, the risk of HT during tPA-mediated thrombolysis in this animal model was decreased. Hung et al. demonstrated that melatonin (5 mg/kg) intravenously injected upon reperfusion provides pluripotent pharmacological properties to negatively regulate postischemic expression and activation of MMP-9 within cerebral infarcts (Hung et al. 2008). As a consequence, brain edema was alleviated, HT was prevented, and there was an improvement in neurologic outcomes in this rat model of transient focal cerebral ischemia/reperfusion (Hung et al. 2008). Tai et al. found that intravenous administration of melatonin (5 mg/kg) at the onset of reperfusion effectively attenuated postischemic activation and MMPs expression (Tai et al. 2010). This occurred via the regulation of the endogenous MMP inhibitor and the plasminogen/plasmin system, which helped preserve the extracellular matrix (ECM) integrity and prevented HT after ischemic stroke (Tai et al. 2010). After melatonin administration, the expression of tissue inhibitors of MMP (TIMP-1) was increased, the activity of plasminogen activator inhibitor (PAI)-1 was enhanced, and the activity of urokinase plasminogen activators (u-PA) was reduced. Together these events contribute to persistent inhibition of enzymatic activity and protein expression of MMP-9 at the subacute stage of transient focal cerebral ischemia in mice (Tai et al. 2010). These findings highlight the beneficial effects of melatonin in preventing cerebral ischemia/reperfusion-induced neurovascular damage and HT. These findings also provide support for its usage as a supplement or add-on to thrombolytic therapies for patients with ischemic stroke.
In conclusion, evidence from basic science research suggests that melatonin is protective against brain injury in hemorrhagic stroke via multiple molecular mechanisms such as antioxidant (Fig. 1), anti-inflammation (Fig. 2), and anti-apoptosis (Fig. 3). Considering that melatonin is an endogenous, non-toxic substance with potent neuroprotective properties, it should be thoroughly tested on patients with hemorrhagic stroke as a co-treatment with conventional therapies (Chahbouni et al. 2010; Weishaupt et al. 2006). Of note, although ICH, SAH and other cerebral hemorrhagic diseases share several common pathological mechanisms, not all therapeutic benefits of melatonin apply to the same degree to these diseases. The effects of melatonin in hemorrhagic stroke therapies should be rigorously tested in experimental models of different types of brain hemorrhage prior to clinical trials to determine their efficacy and safety profile.
Fig. 1.
The antioxidative effects of melatonin in hemorrhagic stroke
Fig. 2.
The anti-inflammatory effect of melatonin in hemorrhagic stroke
Fig. 3.
The anti-apoptotic effect of melatonin in hemorrhagic stroke
Conclusion and Perspective
Hemorrhagic stroke is a complex pathological process, which involves multiple molecular and cellular events that can lead to brain injury. The complicated pathophysiology of hemorrhagic stroke determines that successful neuroprotection could be achieved only by multi-therapeutic approaches. And optimizing pharmacological therapy for secondary brain injury following hemorrhagic stroke requires capitalizing on multiple pathological pathways. Therefore, there is no doubt that a multi-targeted neuroprotective compound would be a potential strategy to treat this devastating disease. The experimental studies discussed throughout this article provide evidence that melatonin is a pleiotropic agent that is capable of interfering with oxidative stress, inflammatory responses, and apoptosis, all of which would be useful in treating common pathological events that take place in ICH, SAH, and other cerebral hemorrhagic diseases. Considering the lack of effective pharmacological strategies against this disease up to date, a safety-validated drug such as melatonin is particularly recommended as a candidate neuroprotectant for clinical evaluation.
Nevertheless, there are still a few critical issues that need to be addressed in order for melatonin to advance and develop into clinical applications involving hemorrhagic stroke. As an example, numerous studies supported neuroprotective benefits of exogenous melatonin in preclinical animal models of neurodegenerative diseases (Naskar et al. 2015). However, clinical trial data have been less positive, with available evidence suggesting minimal to no benefit (Medeiros et al. 2007; Schutte-Rodin et al. 2008). Discordant results between preclinical and clinical studies may be associated with the dose, timing, frequency, duration, and formulation of melatonin administration (Trotti and Karroum 2016). As have been reported, doses in animal studies are relatively large compared to human studies. In clinical practice, benefits might only be seen when using very low doses of melatonin, as clinical doses of melatonin are supra-physiologic, and that high doses of melatonin may result in unwanted circadian phase-shifting (Gehrman et al. 2009). Alternatively, the clinical trials performed in neurodegenerative diseases may have been hampered by the relatively late administration of melatonin in the disease course, when any protective effects of melatonin could come too late to halt disease progression. Also, there may be a true lack of benefits of melatonin in human neurodegenerative diseases, broadly concordant with the only modest benefits on sleep latency, but not other measures (Bolitho et al. 2014). Thus, the neuroprotective properties of melatonin in hemorrhagic stroke should be examined in different animal species exposed to variety of experimental conditions. Furthermore, the optimal dosages, reliability of the routes of drug administration, and the therapeutic time windows of melatonin in the treatment of hemorrhagic stroke must be confirmed. As has been demonstrated, acute cerebral hemorrhage changes the nocturnal surge of plasma melatonin in humans (Pang et al. 1990). Additionally, the extremely short half-life of melatonin in the circulation will be a major obstacle for its potential application in clinical practice, which can be solved by development of more potent melatonin analogs with prolonged effects or design of slow release melatonin preparations (Cardinali et al. 2012). Currently, a double-blind, placebo-controlled study to evaluate the efficacy of melatonin in acute ischemic stroke is ongoing (ClinicalTrials.gov Identifier: NCT01863277). In contrast, no clinical studies have been conducted to investigate the efficacy and safety of melatonin in the treatment of patients with cerebral hemorrhage. In the future, long-term, large-scale, randomized, double-blind, multicenter clinical trials are urgently needed, in order to evaluate the potential usage of this pleiotropic molecule for hemorrhagic stroke.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81371433, No. 81402044), Natural Science Foundation of Zhejiang Province of China (LY14H160017), Zhejiang Provincial Medical Science and Technology Planning Project (No. 201466094), Zhejiang Provincial Welfare Technology Application Research Project (No. 2015C33217).
Compliance with Ethical Standards
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Hai-Jian Wu, Cheng Wu and Huan-Jiang Niu have contributed equally to this work.
Contributor Information
Shu-Xu Yang, Phone: +86-571-86006166, Email: yangsxsy@163.com.
Yi-Rong Wang, Phone: +86-571-86006166, Email: wang.yr@163.com.
References
- Acuna-Castroviejo D, Escames G, Venegas C, Diaz-Casado ME, Lima-Cabello E, Lopez LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci 71(16):2997–3025. doi:10.1007/s00018-014-1579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeoye O, Broderick JP (2010) Advances in the management of intracerebral hemorrhage. Nat Rev Neurol 6(11):593–601. doi:10.1038/nrneurol.2010.146 [DOI] [PubMed] [Google Scholar]
- Albayrak S, Zhao Q, Siesjo BK, Smith ML (1997) Effect of transient focal ischemia on blood-brain barrier permeability in the rat: correlation to cell injury. Acta Neuropathol 94(2):158–163 [DOI] [PubMed] [Google Scholar]
- Ali T, Badshah H, Kim TH, Kim MO (2015) Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-kB/JNK signaling pathway in aging mouse model. J Pineal Res 58(1):71–85. doi:10.1111/jpi.12194 [DOI] [PubMed] [Google Scholar]
- Aydin MV, Caner H, Sen O, Ozen O, Atalay B, Cekinmez M, Altinors N (2005) Effect of melatonin on cerebral vasospasm following experimental subarachnoid hemorrhage. Neurol Res 27(1):77–82. doi:10.1179/016164105X18331 [DOI] [PubMed] [Google Scholar]
- Ayer RE, Sugawara T, Chen W, Tong W, Zhang JH (2008) Melatonin decreases mortality following severe subarachnoid hemorrhage. J Pineal Res 44(2):197–204. doi:10.1111/j.1600-079X.2007.00508.x [DOI] [PubMed] [Google Scholar]
- Ayer A, Hwang BY, Appelboom G, Connolly ES Jr (2012) Clinical trials for neuroprotective therapies in intracerebral hemorrhage: a new roadmap from bench to bedside. Transl Stroke Res 3(4):409–417. doi:10.1007/s12975-012-0207-4 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay D, Biswas K, Bandyopadhyay U, Reiter RJ, Banerjee RK (2000) Melatonin protects against stress-induced gastric lesions by scavenging the hydroxyl radical. J Pineal Res 29(3):143–151 [DOI] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Ginsberg MD (1996) Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res 739(1–2):88–96 [DOI] [PubMed] [Google Scholar]
- Benchenane K, Berezowski V, Fernandez-Monreal M, Brillault J, Valable S, Dehouck MP, Cecchelli R, Vivien D, Touzani O, Ali C (2005) Oxygen glucose deprivation switches the transport of tPA across the blood-brain barrier from an LRP-dependent to an increased LRP-independent process. Stroke 36(5):1065–1070. doi:10.1161/01.STR.0000163050.39122.4f [DOI] [PubMed] [Google Scholar]
- Beslow LA, Smith SE, Vossough A, Licht DJ, Kasner SE, Favilla CG, Halperin AR, Gordon DM, Jones CI, Cucchiara AJ, Ichord RN (2011) Hemorrhagic transformation of childhood arterial ischemic stroke. Stroke 42(4):941–946. doi:10.1161/STROKEAHA.110.604199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolitho SJ, Naismith SL, Rajaratnam SM, Grunstein RR, Hodges JR, Terpening Z, Rogers N, Lewis SJ (2014) Disturbances in melatonin secretion and circadian sleep-wake regulation in Parkinson disease. Sleep Med 15(3):342–347. doi:10.1016/j.sleep.2013.10.016 [DOI] [PubMed] [Google Scholar]
- Caner B, Hou J, Altay O, Fujii M, Zhang JH (2012) Transition of research focus from vasospasm to early brain injury after subarachnoid hemorrhage. J Neurochem 123(Suppl 2):12–21. doi:10.1111/j.1471-4159.2012.07939.x [DOI] [PubMed] [Google Scholar]
- Cardinali DP, Srinivasan V, Brzezinski A, Brown GM (2012) Melatonin and its analogs in insomnia and depression. J Pineal Res 52(4):365–375. doi:10.1111/j.1600-079X.2011.00962.x [DOI] [PubMed] [Google Scholar]
- Carloni S, Favrais G, Saliba E, Albertini MC, Chalon S, Longini M, Gressens P, Buonocore G, Balduini W (2016) Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR-34a/silent information regulator 1 pathway. J Pineal Res 61(3):370–380. doi:10.1111/jpi.12354 [DOI] [PubMed] [Google Scholar]
- Cervantes M, Morali G, Letechipia-Vallejo G (2008) Melatonin and ischemia-reperfusion injury of the brain. J Pineal Res 45(1):1–7. doi:10.1111/j.1600-079X.2007.00551.x [DOI] [PubMed] [Google Scholar]
- Chahbouni M, Escames G, Venegas C, Sevilla B, Garcia JA, Lopez LC, Munoz-Hoyos A, Molina-Carballo A, Acuna-Castroviejo D (2010) Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. J Pineal Res 48(3):282–289. doi:10.1111/j.1600-079X.2010.00752.x [DOI] [PubMed] [Google Scholar]
- Chakraborty J, Nthenge-Ngumbau DN, Rajamma U, Mohanakumar KP (2014) Melatonin protects against behavioural dysfunctions and dendritic spine damage in 3-nitropropionic acid-induced rat model of Huntington’s disease. Behav Brain Res 264:91–104. doi:10.1016/j.bbr.2014.01.048 [DOI] [PubMed] [Google Scholar]
- Chen HY, Chen TY, Lee MY, Chen ST, Hsu YS, Kuo YL, Chang GL, Wu TS, Lee EJ (2006a) Melatonin decreases neurovascular oxidative/nitrosative damage and protects against early increases in the blood-brain barrier permeability after transient focal cerebral ischemia in mice. J Pineal Res 41(2):175–182. doi:10.1111/j.1600-079X.2006.00351.x [DOI] [PubMed] [Google Scholar]
- Chen TY, Lee MY, Chen HY, Kuo YL, Lin SC, Wu TS, Lee EJ (2006b) Melatonin attenuates the postischemic increase in blood-brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J Pineal Res 40(3):242–250. doi:10.1111/j.1600-079X.2005.00307.x [DOI] [PubMed] [Google Scholar]
- Chen J, Chen G, Li J, Qian C, Mo H, Gu C, Yan F, Yan W, Wang L (2014a) Melatonin attenuates inflammatory response-induced brain edema in early brain injury following a subarachnoid hemorrhage: a possible role for the regulation of pro-inflammatory cytokines. J Pineal Res 57(3):340–347. doi:10.1111/jpi.12173 [DOI] [PubMed] [Google Scholar]
- Chen J, Wang L, Wu C, Hu Q, Gu C, Yan F, Li J, Yan W, Chen G (2014b) Melatonin-enhanced autophagy protects against neural apoptosis via a mitochondrial pathway in early brain injury following a subarachnoid hemorrhage. J Pineal Res 56(1):12–19. doi:10.1111/jpi.12086 [DOI] [PubMed] [Google Scholar]
- Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, Zhang J, Tang J, Zhang JH (2014c) Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol 115:64–91. doi:10.1016/j.pneurobio.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Qian C, Duan H, Cao S, Yu X, Li J, Gu C, Yan F, Wang L, Chen G (2015a) Melatonin attenuates neurogenic pulmonary edema via the regulation of inflammation and apoptosis after subarachnoid hemorrhage in rats. J Pineal Res 59(4):469–477. doi:10.1111/jpi.12278 [DOI] [PubMed] [Google Scholar]
- Chen S, Wu H, Tang J, Zhang J, Zhang JH (2015b) Neurovascular events after subarachnoid hemorrhage: focusing on subcellular organelles. Acta Neurochir Suppl 120:39–46. doi:10.1007/978-3-319-04981-6_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SY, Han SH (2003) Melatonin attenuates kainic acid-induced hippocampal neurodegeneration and oxidative stress through microglial inhibition. J Pineal Res 34(2):95–102 [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Costantino G, Gitto E, Mazzon E, Fulia F, Serraino I, Cordaro S, Barberi I, De Sarro A, Caputi AP (2000) Protective effects of melatonin in ischemic brain injury. J Pineal Res 29(4):217–227 [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Asahi M, Wu O, Rosen BR, Lo EH (2002) Rapid breakdown of microvascular barriers and subsequent hemorrhagic transformation after delayed recombinant tissue plasminogen activator treatment in a rat embolic stroke model. Stroke 33(8):2100–2104 [DOI] [PubMed] [Google Scholar]
- Ding K, Wang H, Xu J, Li T, Zhang L, Ding Y, Zhu L, He J, Zhou M (2014) Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: the Nrf2-ARE signaling pathway as a potential mechanism. Free Radic Biol Med 73:1–11. doi:10.1016/j.freeradbiomed.2014.04.031 [DOI] [PubMed] [Google Scholar]
- Dong Y, Fan C, Hu W, Jiang S, Ma Z, Yan X, Deng C, Di S, Xin Z, Wu G, Yang Y, Reiter RJ, Liang G (2016) Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signaling. J Pineal Res 60(3):253–262. doi:10.1111/jpi.12300 [DOI] [PubMed] [Google Scholar]
- Ersahin M, Toklu HZ, Cetinel S, Yuksel M, Yegen BC, Sener G (2009) Melatonin reduces experimental subarachnoid hemorrhage-induced oxidative brain damage and neurological symptoms. J Pineal Res 46(3):324–332. doi:10.1111/j.1600-079X.2009.00664.x [DOI] [PubMed] [Google Scholar]
- Fang Q, Chen G, Zhu W, Dong W, Wang Z (2009) Influence of melatonin on cerebrovascular proinflammatory mediators expression and oxidative stress following subarachnoid hemorrhage in rabbits. Mediators Inflamm 2009:426346. doi:10.1155/2009/426346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer TW, Kleszczynski K, Hardkop LH, Kruse N, Zillikens D (2013) Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J Pineal Res 54(3):303–312. doi:10.1111/jpi.12018 [DOI] [PubMed] [Google Scholar]
- Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH (2013) Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res 4(4):432–446. doi:10.1007/s12975-013-0257-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galano A, Tan DX, Reiter RJ (2013) On the free radical scavenging activities of melatonin’s metabolites. AFMK and AMK. J Pineal Res 54(3):245–257. doi:10.1111/jpi.12010 [DOI] [PubMed] [Google Scholar]
- Gehrman PR, Connor DJ, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S (2009) Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer disease. Am J Geriatr Psychiatry 17(2):166–169. doi:10.1097/JGP.0b013e318187de18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursoy-Ozdemir Y, Can A, Dalkara T (2004) Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke 35(6):1449–1453. doi:10.1161/01.STR.0000126044.83777.f4 [DOI] [PubMed] [Google Scholar]
- Han HS, Suk K (2005) The function and integrity of the neurovascular unit rests upon the integration of the vascular and inflammatory cell systems. Curr Neurovasc Res 2(5):409–423 [DOI] [PubMed] [Google Scholar]
- Hartman RE, Rojas HA, Lekic T, Ayer R, Lee S, Jadhav V, Titova E, Tang J, Zhang JH (2008) Long-term effects of melatonin after intracerebral hemorrhage in rats. Acta Neurochir Suppl 105:99–100 [DOI] [PubMed] [Google Scholar]
- Hayter CL, Bishop GM, Robinson SR (2004) Pharmacological but not physiological concentrations of melatonin reduce iron-induced neuronal death in rat cerebral cortex. Neurosci Lett 362(3):182–184. doi:10.1016/j.neulet.2004.02.024 [DOI] [PubMed] [Google Scholar]
- Huang ZG, Xue D, Preston E, Karbalai H, Buchan AM (1999) Biphasic opening of the blood-brain barrier following transient focal ischemia: effects of hypothermia. Can J Neurol Sci 26(4):298–304 [DOI] [PubMed] [Google Scholar]
- Hung YC, Chen TY, Lee EJ, Chen WL, Huang SY, Lee WT, Lee MY, Chen HY, Wu TS (2008) Melatonin decreases matrix metalloproteinase-9 activation and expression and attenuates reperfusion-induced hemorrhage following transient focal cerebral ischemia in rats. J Pineal Res 45(4):459–467. doi:10.1111/j.1600-079X.2008.00617.x [DOI] [PubMed] [Google Scholar]
- Ikram MA, Wieberdink RG, Koudstaal PJ (2012) International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep 14(4):300–306. doi:10.1007/s11883-012-0252-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, Sharp FR (2014) Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab 34(2):185–199. doi:10.1038/jcbfm.2013.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Jorge S, Guerrero JM, Jimenez-Caliani AJ, Naranjo MC, Lardone PJ, Carrillo-Vico A, Osuna C, Molinero P (2007) Evidence for melatonin synthesis in the rat brain during development. J Pineal Res 42(3):240–246. doi:10.1111/j.1600-079X.2006.00411.x [DOI] [PubMed] [Google Scholar]
- Jou MJ, Peng TI, Yu PZ, Jou SB, Reiter RJ, Chen JY, Wu HY, Chen CC, Hsu LF (2007) Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J Pineal Res 43(4):389–403. doi:10.1111/j.1600-079X.2007.00490.x [DOI] [PubMed] [Google Scholar]
- Kapadia A, Schweizer TA, Spears J, Cusimano M, Macdonald RL (2014) Nonaneurysmal perimesencephalic subarachnoid hemorrhage: diagnosis, pathophysiology, clinical characteristics, and long-term outcome. World Neurosurg 82(6):1131–1143. doi:10.1016/j.wneu.2014.07.006 [DOI] [PubMed] [Google Scholar]
- Keep RF, Hua Y, Xi G (2012) Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 11(8):720–731. doi:10.1016/S1474-4422(12)70104-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleszczynski K, Zillikens D, Fischer TW (2016) Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (gamma-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK). J Pineal Res 61(2):187–197. doi:10.1111/jpi.12338 [DOI] [PubMed] [Google Scholar]
- Kotler M, Rodriguez C, Sainz RM, Antolin I, Menendez-Pelaez A (1998) Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J Pineal Res 24(2):83–89 [DOI] [PubMed] [Google Scholar]
- Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W, Ecass AN, Group Er-PS, Allen K, Mau J, Meier D, del Zoppo G, De Silva DA, Butcher KS, Parsons MW, Barber PA, Levi C, Bladin C, Byrnes G (2010) Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 375(9727):1695–1703. doi:10.1016/S0140-6736(10)60491-6 [DOI] [PubMed] [Google Scholar]
- Leibowitz A, Volkov A, Voloshin K, Shemesh C, Barshack I, Grossman E (2016) Melatonin prevents kidney injury in a high salt diet-induced hypertension model by decreasing oxidative stress. J Pineal Res 60(1):48–54. doi:10.1111/jpi.12287 [DOI] [PubMed] [Google Scholar]
- Lekic T, Hartman R, Rojas H, Manaenko A, Chen W, Ayer R, Tang J, Zhang JH (2010) Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J Neurotrauma 27(3):627–637. doi:10.1089/neu.2009.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZQ, Liang GB, Xue YX, Liu YH (2009) Effects of combination treatment of dexamethasone and melatonin on brain injury in intracerebral hemorrhage model in rats. Brain Res 1264:98–103. doi:10.1016/j.brainres.2009.01.055 [DOI] [PubMed] [Google Scholar]
- Li H, Wang Y, Feng D, Liu Y, Xu M, Gao A, Tian F, Zhang L, Cui Y, Wang Z, Chen G (2014) Alterations in the time course of expression of the Nox family in the brain in a rat experimental cerebral ischemia and reperfusion model: effects of melatonin. J Pineal Res 57(1):110–119. doi:10.1111/jpi.12148 [DOI] [PubMed] [Google Scholar]
- Lin AM, Ho LT (2000) Melatonin suppresses iron-induced neurodegeneration in rat brain. Free Radic Biol Med 28(6):904–911 [DOI] [PubMed] [Google Scholar]
- Liu YJ, Zhuang J, Zhu HY, Shen YX, Tan ZL, Zhou JN (2007) Cultured rat cortical astrocytes synthesize melatonin: absence of a diurnal rhythm. J Pineal Res 43(3):232–238. doi:10.1111/j.1600-079X.2007.00466.x [DOI] [PubMed] [Google Scholar]
- Liu YJ, Meng FT, Wang LL, Zhang LF, Cheng XP, Zhou JN (2012) Apolipoprotein E influences melatonin biosynthesis by regulating NAT and MAOA expression in C6 cells. J Pineal Res 52(4):397–402. doi:10.1111/j.1600-079X.2011.00954.x [DOI] [PubMed] [Google Scholar]
- Macchi MM, Bruce JN (2004) Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol 25(3–4):177–195. doi:10.1016/j.yfrne.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Macdonald RL (2014) Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 10(1):44–58. doi:10.1038/nrneurol.2013.246 [DOI] [PubMed] [Google Scholar]
- Maharaj DS, Maharaj H, Daya S, Glass BD (2006) Melatonin and 6-hydroxymelatonin protect against iron-induced neurotoxicity. J Neurochem 96(1):78–81. doi:10.1111/j.1471-4159.2005.03532.x [DOI] [PubMed] [Google Scholar]
- Martin M, Macias M, Escames G, Reiter RJ, Agapito MT, Ortiz GG, Acuna-Castroviejo D (2000) Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J Pineal Res 28(4):242–248 [DOI] [PubMed] [Google Scholar]
- Martinez-Cruz F, Espinar A, Pozo D, Osuna C, Guerrero JM (2002) Melatonin prevents focal rat cerebellum injury as assessed by induction of heat shock protein (HO-1) following subarachnoid injections of lysed blood. Neurosci Lett 331(3):208–210 [DOI] [PubMed] [Google Scholar]
- Medeiros CA, Carvalhedo de Bruin PF, Lopes LA, Magalhaes MC, de Lourdes Seabra M, de Bruin VM (2007) Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease. A randomized, double blind, placebo-controlled study. J Neurol 254(4):459–464. doi:10.1007/s00415-006-0390-x [DOI] [PubMed]
- Mesenge C, Margaill I, Verrecchia C, Allix M, Boulu RG, Plotkine M (1998) Protective effect of melatonin in a model of traumatic brain injury in mice. J Pineal Res 25(1):41–46 [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Ghosh AK, Dutta M, Mitra E, Mallick S, Saha B, Reiter RJ, Bandyopadhyay D (2015) Mechanisms of isoproterenol-induced cardiac mitochondrial damage: protective actions of melatonin. J Pineal Res 58(3):275–290. doi:10.1111/jpi.12213 [DOI] [PubMed] [Google Scholar]
- Naskar A, Prabhakar V, Singh R, Dutta D, Mohanakumar KP (2015) Melatonin enhances L-DOPA therapeutic effects, helps to reduce its dose, and protects dopaminergic neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice. J Pineal Res 58(3):262–274. doi:10.1111/jpi.12212 [DOI] [PubMed] [Google Scholar]
- Negi G, Kumar A, Sharma SS (2011) Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF-kB and Nrf2 cascades. J Pineal Res 50(2):124–131. doi:10.1111/j.1600-079X.2010.00821.x [DOI] [PubMed] [Google Scholar]
- Okatani Y, Wakatsuki A, Reiter RJ (2002a) Melatonin protects hepatic mitochondrial respiratory chain activity in senescence-accelerated mice. J Pineal Res 32(3):143–148 [DOI] [PubMed] [Google Scholar]
- Okatani Y, Wakatsuki A, Reiter RJ, Miyahara Y (2002b) Hepatic mitochondrial dysfunction in senescence-accelerated mice: correction by long-term, orally administered physiological levels of melatonin. J Pineal Res 33(3):127–133 [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R (2006) Melatonin: nature’s most versatile biological signal? FEBS J 273(13):2813–2838. doi:10.1111/j.1742-4658.2006.05322.x [DOI] [PubMed] [Google Scholar]
- Pang SF, Li Y, Jiang DH, Chang B, Xie BL (1990) Acute cerebral hemorrhage changes the nocturnal surge of plasma melatonin in humans. J Pineal Res 9(3):193–208 [DOI] [PubMed] [Google Scholar]
- Pei Z, Cheung RT (2004) Pretreatment with melatonin exerts anti-inflammatory effects against ischemia/reperfusion injury in a rat middle cerebral artery occlusion stroke model. J Pineal Res 37(2):85–91. doi:10.1111/j.1600-079X.2004.00138.x [DOI] [PubMed] [Google Scholar]
- Poeggeler B, Saarela S, Reiter RJ, Tan DX, Chen LD, Manchester LC, Barlow-Walden LR (1994) Melatonin–a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro. Ann N Y Acad Sci 738:419–420 [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suri MF, Ostrow PT, Kim SH, Ali Z, Shatla AA, Guterman LR, Hopkins LN (2003) Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery 52(5):1041–1047; discussion 1047–1048 [PubMed]
- Radogna F, Cristofanon S, Paternoster L, D’Alessio M, De Nicola M, Cerella C, Dicato M, Diederich M, Ghibelli L (2008) Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl-2. J Pineal Res 44(3):316–325. doi:10.1111/j.1600-079X.2007.00532.x [DOI] [PubMed] [Google Scholar]
- Regrigny O, Delagrange P, Scalbert E, Atkinson J, Lartaud-Idjouadiene I (1998) Melatonin improves cerebral circulation security margin in rats. Am J Physiol 275(1 Pt 2):H139–H144 [DOI] [PubMed] [Google Scholar]
- Regrigny O, Dupuis F, Atkinson J, Liminana P, Scalbert E, Delagrange P, Chillon JM (2001) Cerebral arteriolar structure and function in pinealectomized rats. Am J Physiol Heart Circ Physiol 281(4):H1476–H1480 [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Manchester LC, Tan DX (2010) Neurotoxins: free radical mechanisms and melatonin protection. Curr Neuropharmacol 8(3):194–210. doi:10.2174/157015910792246236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Rosales-Corral S, Manchester LC (2013) The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev Med Chem 13(3):373–384 [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J Pineal Res 61(3):253–278. doi:10.1111/jpi.12360 [DOI] [PubMed] [Google Scholar]
- Rinkel GJ, Algra A (2011) Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol 10(4):349–356. doi:10.1016/S1474-4422(11)70017-5 [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36(1):1–9 [DOI] [PubMed] [Google Scholar]
- Rojas H, Lekic T, Chen W, Jadhav V, Titova E, Martin RD, Tang J, Zhang J (2008) The antioxidant effects of melatonin after intracerebral hemorrhage in rats. Acta Neurochir Suppl 105:19–21 [DOI] [PubMed] [Google Scholar]
- Rosales-Corral SA, Acuna-Castroviejo D, Coto-Montes A, Boga JA, Manchester LC, Fuentes-Broto L, Korkmaz A, Ma S, Tan DX, Reiter RJ (2012) Alzheimer’s disease: pathological mechanisms and the beneficial role of melatonin. J Pineal Res 52(2):167–202. doi:10.1111/j.1600-079X.2011.00937.x [DOI] [PubMed] [Google Scholar]
- Samantaray S, Das A, Thakore NP, Matzelle DD, Reiter RJ, Ray SK, Banik NL (2009) Therapeutic potential of melatonin in traumatic central nervous system injury. J Pineal Res 47(2):134–142. doi:10.1111/j.1600-079X.2009.00703.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M (2008) Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 4(5):487–504 [PMC free article] [PubMed] [Google Scholar]
- Sehba FA, Hou J, Pluta RM, Zhang JH (2012) The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol 97(1):14–37. doi:10.1016/j.pneurobio.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal NK, Srivastava G, Agrawal S, Jain SK, Singh MP (2012) Melatonin as a neuroprotective agent in the rodent models of Parkinson’s disease: is it all set to irrefutable clinical translation? Mol Neurobiol 45(1):186–199. doi:10.1007/s12035-011-8225-x [DOI] [PubMed] [Google Scholar]
- Skaper SD, Ancona B, Facci L, Franceschini D, Giusti P (1998) Melatonin prevents the delayed death of hippocampal neurons induced by enhanced excitatory neurotransmission and the nitridergic pathway. FASEB J 12(9):725–731 [DOI] [PubMed] [Google Scholar]
- Tai SH, Chen HY, Lee EJ, Chen TY, Lin HW, Hung YC, Huang SY, Chen YH, Lee WT, Wu TS (2010) Melatonin inhibits postischemic matrix metalloproteinase-9 (MMP-9) activation via dual modulation of plasminogen/plasmin system and endogenous MMP inhibitor in mice subjected to transient focal cerebral ischemia. J Pineal Res 49(4):332–341. doi:10.1111/j.1600-079X.2010.00797.x [DOI] [PubMed] [Google Scholar]
- Tan DX, Chen LD, Poeggeler BL, Manchester LC, Reiter RJ (1993a) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J 1(4):57–60. doi:10.1111/j.1749-6632.1994.tb21831.x [Google Scholar]
- Tan DX, Poeggeler B, Reiter RJ, Chen LD, Chen S, Manchester LC, Barlow-Walden LR (1993b) The pineal hormone melatonin inhibits DNA-adduct formation induced by the chemical carcinogen safrole in vivo. Cancer Lett 70(1–2):65–71 [DOI] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ (2003) Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res 34(1):75–78 [DOI] [PubMed] [Google Scholar]
- Tang J, Liu J, Zhou C, Ostanin D, Grisham MB, Neil Granger D, Zhang JH (2005) Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. J Neurochem 94(5):1342–1350. doi:10.1111/j.1471-4159.2005.03292.x [DOI] [PubMed] [Google Scholar]
- Terruso V, D’Amelio M, Di Benedetto N, Lupo I, Saia V, Famoso G, Mazzola MA, Aridon P, Sarno C, Ragonese P, Savettieri G (2009) Frequency and determinants for hemorrhagic transformation of cerebral infarction. Neuroepidemiology 33(3):261–265. doi:10.1159/000229781 [DOI] [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333(24):1581–1587. doi:10.1056/NEJM199512143332401 [DOI] [PubMed]
- Trotti LM, Karroum EG (2016) Melatonin for sleep disorders in patients with neurodegenerative diseases. Curr Neurol Neurosci Rep 16(7):63. doi:10.1007/s11910-016-0664-3 [DOI] [PubMed] [Google Scholar]
- Tsai MC, Chen WJ, Tsai MS, Ching CH, Chuang JI (2011) Melatonin attenuates brain contusion-induced oxidative insult, inactivation of signal transducers and activators of transcription 1, and upregulation of suppressor of cytokine signaling-3 in rats. J Pineal Res 51(2):233–245. doi:10.1111/j.1600-079X.2011.00885.x [DOI] [PubMed] [Google Scholar]
- Tunez I, Montilla P, Del Carmen Munoz M, Feijoo M, Salcedo M (2004) Protective effect of melatonin on 3-nitropropionic acid-induced oxidative stress in synaptosomes in an animal model of Huntington’s disease. J Pineal Res 37(4):252–256. doi:10.1111/j.1600-079X.2004.00163.x [DOI] [PubMed] [Google Scholar]
- Turjanski AG, Rosenstein RE, Estrin DA (1998) Reactions of melatonin and related indoles with free radicals: a computational study. J Med Chem 41(19):3684–3689. doi:10.1021/jm980117m [DOI] [PubMed] [Google Scholar]
- Ueda Y, Masuda T, Ishida A, Misumi S, Shimizu Y, Jung CG, Hida H (2014) Enhanced electrical responsiveness in the cerebral cortex with oral melatonin administration after a small hemorrhage near the internal capsule in rats. J Neurosci Res 92(11):1499–1508. doi:10.1002/jnr.23434 [DOI] [PubMed] [Google Scholar]
- van Gijn J, Kerr RS, Rinkel GJ (2007) Subarachnoid haemorrhage. Lancet 369(9558):306–318. doi:10.1016/S0140-6736(07)60153-6 [DOI] [PubMed] [Google Scholar]
- Wang J, Dore S (2007) Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab 27(5):894–908. doi:10.1038/sj.jcbfm.9600403 [DOI] [PubMed] [Google Scholar]
- Wang X, Lo EH (2003) Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol 28(3):229–244. doi:10.1385/MN:28:3:229 [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma C, Meng CJ, Zhu GQ, Sun XB, Huo L, Zhang J, Liu HX, He WC, Shen XM, Shu Z, Chen G (2012) Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J Pineal Res 53(2):129–137. doi:10.1111/j.1600-079X.2012.00978.x [DOI] [PubMed] [Google Scholar]
- Wang X, Wang ZH, Wu YY, Tang H, Tan L, Wang X, Gao XY, Xiong YS, Liu D, Wang JZ, Zhu LQ (2013a) Melatonin attenuates scopolamine-induced memory/synaptic disorder by rescuing EPACs/miR-124/Egr1 pathway. Mol Neurobiol 47(1):373–381. doi:10.1007/s12035-012-8355-9 [DOI] [PubMed] [Google Scholar]
- Wang Z, Wu L, You W, Ji C, Chen G (2013b) Melatonin alleviates secondary brain damage and neurobehavioral dysfunction after experimental subarachnoid hemorrhage: possible involvement of TLR4-mediated inflammatory pathway. J Pineal Res 55(4):399–408. doi:10.1111/jpi.12087 [DOI] [PubMed] [Google Scholar]
- Wang W, Li M, Chen Q, Wang J (2015) Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke: mechanisms, models, and biomarkers. Mol Neurobiol 52(3):1572–1579. doi:10.1007/s12035-014-8952-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman JK, Schlichter LC (2007) Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Exp Neurol 207(2):227–237. doi:10.1016/j.expneurol.2007.06.025 [DOI] [PubMed] [Google Scholar]
- Weishaupt JH, Bartels C, Polking E, Dietrich J, Rohde G, Poeggeler B, Mertens N, Sperling S, Bohn M, Huther G, Schneider A, Bach A, Siren AL, Hardeland R, Bahr M, Nave KA, Ehrenreich H (2006) Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J Pineal Res 41(4):313–323. doi:10.1111/j.1600-079X.2006.00377.x [DOI] [PubMed] [Google Scholar]
- Wu H, Shao A, Zhao M, Chen S, Yu J, Zhou J, Liang F, Shi L, Dixon BJ, Wang Z, Ling C, Hong Y, Zhang J (2016) Melatonin attenuates neuronal apoptosis through up-regulation of K+-Cl− cotransporter KCC2 expression following traumatic brain injury in rats. J Pineal Res 61(2):241–250. doi:10.1111/jpi.12344 [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 5(1):53–63. doi:10.1016/S1474-4422(05)70283-0 [DOI] [PubMed] [Google Scholar]
- Zacharia BE, Hickman ZL, Grobelny BT, DeRosa P, Kotchetkov I, Ducruet AF, Connolly ES Jr (2010) Epidemiology of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am 21(2):221–233. doi:10.1016/j.nec.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li X, Grailer JJ, Wang N, Wang M, Yao J, Zhong R, Gao GF, Ward PA, Tan DX, Li X (2016) Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J Pineal Res 60(4):405–414. doi:10.1111/jpi.12322 [DOI] [PubMed] [Google Scholar]
- Zhao L, An R, Yang Y, Yang X, Liu H, Yue L, Li X, Lin Y, Reiter RJ, Qu Y (2015) Melatonin alleviates brain injury in mice subjected to cecal ligation and puncture via attenuating inflammation, apoptosis, and oxidative stress: the role of SIRT1 signaling. J Pineal Res 59(2):230–239. doi:10.1111/jpi.12254 [DOI] [PubMed] [Google Scholar]
- Zhou C, Yamaguchi M, Kusaka G, Schonholz C, Nanda A, Zhang JH (2004) Caspase inhibitors prevent endothelial apoptosis and cerebral vasospasm in dog model of experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab 24(4):419–431. doi:10.1097/00004647-200404000-00007 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW (2014) Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol 115:25–44. doi:10.1016/j.pneurobio.2013.11.003 [DOI] [PubMed] [Google Scholar]