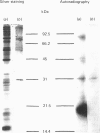
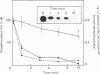
Abstract
A new improved method for purification of the enzyme 1-O-alkyl-2-lyso-sn-glycero-3-phosphocholine: acetyl-CoA acetyltransferase (EC 2.3.1.67) from rat spleen is described. The catalytic subunit of cyclic AMP-dependent protein kinase in the presence of MgATP stimulated about 3-fold the activity of this partially purified enzyme activity. When [gamma-32P]ATP was included in the assay mixture, the analysis of phosphoprotein products by SDS/polyacrylamide-gel electrophoresis and autoradiography showed the incorporation of [32P]phosphate into a single protein band of about 30 kDa. Analysis of the phosphorylated amino acids indicated that the phosphate was incorporated into a serine residue. Activation of the acetylation reaction by the protein kinase was reversible. The reversal of the activation was coincident with the loss of the [32P]phosphate incorporated into the 30 kDa protein band, which suggests that the acetyltransferase is regulated by a phosphorylation-dephosphorylation mechanism dependent on cyclic AMP.
Full text
PDF

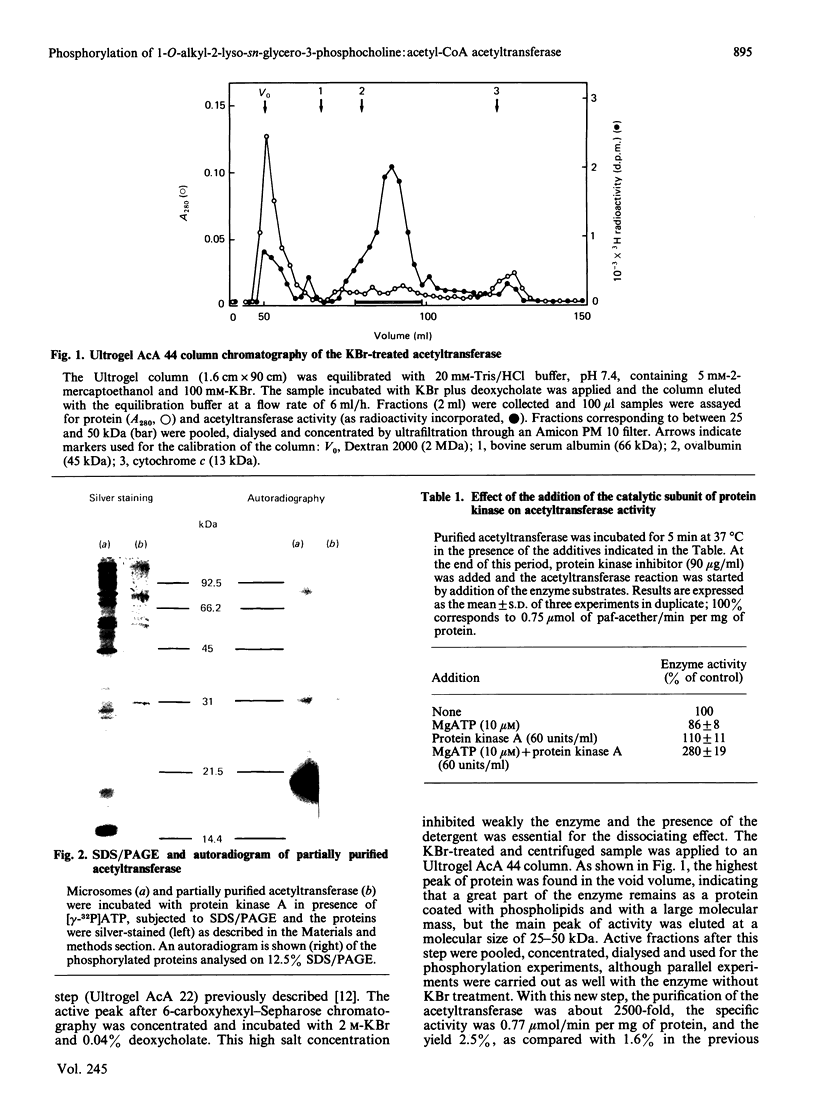
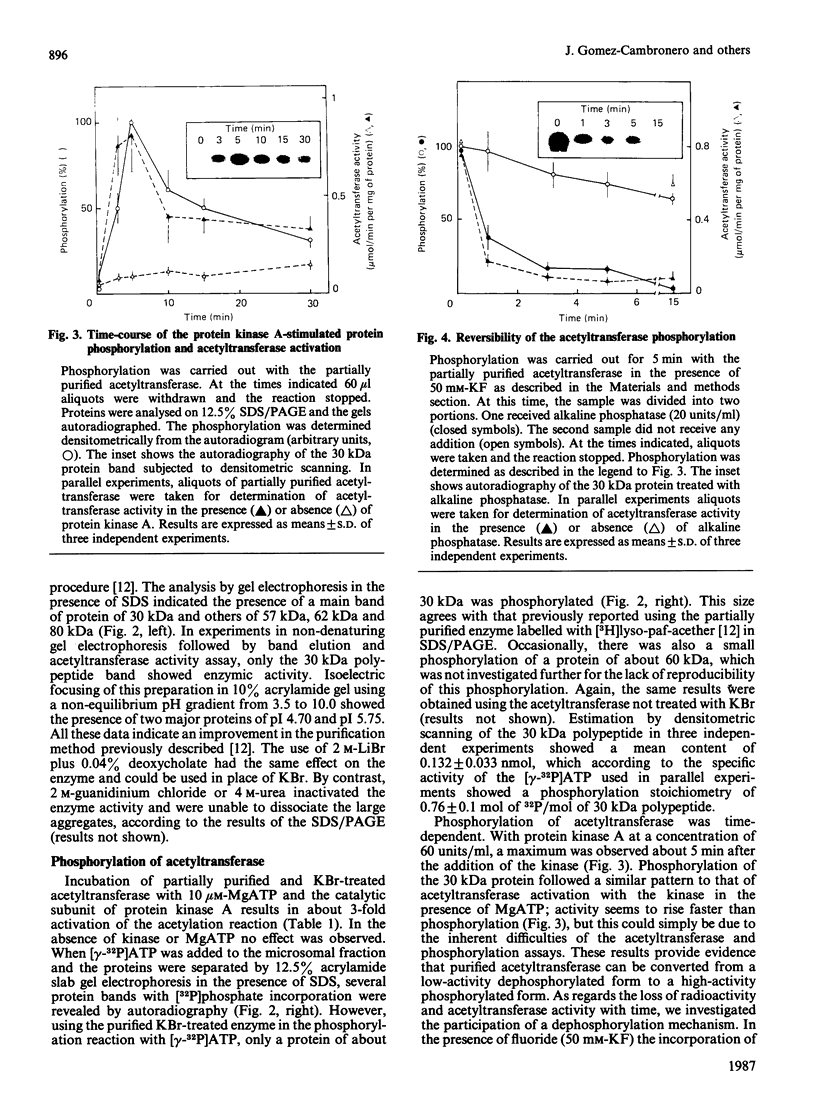

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad H., Saleemuddin M. A Coomassie blue-binding assay for the microquantitation of immobilized proteins. Anal Biochem. 1985 Aug 1;148(2):533–541. doi: 10.1016/0003-2697(85)90264-7. [DOI] [PubMed] [Google Scholar]
- Alonso F., Gil M. G., Sánchez-Crespo M., Mato J. M. Activation of 1-alkyl-2-lysoglycero-3-phosphocholine. Acetyl-CoA transferase during phagocytosis in human polymorphonuclear leukocytes. J Biol Chem. 1982 Apr 10;257(7):3376–3378. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Domenech C., Machado-De Domenech E., Söling H. D. Regulation of acetyl-CoA:1-alkyl-sn-glycero-3-phosphocholine O2-acetyltransferase (lyso-PAF-acetyltransferase) in exocrine glands. Evidence for an activation via phosphorylation by calcium/calmodulin-dependent protein kinase. J Biol Chem. 1987 Apr 25;262(12):5671–5676. [PubMed] [Google Scholar]
- Gomez-Cambronero J., Velasco S., Sanchez-Crespo M., Vivanco F., Mato J. M. Partial purification and characterization of 1-O-alkyl-2-lyso-sn-glycero-3-phosphocholine:acetyl-CoA acetyltransferase from rat spleen. Biochem J. 1986 Jul 15;237(2):439–445. doi: 10.1042/bj2370439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Cambronero J., Iñarrea P., Alonso F., Sánchez Crespo M. The role of calcium ions in the process of acetyltransferase activation during the formation of platelet-activating factor (PAF-acether). Biochem J. 1984 Apr 15;219(2):419–424. doi: 10.1042/bj2190419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Cambronero J., Nieto M. L., Mato J. M., Sánchez-Crespo M. Modulation of lyso-platelet-activating factor: acetyl-CoA acetyltransferase from rat splenic microsomes. The role of calcium ions. Biochim Biophys Acta. 1985 Jun 30;845(3):511–515. doi: 10.1016/0167-4889(85)90218-6. [DOI] [PubMed] [Google Scholar]
- Gómez-Cambronero J., Velasco S., Mato J. M., Sánchez-Crespo M. Modulation of lyso-platelet activating factor: acetyl-CoA acetyltransferase from rat splenic microsomes. The role of cyclic AMP-dependent protein kinase. Biochim Biophys Acta. 1985 Jun 30;845(3):516–519. doi: 10.1016/0167-4889(85)90219-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. C., Malone B., Snyder F. A new de novo pathway for the formation of 1-alkyl-2-acetyl-sn-glycerols, precursors of platelet activating factor. Biochemical characterization of 1-alkyl-2-lyso-sn-glycero-3-P:acetyl-CoA acetyltransferase in rat spleen. J Biol Chem. 1986 Apr 25;261(12):5373–5377. [PubMed] [Google Scholar]
- Lenihan D. J., Lee T. C. Regulation of platelet activating factor synthesis: modulation of 1-alkyl-2-lyso-sn-glycero-3-phosphocholine:acetyl-CoA acetyltransferase by phosphorylation and dephosphorylation in rat spleen microsomes. Biochem Biophys Res Commun. 1984 May 16;120(3):834–839. doi: 10.1016/s0006-291x(84)80182-5. [DOI] [PubMed] [Google Scholar]
- Ninio E., Mencia-Huerta J. M., Heymans F., Benveniste J. Biosynthesis of platelet-activating factor. I. Evidence for an acetyl-transferase activity in murine macrophages. Biochim Biophys Acta. 1982 Jan 15;710(1):23–31. doi: 10.1016/0005-2760(82)90185-0. [DOI] [PubMed] [Google Scholar]
- Pajares M. A., Villalba M., Mato J. M. Purification of phospholipid methyltransferase from rat liver microsomal fraction. Biochem J. 1986 Aug 1;237(3):699–705. doi: 10.1042/bj2370699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbes G., Ninio E., Fontan P., Record M., Chap H., Benveniste J., Douste-Blazy L. Evidence that biosynthesis of platelet-activating factor (paf-acether) by human neutrophils occurs in an intracellular membrane. FEBS Lett. 1985 Oct 28;191(2):195–199. doi: 10.1016/0014-5793(85)80007-7. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Weissmann G. Stimuli which provoke secretion of azurophil enzymes from human neutrophils induce increments in adenosine cyclic 3'-5'-monophosphate. Biochim Biophys Acta. 1981 Jan 21;672(2):197–206. doi: 10.1016/0304-4165(81)90393-7. [DOI] [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Thomas G., Thomas G., Luther H. Transcriptional and translational control of cytoplasmic proteins after serum stimulation of quiescent Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5712–5716. doi: 10.1073/pnas.78.9.5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykle R. L., Malone B., Snyder F. Enzymatic synthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, a hypotensive and platelet-aggregating lipid. J Biol Chem. 1980 Nov 10;255(21):10256–10260. [PubMed] [Google Scholar]
- Yeaman S. J., Cohen P., Watson D. C., Dixon G. H. The substrate specificity of adenosine 3':5'-cyclic monophosphate-dependent protein kinase of rabbit skeletal muscle. Biochem J. 1977 Feb 15;162(2):411–421. doi: 10.1042/bj1620411. [DOI] [PMC free article] [PubMed] [Google Scholar]