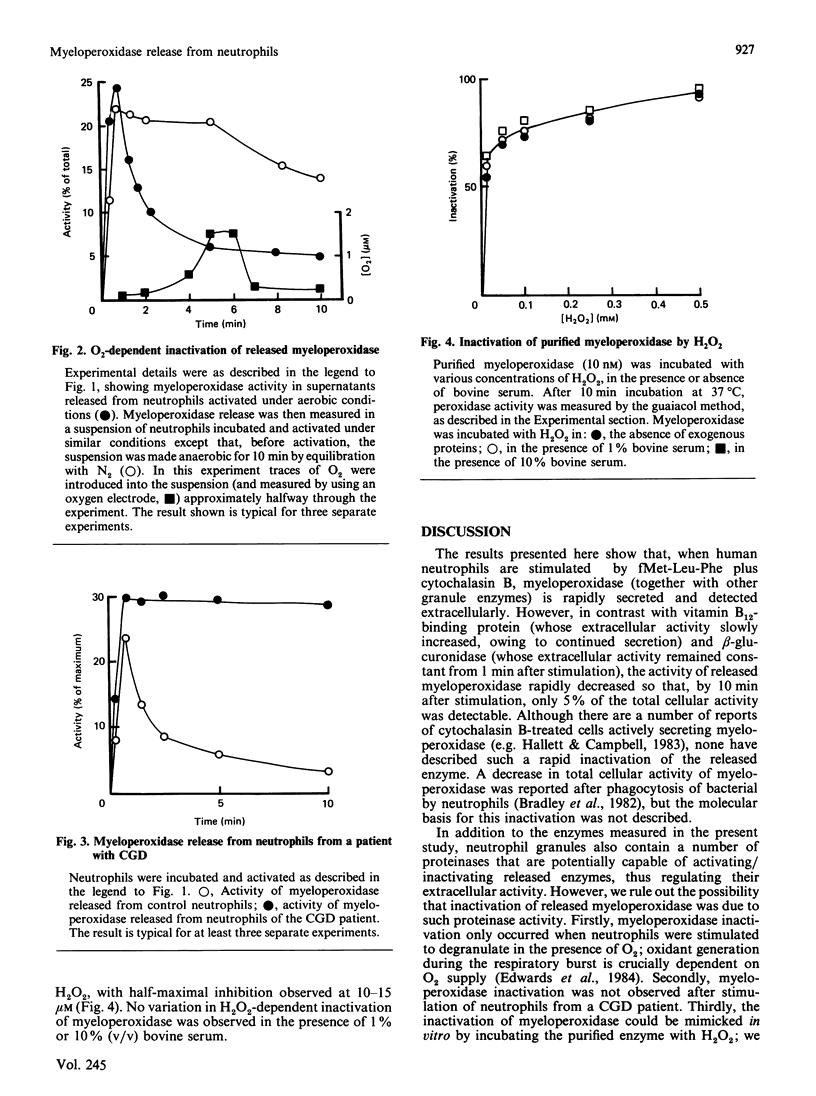
Abstract
Within 1 min of stimulation of human neutrophils by the chemotactic peptide (N-formyl-L-methionyl-L-leucyl-L-phenylalanine) plus cytochalasin B, myeloperoxidase (together with other granule enzymes) was secreted and detected extracellularly. In contrast with the other granule constituents assayed (vitamin B12-binding protein and beta-glucuronidase), the activity of released myeloperoxidase rapidly decreased, so that, by 10 min after stimulation, only about 5% of the total cellular activity was detected. This inactivation was shown to be dependent on oxidant generation during the respiratory burst, since inactivation was not observed (a) after stimulation of anaerobic suspensions or (b) after release from neutrophils from a patient with chronic granulomatous disease; purified myeloperoxidase was rapidly inactivated after incubation with H2O2, presumably owing to the formation of an inactive enzyme-H2O2 complex. These results show that experiments designed to assess the role of myeloperoxidase in neutrophil functions which utilize assays based on peroxidase activity will grossly underestimate this enzyme if oxidant generation during the respiratory burst has also been activated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bradley P. P., Christensen R. D., Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982 Sep;60(3):618–622. [PubMed] [Google Scholar]
- Cech P., Papathanassiou A., Boreux G., Roth P., Miescher P. A. Hereditary myeloperoxidase deficiency. Blood. 1979 Mar;53(3):403–411. [PubMed] [Google Scholar]
- Clark R. A., Borregaard N. Neutrophils autoinactivate secretory products by myeloperoxidase-catalyzed oxidation. Blood. 1985 Feb;65(2):375–381. [PubMed] [Google Scholar]
- Dri P., Soranzo M. R., Cramer R., Menegazzi R., Miotti V., Patriarca P. Role of myeloperoxidase in respiratory burst of human polymorphonuclear leukocytes. Studies with myeloperoxidase-deficient subjects. Inflammation. 1985 Mar;9(1):21–31. doi: 10.1007/BF00915408. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Hallett M. B., Campbell A. K. Oxygen-radical production during inflammation may be limited by oxygen concentration. Biochem J. 1984 Feb 1;217(3):851–854. doi: 10.1042/bj2170851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Lloyd D. Formation of myeloperoxidase compound II during aerobic stimulation of rat neutrophils. Biosci Rep. 1986 Mar;6(3):275–282. doi: 10.1007/BF01115156. [DOI] [PubMed] [Google Scholar]
- Edwards S. W. Luminol- and lucigenin-dependent chemiluminescence of neutrophils: role of degranulation. J Clin Lab Immunol. 1987 Jan;22(1):35–39. [PubMed] [Google Scholar]
- Edwards S. W., Swan T. F. Regulation of superoxide generation by myeloperoxidase during the respiratory burst of human neutrophils. Biochem J. 1986 Jul 15;237(2):601–604. doi: 10.1042/bj2370601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTLIEBLAU K. S., WASSERMAN L. R., HERBERT V. RAPID CHARCOAL ASSAY FOR INTRINSIC FACTOR (IF), GASTRIC JUICE UNSATURATED B12 BINDING CAPACITY, ANTIBODY TO IF, AND SERUM UNSATURATED B12 BINDING CAPACITY. Blood. 1965 Jun;25:875–884. [PubMed] [Google Scholar]
- Hallett M. B., Campbell A. K. Two distinct mechanisms for stimulation of oxygen-radical production by polymorphonuclear leucocytes. Biochem J. 1983 Nov 15;216(2):459–465. doi: 10.1042/bj2160459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Olsen R. L., Steigen T. K., Holm T., Little C. Molecular forms of myeloperoxidase in human plasma. Biochem J. 1986 Jul 15;237(2):559–565. doi: 10.1042/bj2370559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pember S. O., Shapira R., Kinkade J. M., Jr Multiple forms of myeloperoxidase from human neutrophilic granulocytes: evidence for differences in compartmentalization, enzymatic activity, and subunit structure. Arch Biochem Biophys. 1983 Mar;221(2):391–403. doi: 10.1016/0003-9861(83)90158-3. [DOI] [PubMed] [Google Scholar]
- Peppin G. J., Weiss S. J. Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4322–4326. doi: 10.1073/pnas.83.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmaszyńska T., Zgliczyński J. M. Myeloperoxidase of human neutrophilic granulocytes as chlorinating enzyme. Eur J Biochem. 1974 Jun 1;45(1):305–312. doi: 10.1111/j.1432-1033.1974.tb03555.x. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Peppin G., Ortiz X., Ragsdale C., Test S. T. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985 Feb 15;227(4688):747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta. 1985 Jun 18;840(2):204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Garcia R. C., Segal A. W. Production of the superoxide adduct of myeloperoxidase (compound III) by stimulated human neutrophils and its reactivity with hydrogen peroxide and chloride. Biochem J. 1985 Jun 15;228(3):583–592. doi: 10.1042/bj2280583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Myeloperoxidase as an effective inhibitor of hydroxyl radical production. Implications for the oxidative reactions of neutrophils. J Clin Invest. 1986 Aug;78(2):545–550. doi: 10.1172/JCI112607. [DOI] [PMC free article] [PubMed] [Google Scholar]


