Keywords: cardiovascular risk, type 1/type 2 diabetes mellitus, epidemiology, impaired fasting glucose, obesity
Abstract
The CA.ME.LI.A (CArdiovascular risks, MEtabolic syndrome, LIver and Autoimmune disease) epidemiological study was conducted in Abbiategrasso (Milan, Italy) to identify risk factors for metabolic and cardiovascular disease in an apparently healthy population of northern Italy. The population (n = 2,545, 1,251 men, 1,254 women) was stratified according to body mass index [normal body weight (NBW): <25 kg/m2; overweight-obese (OWO): ≥25 kg/m2] and according to fasting blood glucose [normal fasting glucose: <100 mg/dL; impaired fasting glucose (IFG): 100–125 mg/dL; diabetes mellitus (DM): ≥126 mg/dL]. The incidence of cardiovascular (CV) events and overall mortality were studied by the Kaplan–Meier method using the log rank test. Univariate analysis was conducted with time-dependent Cox models. During the 7-yr follow-up period, 80 deaths and 149 CV events occurred. IFG [hazard ratio (HR): 2.81; confidence interval (CI): 1.37–5.77; P = 0.005], DM (HR: 4.88; CI: 1.47–16; P = 0.010), or OWO (HR: 2.78; CI:1.68–4.59; P < 0.001) all produced significant increases in CV events and deaths. In the combination IFG/OWO (HR: 5.51; CI: 3.34–9.08; P < 0.001), there was an apparent additive effect of the two conditions, whereas in the combination DM/OWO (HR: 12.71; CI: 7.48–22; P < 0.001), there was an apparent multiplicative effect on the risk for CV events and deaths. In males, the DM/NBW group had a higher incidence of cardiovascular events and deaths than the IFG/OWO group. In contrast, in females, the IFG/OWO group had a higher incidence of cardiovascular events and deaths than the DM/NBW group. In women, there was a greater incidence of CV events in the IFG/OWO group (HR: 6.23; CI: 2.88–13; P < 0.001) than in men in the same group (HR: 4.27; CI: 2.15–8.47; P < 0.001). Consistent with these data, also all-cause mortality was progressively increased by IFG/DM and OWO, with an apparently exponential effect in the combination DM/OWO (HR: 11.78; CI: 6.11–23; P < 0.001). IFG/DM and OWO, alone or in combination, had major effects in increasing mortality for all causes and CV events. The relative contributions of hyperglycemia and overweight/obesity on cardiovascular events and deaths were apparently, to a certain extent, sex dependent. Females were more affected by overweight/obesity either alone or combined with IFG, as compared with males.
NEW & NOTEWORTHY For the first time, the combined effects of glucose tolerance and BMI have been investigated in an apparently healthy large population sample of a city in the north of Italy. We found that there are synergistic effects of glucose levels with BMI to increase not only cardiovascular events and deaths but also cancer-related deaths and all-cause mortality.
INTRODUCTION
Obesity and overweight have reached epidemic proportions, and their growth shows no signs of slowing down.
It has been estimated that in 2022, 2.5 billion adults were overweight or obese (1/3 of the entire world), and of these, 890 million (12%) were obese, also affected by associated comorbidities (1). Obesity is associated with a two- to threefold increased risk for death from all causes, including cardiovascular diseases, chronic kidney disease, sleep apnea, cancer, and type 2 diabetes mellitus (T2DM) (2, 3). Of significance, obesity carries also an important psychosocial burden, thus impacting numerous areas of psychosocial functioning (4).
Diabetes mellitus has emerged as one of the most serious and common chronic diseases of our times, causing life-threatening, disabling, and costly complications also significantly reducing life expectancy (5–9). Just over half a billion people are living with diabetes (type 1 or type 2) worldwide, and its prevalence is expected to rise to 12.2% (783.2 million) in 2045 (10). In addition, 541 million people are estimated to be affected by prediabetes, either impaired fasting glucose (IFG), which is characterized by fasting plasma glucose level between 100 and 125 mg/dL, or impaired glucose tolerance (IGT), that is a glucose level between 140 and 200 mg/dL at 2 h after a 75 g glucose tolerance test (10). Type 2 diabetes mellitus can be diagnosed from fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) and/or >200 mg/dL after an oral glucose tolerance test or hemoglobin A1c >6.4% (46 mmol/mol) (11).
In the early 2000s, the term “diabesity” was proposed to indicate the intimate interactive role that obesity has with the development of type 2 diabetes mellitus (12). Chronic subinflammation in the adipose tissue, leading to imbalanced secretion of adipokines, resulting in increased levels of proinflammatory cytokines and decreased levels of anti-inflammatory adipokines, is considered a crucial risk factor for the development of insulin resistance and type 2 diabetes mellitus in obese individuals (13–16).
Individuals with IGT and/or IFG are at higher risk to develop diabetes mellitus and metabolic syndrome X of insulin resistance and to experience cardiovascular (CV) events (myocardial infarction, stroke, CV death) later in life (17–22). Also, obesity is a well-established independent risk factor for development of a variety of cardiovascular diseases (CVD), including heart failure, coronary heart disease, atrial fibrillation, and arterial hypertension (3, 23–25).
CA.ME.LI.A (CArdiovascular risks, MEtabolic syndrome, LIver and Autoimmune disease) is an epidemiological study conducted between 2009 and 2011 in the city of Abbiategrasso (Milan, Italy). The prevalence of cardiovascular risk factors, metabolic syndrome X of insulin resistance, and liver diseases in the CA.ME.LI.A study population has been reported elsewhere (26). Here, we present the findings of the 7-yr follow-up conducted in the CA.ME.LI.A population. The study aims to investigate the impact of hyperglycemia (IFG and DM) and increased body mass (overweight/obesity) on the incidence of cardiovascular events and all-cause mortality, including cardiovascular events (heart and cerebrovascular), cancer, and other diseases.
MATERIALS AND METHODS
The CA.ME.LI.A Population
The CA.ME.LI.A study organization, planning, medical examination, and participants’ baseline clinical, ultrasound, and biochemical data have been previously reported (26). The participants’ baseline data were collected between May 2009 and September 2011 (26). The data from the follow-up study period are reported here. All events that occurred from the day of enrollment until August 30, 2017, retrievable in the digital records of the Milan 1 Health District, based on the disease diagnosis coding system of the hospital discharge forms (in Italian: “Scheda Dimissione Ospedaliera” SDO), were considered. The compilation of the “SDO” (hospital discharge form) was based on ICD-9-CM (International Classification of Diseases, 9th Revision, Clinical Modification) codes as previously reported (26). For the present report, causes of death were then coded according to the ICD-10 (International Classification of Diseases, 10th Revision) coding system.
To study the relationships between various degrees of glucose tolerance and overweight/obesity, the population was stratified according to body mass index (BMI) and fasting plasma glucose (3, 27, 28). BMI: 1) normal body weight subjects (NBW): BMI of <25 kg/m2; 2) overweight and obese subjects (OWO): BMI of ≥25 kg/m2. Fasting plasma glucose: 1) normal fasting glucose subjects (NFG): fasting plasma glucose level of <100 mg/dL; 2) impaired fasting glucose subjects (IFG): fasting plasma glucose level of 100–125 mg/dL; and 3) type 2 diabetes mellitus (DM) subjects: fasting plasma glucose level of ≥126 mg/dL.
To verify the potential interaction of BMI with fasting plasma glucose, analyses were performed by considering all possible (six) combinations: 1) NFG/NBW, 2) NFG/OWO, 3) IFG/NBW, 4) IFG/OWO, 5) DM/NBW, and 6) DM/OWO.
Data Analysis, Visualization, and Interpretation
Data were expressed as counts, percentages, prevalence ratios, and 95% confidence intervals (CIs). Continuous variables are summarized as mean values and standard deviations (SDs). In univariate analyses, the significance level of differences among groups was assessed by the nonparametric Mann–Whitney or Kruskal–Wallis test. To assess the presence of a trend across groups of patients ordered according to their glucose tolerance and BMI levels, the Kruskal–Wallis test was preliminarily performed on the variable of interest, followed, in the case of a significant result, by a nonparametric test for trend based on a method described by Cuzick (29). For categorical variables, the Fisher’s exact test was employed. A modified Poisson approach was used to estimate relative risks, either as a crude value or after covariate adjustment, and their CI and significance levels were assessed using robust error variances.
The study of the incidence of events was carried out using the Kaplan–Meier method, with the log-rank test to assess statistical significance levels. For this study, time 0 was the date of enrollment in the CA.ME.LI.A study. The predefined final observation dates were considered that of the first cardiovascular event, or the date of death for cardiovascular causes, or the death for all other causes.
For the univariate analysis of the association between any continuous or categorical variable, considered as the risk factor, and the incidence of each outcome event, the aforementioned risk factors were introduced in single-variable, time-dependent Cox models.
To identify the variables retaining an independent prognostic value, those associated (P < 0.10) in the univariate analyses with the incidence of an outcome event were studied within the framework of multivariate analysis by means of time-dependent multivariate Cox models.
The study protocol established that, in consideration of the multiple associations between the variables under study and the sex of the subjects, the main analyses were conducted separately in the two sexes. To contextualize the study data, assuming an incidence of events or cardiovascular death of 10% during 7 yr after enrollment, and to demonstrate significance (P < 0.05, power of 90%), a difference of ≥5% between the incidence of events in the two groups, it was calculated that 1,189 subjects would be needed using the two-tailed log-rank test (30). In the present study, 1,257 male and 1,297 female subjects were enrolled for all analyses. The level of statistical significance was P < 0.05 in two-tailed tests.
Collected data were exported, and statistical analyses were carried out using the Stata software (StataCorp. 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC).
RESULTS
The study population included 2,554 subjects (1,257 men and 1,297 women) residing in the city of Abbiategrasso (Milano, Italy). The follow-up period extended from May 2009 to August 30, 2017, with a median duration of 7.4 yr, an interquartile range of 6.8–7.8 yr, and a maximum follow-up duration of 8.3 yr. Overall, the population was followed for 17,776 person-years. During this period, 35 subjects were lost to follow-up.
Analysis of the Population According to Glucose Tolerance and BMI
The population included 2,554 individuals (51% women and 49% men) aged 18–77 yr, (mean ± SD = all patients: 47.7 ± 14.9 yr; women: 48.0 ± 15.1 yr; men: 47.3 ± 15.1 yr; P = 0.221). After stratification in different age-groups (<35, 35–44, 45–54, 55–64, and ≥65 yr), gender distribution did not differ significantly, but a highly significant difference emerged when considering fasting plasma glucose and BMI categories (Supplemental Table S1). Such gender difference was evident in the older age class (≥ 65 yr), in which women were more prevalent. Regarding glucose levels, around two-third (70.4%) of the study participants had NFG, followed by IFG (22.9%) and DM (6.7%) categories (Table 1). Considering body mass index, the population was divided in NBW (48.9%) and OWO (51.1%). Regarding gender distribution, males were more prevalent in the IFG, DM, and OWO categories, whereas females were more prevalent in the NFG and NBW categories (P ≤ 0.0001) (Table 1 and Supplemental Fig. S1, A and B). Participants’ age increased as fasting glucose levels and BMI increased, with some differences in males and females (Supplemental Fig. S1, C and D).
Table 1.
Gender distribution of the CA.ME.LI.A population based on glucose tolerance and BMI
| Total Population | Men | Women | P Value | |
|---|---|---|---|---|
| n | 2,553 | 1,257 | 1,296* |
|
| NFG | 1,796 (70.4%) | 770 (61.3%) | 1,026 (79.2%) | <0.0001 |
| IFG | 585 (22.9%) | 377 (30.0%) | 208 (16.0%) | |
| DM | 172 (6.7%) | 110 (8.7%) | 62 (4.8%) | |
| NBW (BMI of <25 kg/m2) | 1,250 (48.9%) | 492 (39.1%) | 758 (58.4%) | <0.0001 |
| OWO (BMI of ≥25 kg/m2) | 1,304 (51.1%) | 765 (60.9%) | 539 (41.6%) |
BMI, body mass index; CA.ME.LI.A, CArdiovascular risks, MEtabolic syndrome, LIver and Autoimmune disease; DM, diabetes mellitus; IFG, impaired fasting glucose; NBW, normal body weight; NFG, normal fasting glucose; OWO, overweight-obese.
*One woman lacking glycemic data.
In the population with BMI of <25 kg/m2 (NBW), 30%–40% of subjects, in both sexes, were younger than 35 yr. In men with BMI of >25 kg/m2 (OWO), the frequency of older subjects progressively increased up to the 45–54 yr class, but it subsequently decreased. Among OWO women, the trend toward increasing frequency of aged subjects was consistent over the whole age class spectrum (Supplemental Fig. S1D). The cause of the fluctuating trend of the age distribution in overweight/obese class among men, first “in crescendo” and then “in diminuendo,” could be ascribed possibly to the higher mortality rate of men versus women after 55 yr. In women with BMI of ≥25 kg/m2, the trend is opposite to that seen for BMI of <25 kg/m2, and the frequency of subjects included in the age categories increased with age.
In the different glucose categories (NFG, IFG, DM), NFG females had a lower BMI than NFG males (P ≤ 0.0001) (Supplemental Fig. S2). IFG males and females had similar BMI, whereas DM females had higher BMI than males. Of note, BMI of females showed greater variability than that of males.
The Interrelationship between Glucose Levels and BMI
To better understand the relationship between impaired fasting glucose and diabetes mellitus (IFG/DM) with overweight/obesity, the CA.ME.LI.A population was stratified into six subgroups, based on fasting glucose levels and BMI. The number of males was higher in the five groups with either increased glucose or increased BMI, whereas in the group with both normal glucose tolerance and normal body weight (NFG/NBW), females were significantly more represented than males (P ≤ 0.0001) (Table 2).
Table 2.
CA.ME.LI.A population stratified by gender, BMI, and glucose tolerance
| Total Population | Men | Women | P Value | |
|---|---|---|---|---|
| n | 2,545* | 1,251 | 1,294 |
|
| NFG/NBW | 1,039 (40.8%) | 372 (29.7%) (14.5%) | 667 (51.6%) (26.2%) | <0.0001 |
| NFG/OWO | 754 (29.6%) | 395 (31.6%) (15.5%) | 359 (27.7%) (14.0%) | |
| IFG/NBW | 183 (7.2%) | 105 (8.4%) (4.1%) | 78 (6.0%) (3.0%) | |
| IFG/OWO | 398 (15.7%) | 269 (21.5%) (10.5%) | 129 (10.0%) (5.1%) | |
| DM/NBW | 28 (1.1%) | 15 (1.2%) (0.5%) | 13 (1.0%) (0.5%) | |
| DM/OWO | 143 (5.6%) | 95 (7.6%) (3.7%) | 48 (1.9%) (3.7%) |
The % was calculated on the total number of subjects in the group (column). For men and women columns, an additional % vs. the whole population is also reported on the left. BMI, body mass index; CA.ME.LI.A, CArdiovascular risks, MEtabolic syndrome, LIver and Autoimmune disease; DM, diabetes mellitus; IFG, impaired fasting glucose; NBW, normal body weight; NFG, normal fasting glucose; OWO, overweight-obese.
*Nine patients of the whole CA.ME.LI.A population were excluded from the analysis for the lack of data, either BMI or glucose tolerance.
Table 3 and Supplemental Table S2 (a and b) and Supplemental Table S3 show anthropometric, clinical, and laboratory characteristics of the six subgroups in the CA.ME.LI.A population. Mean glucose levels increased in parallel with age. OWO was associated with increased glucose levels in NFG, IFG, and DM, as compared with NBW subjects. Values of insulin and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) progressively increased going from NFG to IFG and to DM. For each glucose category, i.e., NFG, IFG, and DM, those who had a BMI of <25 kg/m2 showed lower insulin and HOMA IR as compared with those with a BMI of ≥25 kg/m2. A similar trend was also present for triglyceride levels, with the highest level in DM/OWO subjects. HDL cholesterol showed a trend toward decreasing values with increasing glucose levels, both in NBW and OWO subjects. Incidence of C-reactive protein (CRP) >5 mg/L, of metabolic (dysfunction)-associated fatty liver disease (MAFLD; previously called nonalcoholic fatty liver disease) (31, 32), and of arterial hypertension showed a significant progressive increase from NFG to IFG and to DM. Both conditions were further markedly increased in each class of glucose levels when OWO was also present (all, P < 0.0001).
Table 3.
Clinical and laboratory characteristics of CA.ME.LI.A population stratified by glucose tolerance and BMI
| NFG/NBW | NFG/OWO | IFG/NBW | IFG/OWO | DM/NBW | DM/OWO | |
|---|---|---|---|---|---|---|
| n | 1,039 |
754 |
183 |
398 |
28 | 143 |
| Age, yr (P ≤ 0.0001) | 40.6 ± 13.6 | 48.9 ± 14 | 51.6 ± 14.5 | 55.9 ± 12 | 56.9 ± 13.1 | 62.1 ± 9.4 |
| Weight, kg (P ≤ 0.0001) | 60.9 ± 9.2 | 80.6 ± 12.7 | 64.7 ± 9.7 | 84.4 ± 13.0 | 63.8 ± 8.3 | 86.5 ± 16.3 |
| Height, cm (P = 0.07) | 166.4 ± 9.7 | 167.3 ± 10.4 | 168.1 ± 10.0 | 167.8 ± 10.0 | 167.4 ± 8.9 | 165.5 ± 10.4 |
| SBP, mmHg (P ≤ 0.0001) | 114.1 ± 14.7 | 125.6 ± 16.1 | 123.9 ± 17.3 | 133.1 ± 18.3 | 125.3 ± 19.1 | 138.2 ± 19.4 |
| DBP, mmHg (P ≤ 0.0001) | 72.3 ± 9.5 | 79.0 ± 9.5 | 75.7 ± 10.0 | 82.8 ± 10.1 | 74.1 ± 9.9 | 81.1 ± 10.9 |
| Waist circumference, cm (P ≤ 0.0001) | 81.8 ± 7.8 | 98.4 ± 9.3 | 86.1 ± 8.4 | 102.1 ± 10.3 | 83.7 ± 8.5 | 107.5 ± 12.6 |
| Glucose, mg/dL (P ≤ 0.0001) | 87.9 ± 6.7 | 90.9 ± 5.6 | 105.8 ± 6.1 | 107.9 ± 6.4 | 143.1 ± 40.5 | 159.0 ± 49.5 |
| Insulin, U/L (P ≤ 0.0001) | 4.3 ± 2.1 | 6.6 ± 3.4 | 5.7 ± 3.5 | 9.3 ± 7.4 | 9.0 ± 7.7 | 12.4 ± 10.4 |
| HOMA index (P ≤ 0.0001) | 0.9 ± 0.5 | 1.5 ± 0.8 | 1.5 ± 1.0 | 2.5 ± 2.1 | 3.1 ± 2.7 | 5.2 ± 6.1 |
| Total cholesterol, mg/dL (P ≤ 0.0001) | 198.1 ± 39.1 | 211.6 ± 40.0 | 209.0 ± 39.6 | 211.0 ± 40.8 | 196.5 ± 34.5 | 201.1 ± 40.7 |
| HDL cholesterol, mg/dL (P ≤ 0.0001) | 59.6 ± 13.4 | 53.0 ± 13.0 | 58.0 ± 14.1 | 49.6 ± 11.9 | 51.5 ± 14.1 | 46.7 ± 10.5 |
| LDL, mg/dL (P ≤ 0.0001) | 127.4 ± 30.8 | 144.0 ± 32.1 | 137.7 ± 33.2 | 144.2 ± 32.9 | 130.8 ± 27.8 | 137.4 ± 33.3 |
| Triglycerides, mg/dL (P ≤ 0.0001) | 85.1 ± 40.7 | 115.7 ± 67.6 | 99.4 ± 61.3 | 140.7 ± 88.1 | 121.0 ± 70.1 | 154.1 ± 106.9 |
| Hematocrit, % (P ≤ 0.0001) | 41.5 ± 3.7 | 42.5 ± 3.7 | 43.3 ± 3.5 | 43.6 ± 3.5 | 42.3 ± 2.8 | 42.9 ± 4.0 |
| Red blood cell count, n/mm3 (P = 0.0001) | 4.8 ± 0.5 | 4.9 ± 0.5 | 4.9 ± 0.5 | 5.1 ± 0.5 | 4.9 ± 0.5 | 4.9 ± 0.5 |
| Hb, g/dL (P ≤ 0.0001) | 14.0 ± 1.4 | 14.4 ± 1.4 | 14.6 ± 1.3 | 14.8 ± 1.3 | 14.2 ± 1.1 | 14.5 ± 1.5 |
| White blood cell count, n/mm3 (P ≤ 0.0001) | 6.4 ± 1.7 | 6.7 ± 1.7 | 6.7 ± 1.8 | 6.9 ± 1.7 | 7.7 ± 4.4 | 7.1 ± 1.6 |
| Platelet count, n/mm3 (P ≤ 0.0001) | 270 ± 63 | 266 ± 64 | 267 ± 67 | 259 ± 65 | 263 ± 53 | 250 ± 68 |
| hsCRP, mg/L (P ≤ 0.0001) | 1.7 ± 3.0 | 3.0 ± 4.1 | 2.3 ± 5.5 | 3.6 ± 5.0 | 1.1 ± 0.8 | 4.1 ± 4.4 |
| hCys, µmol/L (P ≤ 0.0001) | 13.3 ± 7.8 | 14.2 ± 9.7 | 14.6 ± 8.6 | 14.6 ± 8.2 | 12.0 ± 3.7 | 14.2 ± 5.5 |
| hsCRP > 5 mg/L (P ≤ 0.0001) | 65 (6.3%) | 102 (13.5%) | 15 (8.2%) | 72 (18.1%) | 0 (0%) | 35 (24.5%) |
| Arterial hypertension# (P ≤ 0.0001) | 140 (13.5%) | 273 (36.2%) | 68 (37.2%) | 241 (60.5%) | 12 (42.9%) | 117 (81.8%) |
Data are means ± SD or counts (percentage). BMI, body mass index; CA.ME.LI.A, CArdiovascular risks, MEtabolic syndrome, LIver and Autoimmune disease; DM, diabetes mellitus; hCys, hyperhomocysteinemia; hsCRP, high-sensitivity C‐reactive protein; IFG, impaired fasting glucose; NBW, normal body weight; NFG, normal fasting glucose; OWO, overweight-obese.
#Arterial hypertension was diagnosed in subjects who presented at least one of the following: systolic arterial pressure ≥140 mmHg, diastolic arterial pressure ≥90 mmHg, or treatment with antihypertensive therapy.
The distribution of MAFLD was different in the six subgroups. In NBW subjects, the percentage of subjects with MAFLD increased considerably from NFG to IFG to DM class (10.3% to 21% to 48%; P < 0.0001). In OWO/NFG subjects, the percentage with MAFLD was 50.3, increasing progressively to 68.4 in IFG/OWO and 82.9 in OWO/DM (P < 0.0001). Females had a lower percent incidence of MAFLD than males in the NFG/NBW (7.6% vs. 15.4%), NFG/OWO (46.7% vs. 54.2%), IFG/NBW (16% vs. 25.6%), and IFG/OWO (61.9% vs. 72.1%) groups, whereas they had a higher incidence in the DM/NBW (50% vs. 46.6%) and DM/OWO (88.1% vs. 79.4%) groups (Table 4). The thickness of visceral adipose tissue (VAT) progressively increased in male and female subjects in parallel with glucose and BMI increases. VAT was more represented in men than in women in the whole population as well as after dividing subjects according to fasting glucose levels or BMI (P ≤ 0.0001). Similar trends were observed in the distribution of the thickness of subcutaneous adipose tissue (SAT) in the entire population, with significantly more SAT in females as compared with males, in each of the six subgroups (Table 4 and Supplemental Fig. S3).
Table 4.
Incidence of MAFLD and thickness of VAT and SAT in the population stratified by glucose tolerance and BMI
| NFG/NBW | NFG/OWO |
IFG/NBW |
IFG/OWO |
DM/NBW |
DM/OWO |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Counts (%) | Counts (%) | χ2* | Counts (%) | χ2* | Counts (%) | χ2* | Counts (%) | χ2* | Counts (%) | χ2* | |
| n | 1,039; M = 372, F = 667 | 754; M = 395, F = 359 | 183; M = 105, F = 78 | 398; M = 269, F = 129 | 28; M = 15, F = 13 | 143; M = 95, F = 48 | |||||
| MAFLD |
|
||||||||||
| All | 103 (10.3%) | 324 (50.3%) | <0.0001 | 33 (21.0%) | 0.0021 | 197 (68.4%) | <0.0001 | 12 (48.0%) | <0.0001 | 87 (82.9%) | <0.0001 |
| Female | 50 (7.6%) | 156 (46.7%) | <0.0001 | 12 (16%) | 0.0275 | 65 (61.9%) | <0.0001 | 6 (50.0%) | 0.0003 | 37 (88.1%) | <0.0001 |
| Male | 53 (15.4%) | 168 (54.2%) | <0.0001 | 21 (25.6%) | 0.1694 | 132 (72.1%) | <0.0001 | 6 (46.1%) | 0.0161 | 50 (79.4%) | <0.0001 |
| Means ± SD | Means ± SD | P§ | Means ± SD | P§ | Means ± SD | P§ | Means ± SD | P§ | Means ± SD | P§ | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VAT, mm | |||||||||||
| All | 22.8 ± 11.8 | 39.1 ± 17.3 | <0.0001 | 28.1 ± 13.8 | <0.0001 | 46.2 ± 20.4 | <0.0001 | 30.8 ± 15.5 | 0.0197 | 54.0 ± 20.9 | <0.0001 |
| Female | 19.8 ± 9.6 (64.2%) | 34.2 ± 16.8 (47.6%) | <0.0001 | 21.2 ± 10.1 (42.6%) | 0.9175 | 42.2 ± 20.8 (32.4) | <0.0001 | 27.5 ± 14.5 (46.4%) | 0.2120 | 54.0 ± 20.1 (33.6%) | <0.0001 |
| Male | 28.2 ± 13.0 (35.8%) | 43.5 ± 16.5 (52.4) | <0.0001 | 33.2 ± 13.9 (57.4%) | 0.1694 | 48.0 ± 20.0 (67.6%) | <0.0001 | 33.5 ± 16.5 (53.4%) | 0.4980 | 54.0 ± 21.4 (66.4%) | <0.0001 |
| SAT, mm | |||||||||||
| All | 9.8 ± 5.8 | 16.4 ± 5.7 | <0.0001 | 10.8 ± 4.1 | 0.1110 | 15.8 ± 6.1 | <0.0001 | 8.1 ± 3.5 | 0.4692 | 15.6 ± 6.1 | <0.0001 |
| Female | 10.2 ± 5.2 (64.2%) | 18.3 ± 5.2 (47.6%) | <0.0001 | 11.1 ± 4.1 (42.6%) | 0.9105 | 19.4 ± 5.4 (32.4) | <0.0001 | 9.5 ± 3.6 (46.4%) | 0.9968 | 19.0 ± 5.8 (33.6%) | <0.0001 |
| Male | 9.2 ± 6.8 (35.8%) | 14.8 ± 5.6 (52.4) | <0.0001 | 10.6 ± 4.1 (57.4%) | 0.1448 | 14.1 ± 5.6 (67.6%) | <0.0001 | 6.9 ± 3.1 (53.4%) | 0.5195 | 13.9 ± 5.6 (66.4%) | <0.0001 |
In males, echographic data are missing in 256 of 1,251 subjects, and in females, echographic data are missing in 71 of 1,294 subjects. DM, diabetes mellitus; IFG, impaired fasting glucose; MAFLD, metabolic (dysfunction)-associated fatty liver disease; NBW, normal body weight; NFG, normal fasting glucose; OWO, overweight-obese; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
*Chi-square test (Fisher’s exact test). The data (P value) represent the comparison of each group with the reference NFG/NBW group;
§One-way ANOVA (Šídák’s multiple-comparisons test). The data (P value) represent the comparison of each group with the reference NFG/NBW group.
Association between Glucose Tolerance and/or Obesity with Death for All Causes
Mortality at the 7-yr follow-up of the CA.ME.LI.A study, by considering the stratification in the six subgroups, is reported in Table 5. A total of 80/2,545 (3.1%) apparently healthy subjects died. Among them, cancers accounted for 41.2% (n = 33) of all deaths, whereas cardio-cerebrovascular causes accounted for 17.5% (n = 14). Other diseases [20% (n = 16)] and unspecified causes [21.3% (n = 17)] accounted for the remaining deaths.
Table 5.
Causes of death in subjects with different levels of glucose tolerance (normal, impaired, and diabetes) and body mass index (normal and overweight/obese)
| Causes of Death | NFG/NBW | NFG/OWO | IFG/NBW | IFG/OWO | DM/NBW | DM/OWO |
|---|---|---|---|---|---|---|
| All causes (n = 80) | 15/1,039 (1.44%) | 16/754 (2.12%) | 7/183 (3.8%) | 19/398 (4.7%) | 1/28 (3.5%) | 22/143 (15.3%) |
| Cardio- and cerebrovascular (n = 14) | 2/1,039 (0.19%) | 4/754 (0.5%) | 0 | 1/398 (0.25%) | 0 | 7/143 (4.8%) |
| Cancer (n = 33) | 6/1,039 (0.6%) | 4/754 (0.5%) | 5/183 (2.7%) | 8/398 (2.0%) | 0 | 10/143 (6.9%) |
| Other diseases (n = 16) | 5/1,039 (0.6%) | 1/754 (1%) | 1/183 (1%) | 4/398 (2.5%) | 1/28 (3.5%) | 4/143 (3.4%) |
| Unspecified cause (n = 17) | 2/1,039 (0.2%) | 7/754 (0.9%) | 1/183 (0.6%) | 6/398 (1.5%) | 0 | 1/143 (0.7%) |
DM, diabetes mellitus; IFG, impaired fasting glucose; NBW, normal body weight; NFG, normal fasting glucose; OWO, overweight-obese.
Mortality from all causes increased as plasma glucose levels increased and further increased, within the same glucose level class, in the presence of overweight/obesity. The increase in death from NFG/NBW to DM/OWO seemed to display an exponential trend, with an apparent 15-fold increase (Table 6).
Table 6.
All-cause deaths’ HRs in the study population
| Glucose Tolerance/BMI Category | No. of Deaths | Hazard Ratio | 95% CI | P |
|---|---|---|---|---|
| Whole population | ||||
| NGT/NBW (n = 1,039)a | 15 | 1 | ||
| NGT/OWO (n = 754) | 16 | 1.45 | 0.72–2.93 | 0.302 |
| IFG/NBW (n = 183) | 7 | 2.70 | 1.10–6.62 | 0.030 |
| IFG/OWO (n = 398) | 19 | 3.38 | 1.72–6.66 | <0.001 |
| DM/NBW (n = 28) | 1 | 2.49 | 0.33–19 | 0.377 |
| DM/OWO (n = 143) | 22 | 11.78 | 6.11–23 | <0.001 |
| All subjects (n = 2,545) | 80 | |||
| Men | ||||
| NGT/NBW (n = 372)a | 5 | 1 | ||
| NGT/OWO (n = 395) | 10 | 1.84 | 0.265 | 0.265 |
| IFG/NBW (n = 105) | 2 | 1.43 | 0.28–7.35 | 0.671 |
| IFG/OWO (n = 269) | 17 | 4.85 | 1.78–13.15 | 0.002 |
| DM/NBW (n = 15) | 0 | 0 | ||
| DM/OWO (n = 95) | 17 | 14.99 | 5.53–41 | <0.001 |
| All subjects (n = 1,257) | 51 | |||
| Women | ||||
| NGT/NBW (n = 667)a | 10 | 1 | ||
| NGT/OWO (n = 359) | 6 | 1.10 | 0.40–3.02 | 0.858 |
| IFG/NBW (n = 78) | 5 | 4.41 | 1.51–12.92 | 0.007 |
| IFG/OWO (n = 129) | 2 | 1.04 | 0.23–4.73 | 0.963 |
| DM/NBW (n = 13) | 1 | 5.43 | 0.70–42 | 0.107 |
| DM/OWO (n = 48) | 5 | 7.37 | 2.52–21 | <0.001 |
| All subjects (n = 1,294) | 29 | |||
BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; IFG, impaired fasting glucose; NBW, normal body weight; NFG, normal fasting glucose; OWO, overweight-obese.
aReference category for hazard ratio and 95% CI calculations.
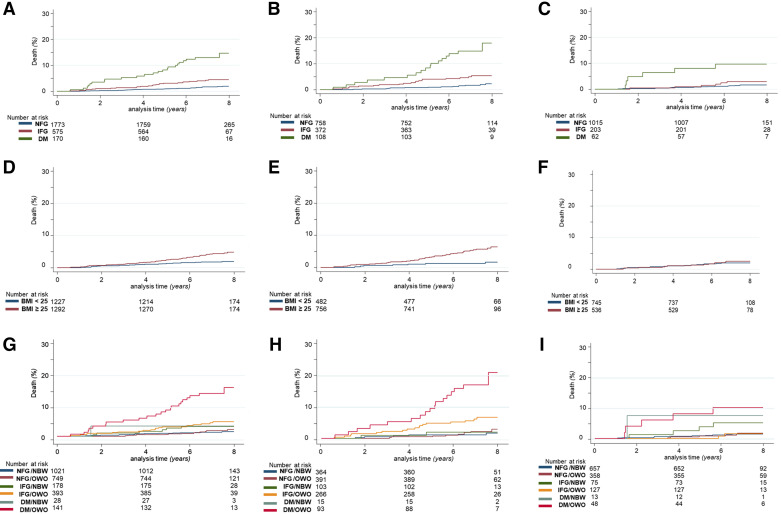
Figure 1 shows the Kaplan–Meier curves for the incidence of death from all causes according to glucose levels (panels A, B, and C), BMI (D, E, and F), and the combination of the two (G, H, and I). The effect of increased glucose levels on death rates is evident both when considering either the population as a whole or males and females separately. The increase in mortality of the IFG group compared with the NFG class is significant (P < 0.005) and similar between males and females. The increase in mortality of the DM group is significantly higher as compared with both NFG and IFG (both P < 0.0005) and more pronounced in males than in females (17.74% vs. 9.68%, P < 0.010) (Fig. 1, A–C, and Supplemental Fig. S4, A–C).
Figure 1.
Incidence of deaths for all causes in the follow-up period of CA.ME.LI.A population according to—A: glucose tolerance in the whole population: NFG = 1.75%, IFG = 4.52%, DM = 13.53%; B: glucose tolerance in the male population: NFG = 1.9%, IFG = 5.11%, = DM 17.74%; C: glucose tolerance in the female population: NFG = 1.58%, IFG = 3.45%, DM = 9.68%; D: BMI in the whole population: BMI of <25 (NBW) = 1.87%, BMI of >25 (OWO) = 4.41%; E: BMI in the male population: BMI of <25 (NBW) = 1.45%, BMI of >25 (OWO) = 5.82%; F: BMI in the female population: BMI of <25 (NBW) = 2.15%, BMI of >25 (OWO) = 2.43% (In OWO, P = 0.003 between sexes); G: BMI and glucose tolerance in the whole population: NFG/NBW = 1.47%, NFG/OWO = 2.14%, DM/NBW = 3.57%, IFG/NBW = 3.93, IFG/OWO = 4.83%, DM/OWO = 15.6%; H: BMI and glucose tolerance in the male population: NFG/NBW = 1.37%, NFG/OWO = 2.56%, DM/NBW = 0%, IFG/NBW = 1.37%, IFG/OWO = 6.39%, DM/OWO = 18.28%; I: BMI and glucose tolerance in the female population: NFG/NBW = 1.52%, NFG/OWO = 1.68%, DM/NBW = 3.69%, IFG/NBW = 6.67, IFG/OWO = 1.57%, DM/OWO = 10.42%. BMI, body mass index; CA.ME.LI.A, CArdiovascular risks, MEtabolic syndrome, LIver and Autoimmune disease; DM, diabetes mellitus; IFG, impaired fasting glucose; NBW, normal body weight; NFG, normal fasting glucose; OWO, overweight-obese.
The effect of BMI on mortality, in contrast, shows sex-related differences. In the population as a whole, the increase in mortality in the OWO class as compared with the NBW class is significant (P < 0.0005). Interestingly, this increase is due to increased male mortality (P < 0.0005 in males), whereas in females, there is a nonsignificant difference between the two BMI classes (2.15% in NBW vs. 2.43% in OWO; P = nonsignificant) (Fig. 1, D–F, and Supplemental Fig. S4, D–F).
Considering the combined effects of glucose tolerance and BMI, when comparing the NFG/NBW with NFG/OWO, mortality was similar in males and females. Among females, however, having a BMI of <25 in the presence of IFG (IFG/NBW) resulted in increased mortality as compared with NFG/NBW and NFG/OWO (P < 0.005). In males, IFG/OWO showed increased mortality as compared with NFG/NBW and NFG/OWO (P < 0.0005). In both sexes, DM associated with NBW did not lead to a significant difference in mortality, possibly due to the low number of subjects in this category (n = 28). As expected, the DM/OWO class had the highest number of deaths in both males (18.3%) and females (10.4%) (P < 0.0005 in both) (Fig. 1, G–I, and Supplemental Fig. S4, G–I).
In Supplemental Table S4, odds ratio (OR) after comparison of mortality for all causes, for cardio-cerebrovascular causes, for cancer, and for all other causes, in each of the six classes versus the others (paired comparison), are reported. Chi-square (and Fisher’s exact) tests were performed to assess whether the discrepancy between the different classes was more than expected by chance. In particular, the OR (95% CI) of mortality was statistically significant (P < 0.0001) for malignant neoplasm and cardiovascular causes when DM/OWO was compared with NFG/NBW, NFG/OWO, and IFG/OWO and was significant for malignant neoplasm when DM/OWO was compared with IFG/NBW.
Association of Glucose Tolerance and/or Obesity with Cardiovascular Events and Deaths
During the 7-yr follow-up period, 163 cardiovascular (CV) events (14 deaths from CV causes) occurred during an overall observation period of 17,776 person-years (Supplemental Table S5) The incidence rate of CV events in the whole population was 0.92 cases per 100 patient-years and was significantly higher in men, with an incidence rate ratio of 1.98 (95% CI: 1.42–2.78; P < 0.0001).
Supplemental Fig. S5A shows the Kaplan–Meier plot of the incidence of CV events and deaths among males and females (P < 0.0001). The two populations have also been analyzed separately (Supplemental Fig. 5, B and C, and Supplemental Table S6) to show the survival plots after the population was stratified into five age categories (≤35, 35–44, 45–54, 55–64, and ≥65 yr). In men, the incidence of CV events was consistently more frequent in all age categories, with a more marked difference with women in the oldest age-group (≥65 yr).
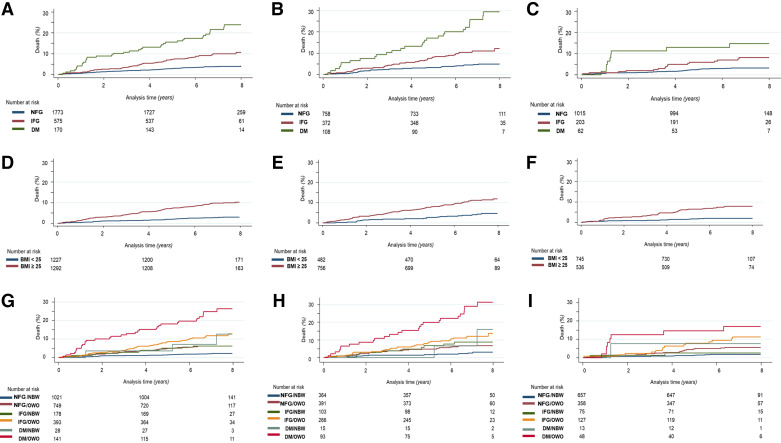
The incidence of CV events and deaths was significantly higher in the IFG and DM classes as compared with the NFG class and in DM as compared with IFG (all, P < 0.0005); the same trend was found in males and females but with higher values for all classes in males (Fig. 2, A–C; Supplemental Fig. S6, A–C, and Supplemental Table S7).
Figure 2.
Incidence of cardiovascular (CV) events and death from CV causes in the follow-up period of CA.ME.LI.A population according to—A: glucose tolerance in the whole population: NFG = 3.84%, IFG = 9.74%, DM = 21.51%; B: glucose tolerance in the male population: NFG = 4.81%, IFG = 10.88%, DM = 25.45%; C: glucose tolerance in the female population: NFG = 3.12%, IFG = 7.69%, DM = 14.55%; D: BMI in the whole population: BMI of <25 (NBW) = 2.96%, BMI of >25 (OWO) = 9.66%; E: BMI in the male population: BMI of <25 (NBW) = 4.47%, BMI of >25 (OWO) = 10.98%; F: BMI in the female population: BMI of <25 (NBW) = 2.15%, BMI of >25 (OWO) = 7.8%; G: BMI and glucose tolerance in the whole population: NFG/NBW = 2.6%, NFG/OWO = 7.0%, DM/NBW = 10.70%, IFG/NBW = 7.5, IFG/OWO = 14.8%, DM/OWO = 24.0%; H: BMI and glucose tolerance in the male population: NFG/NBW = 1.37%, NFG/OWO = 3.76%, DM/NBW = 14.12%, IFG/NBW = 6.37%, IFG/OWO = 9.39%, DM/OWO = 30.28%; I: BMI and glucose tolerance in the female population: NFG/NBW = 1.80%, NFG/OWO = 5.57%, DM/NBW = 7.69%, IFG/NBW = 2.56, IFG/OWO = 10.85%, DM/OWO = 16.67%. BMI, body mass index; CA.ME.LI.A, CArdiovascular risks, MEtabolic syndrome, LIver and Autoimmune disease; DM, diabetes mellitus; IFG, impaired fasting glucose; NBW, normal body weight; NFG, normal fasting glucose; OWO, overweight-obese.
The same analysis was carried out by considering BMI, independently from glucose tolerance, comparing NBW versus OWO. Incidence of CV events and deaths according to BMI in the whole population was always higher in OWO than in NBW subjects (NBW: 2.96%; OWO: 9.66%), with similar trends in both females and males (P < 0.0001). However, there was a higher prevalence of events in men than in women in both BMI categories. Of interest is the observation that also in the NBW group, men had a prevalence of events more than twice higher than women (4.5% vs. 2.0%, P < 0.0001) (Fig. 2, D–F; Supplemental Fig. 6, D–F, and Supplemental Table S8).
The presence of impaired fasting glucose or diabetes increased CV events and mortality as compared with NFG, although to a different extent (DM > IFG). Similarly also OWO as compared with NBW showed a similar effect to increase CV events and deaths. Then we investigated the impact on cardiovascular events and deaths, when hyperglycemia (IFG or DM) was present along with overweight/obesity.
Table 7 reports the incidence of CV events and deaths in the population stratified into the six subgroups based on glucose tolerance and BMI categories. The Kaplan–Meier survival curves are shown in Fig. 2, G–I. In the whole population, the incidence of CV events and deaths increased progressively from about twice in NGT/OWO to more than 12-fold in DM/OWO as compared with the NFG/NBW group (Table 7). A similar trend toward worsening CV morbidity/mortality across the six glucose tolerance and BMI levels was present when men and women were analyzed separately. In addition, when sex was included in Cox modeling, the female sex emerged as a factor conditioning a more favorable CV prognosis (HR to men: 0.70; 95% CI: 0.50–0.97; P = 0.034).
Table 7.
Cerebro-cardiovascular deaths and events’ HRs based on glucose tolerance and BMI during the follow-up
| Glucose Tolerance/BMI Category | No. of CV Events | Hazard Ratio | 95% CI | P |
|---|---|---|---|---|
| Whole population | ||||
| NFG/NBW (n = 1,039)a | 23 | 1 | ||
| NFG/OWO (n = 754) | 46 | 2.78 | 1.68–4.59 | <0.001 |
| IFG/NBW (n = 183) | 11 | 2.81 | 1.37–5.77 | 0.005 |
| IFG/OWO (n = 398) | 46 | 5.51 | 3.34–9.08 | <0.001 |
| DM/NBW (n = 28) | 3 | 4.88 | 1.47–16 | 0.010 |
| DM/OWO (n = 143) | 34 | 12.71 | 7.48–22 | <0.001 |
| All subjects (n = 2,545) | 163 | |||
| Men | ||||
| NFG/NBW (n = 372)a | 11 | 1 | ||
| NFG/OWO (n = 395) | 26 | 2.25 | 1.11–4.54 | 0.025 |
| IFG/NBW (n = 105) | 9 | 2.97 | 1.23–7.18 | 0.015 |
| IFG/OWO (n = 269) | 32 | 4.27 | 2.15–8.47 | <0.001 |
| DM/NBW (n = 15) | 2 | 4.41 | 0.97–20 | 0.053 |
| DM/OWO (n = 95) | 26 | 11.3 | 5.58–22 | <0.001 |
| All subjects (n = 1,257) | 106 | |||
| Women | ||||
| NFG/NBW (n = 667)a | 12 | 1 | ||
| NFG/OWO (n = 359) | 20 | 3.10 | 1.52–6.35 | 0.002 |
| IFG/NBW (n = 78) | 2 | 1.49 | 0.33–8.67 | 0.599 |
| IFG/OWO (n = 129) | 14 | 6.23 | 2.88–13 | <0.001 |
| DM/NBW (n = 13) | 1 | 4.40 | 0.47–34 | 0.155 |
| DM/OWO (n = 48) | 8 | 10.27 | 4.20–25 | <0.001 |
| All subjects (n = 1,294) | 57 | |||
CV events are first hospitalization for CV problems or deaths from CV cause. BMI, body mass index; CI, confidence interval; CV, cardiovascular; DM, diabetes mellitus; HR, hazard ratio; IFG, impaired fasting glucose; NBW, normal body weight; NFG, normal fasting glucose; OWO, overweight-obese.
aReference category for hazard ratio and 95% CI calculations.
Figure 2, G–I, shows that the incidence of cardiovascular events and deaths was significantly lower in NFG/OWO versus DM/OWO subjects in the whole population (P < 0.0001), as well as in men (P ≤ 0.0001) and women (P = 0.002). Likewise also NFG/OWO had significantly less events as compared with IFG/OWO subjects in the whole population (P = 0.0009), in men (P = 0.01), and in women (P = 0.04). In women, there was a greater incidence of CV events in the IFG/OWO group (HR: 6.23 compared with NFG/NBW) than in men in the same group (HR: 4.27 compared with NFG/NBW). In men, the increase in CV events was similar in NFG/OWO and IFG/NBW groups (HR: 2.25 and 2.97, respectively), as compared with NFG/NBW, and in IFG/OWO and DM/NBW (HR: 4.27 and 4.41, respectively), as compared with NFG/NBW, with the highest increase being observed in the DM/OWO group (HR: 11.3). In women, an increase in glucose levels did not result in such a higher CV risk increase as in men, whereas a higher BMI resulted in a greater CV risk increase than in men. In women, NFG/OWO and IFG/NBW had significantly different CV risk increase (HR: 3.10 and 1.49, respectively), with a similar trend observed in IFG/OWO and DM/NBW groups (HR: 6.23 and 4.40, respectively). Finally, in women, the group at highest risk was the DM/OWO (HR: 10.27, compared with NFG/NBW) (Table 7).
Univariate and Multivariate Analysis
A multivariate analysis was performed to assess whether overweight/obesity, impaired fasting glucose, and diabetes were significantly associated with cardiovascular deaths and events even after correction for other variables.
Before the multivariate analysis, univariate analyses were performed to understand the distribution of values for each variable of major interest. The variables used are continuous, when possible, or categorical. Only those found to be significant (P < 0.10 level) in the univariate Cox analyses have been included in the multivariate analysis (Table 7). Male and female populations were analyzed separately, as sex was significantly associated with most study variables.
In men, LDL cholesterol (P = 0.28) and SAT (P = 0.513) did not show statistically significant associations with cardiovascular events and are not reported. All variables significantly associated with the events in the univariate analyses are reported in Table 8 and were included in the multivariate analysis.
Table 8.
Univariate and multivariate analysis of cardiovascular events and deaths versus glucose intolerance/obesity
| Variables | General Population (n = 2,545) |
Men (n = 1,251) |
Women (n = 1,294) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis |
Multivariate Analysis |
Univariate Analysis |
Multivariate Analysis |
Univariate Analysis |
Multivariate Analysis |
|||||||
| P | P | HR | 95% CI | P | P | HR | 95% CI | P | P | HR | 95% CI | |
| Age, yr | <0.0001 | <0.0001 | 1.08 | 1.0–1.1 | <0.0001 | <0.0001 | 1.08 | 1.0–1.1 | <0.0001 | <0.0001 | 1.09 | 1.0–1.1 |
| hsCRP elevateda, mg/L | <0.0001 | 0.002 | 1.90 | 1.3–2.9 | <0.0001 | <0.0001 | 3.06 | 1.8–5.3 | ns | ns | ns | ns |
| HDL, mg/dL | <0.0001 | <0.0001 | 0.97 | 0.93–0.98 | 0.003 | 0.041 | 0.97 | 0.9–1.0 | ns | ns | ns | ns |
| LDL, mg/dL | 0.013 | 0.251 | 1.00 | 0.99–1.00 | ns | ns | ns | ns | 0.001 | 0.681 | 1.00 | 1.0–1.1 |
| Diabetes mellitus | <0.0001 | <0.0001 | 3.51 | 2.4–5.1 | <0.0001 | 0.025 | 2.02 | 1.1–3.2 | ns | ns | ns | ns |
| Glucose intolerance (100–125 mg/dL) | <0.0001 | <0.0001 | 2.13 | 1.5–3.1 | <0.0001 | 0.001 | 2.11 | 1.4–3.3 | <0.0001 | 0.052 | 1.84 | 1.0–3.4 |
| BMI, kg/m2 | <0.0001 | <0.0001 | 2.78 | 1.9–4.1 | <0.0001 | 0.7 | 0.98 | 0.9–1.1 | ns | ns | ns | ns |
| Triglycerides, mg/dL | <0.0001 | 0.375 | 1.00 | 0.99–1.00 | 0.001 | 0.224 | 1.00 | 0.99–1.0 | 0.003 | 0.518 | 1.00 | 1.0–1.1 |
| Hypertension | <0.0001 | 0.083 | 1.52 | 0.95–2.44 | <0.0001 | 0.130 | 1.59 | 0.9–2.9 | <0.0001 | 0.311 | 1.48 | 0.7–3.1 |
| Metabolic syndrome | <0.0001 | 0.250 | 0.75 | 0.46–1.22 | <0.0001 | 0.114 | 0.57 | 0.3–1.1 | <0.0001 | 0.672 | 0.85 | 0.4–1.8 |
| HOMA index | <0.0001 | 0.095 | 1.06 | 0.99–1.14 | <0.0001 | 0.068 | 1.07 | 1.0–1.2 | <0.0001 | 0.702 | 1.02 | 0.9–1.2 |
| MAFLD | <0.0001 | 0.758 | 1.06 | 0.71–1.59 | 0.006 | 0.735 | 1.09 | 0.7–1.8 | <0.0001 | 0.442 | 1.27 | 0.7–2.4 |
| VAT elevatedb | 0.0018 | 0.367 | 0.80 | 0.49–1.30 | 0.005 | 0.517 | 1.25 | 0.6–2.5 | ns | ns | ns | ns |
BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; hsCRP, high-sensitivity C-reactive protein; MAFLD, metabolic (dysfunction)-associated fatty liver disease; ns, not significant; VAT, visceral adipose tissue.
aValues above the upper reference limit for CRP (>5 mg/L).
bValues in the three upper quartiles of the VAT distribution (Q2, Q3, Q4) >16 mm.
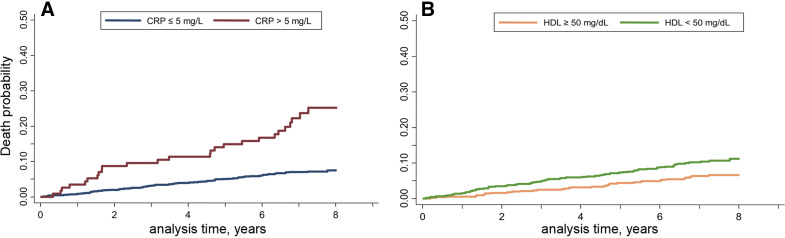
In men, only age (<0.0001), the presence of elevated CRP levels (<0.0001), HDL cholesterol (P = 0.03), diabetes (P = 0.02), and BMI retained a statistically significant association with CV events after correction for the other variables. Figure 3A shows the survival curves of men according to high-sensitivity CRP, where death probability is higher with CRP values above the cutoff (P < 0.0001). Figure 3B shows survival plots based on HDL cholesterol, highlighting a significantly higher incidence of CV events in subjects with levels under the 40 mg/dL cutoff (P = 0.020).
Figure 3.
Incidence of cardiovascular events in the male CA.ME.LI.A population stratified by CRP values (A, cutoff = 5 mg/dL) and HDL values (B, cutoff = 40 mg/dL). CA.ME.LI.A, CArdiovascular risks, MEtabolic syndrome, LIver and Autoimmune disease; CRP, C-reactive protein.
In contrast, CRP (P = 0.122), HDL (P = 0.086), and high VAT (P = 0.46) and SAT (P = 0.116) were not included in Table 8, for females, as they did not show a statistically significant association with cardiovascular events. All the other variables with a significant association with CV events have been included in the table. Multivariate analysis shows that all the variables lost their significant effect after correction for the other factors, with the exception of age, which maintains its high level of statistical significance (<0.0001).
DISCUSSION
Overweight and obesity have reached epidemic proportions around the world, affecting almost 60% of adults and one in three school-aged children (33, 34). The data collected on the prevalence of obesity in the study population are consistent with the ISTAT (Italian National Institute of Statistics) data collected in the Italian population during that period showing a prevalence of obesity and overweight of 45.9% (consistent with this reported prevalence, in our study population it was 51.1%), of which 37.0% in females and 55.6% in males (in our study population, females were 41.6% and males were 60.9%) (35). Regarding the prevalence of diabetes mellitus (type 1 and type 2) found by ISTAT in the Italian general population during the study period, this accounted for 5.3% (5.4% for males and 5.2% for females) (36); our study population showed comparable figures for the total prevalence of diabetes (type 1 and type 2: 6.7%), with greater intersex variability (8.7% for males and 4.8% for females). In addition, 22.9% had impaired fasting glucose, i.e., fasting blood sugar levels between 100 and 125 mg/dL. The sum of the two percentages (29.6%) shows that almost one-third of the general population in this study had altered glucose levels (prediabetes or diabetes). Of note, in the ISTAT database, the incidence of impaired fasting glucose (prediabetes) is not present (36).
Sex impacts the pathogenesis of numerous diseases, including metabolic disorders such as diabetes. In most parts of the world, diabetes is more prevalent in men than in women, especially in middle-aged populations (37). In our study population, men with IFG and men with DM were about double than women in the same categories (30.0% and 8.7% vs. 16.0% and 4.8%). Sex-specific differences have been found in glucose homeostasis, insulin secretion and action, and the incidence and progression of diabetes, which might help explain this different distribution (38). Only in people aged ≥65, there were more women with DM than men, and this was probably due to women’s greater longevity (39).
With increasing age, worsening of glucose tolerance (IFG and DM) emerged, confirming the importance of advanced age as a risk factor for glucose intolerance, independently from BMI (40). IFG and DM subjects had a significantly higher BMI than NFG subjects, especially in men. This observation is consistent with overweight/obesity being an independent and dose-dependent risk factor for type 2 diabetes mellitus (40).
Overweight/obesity also correlated with an increased thickness of VAT and SAT as compared with NBW. VAT was increased in subjects with impaired fasting glucose and diabetes as compared with NFG subjects, both in women and men, although VAT was thicker in men as compared with women. VAT increased significantly in size in the diabetic and intolerant population in both men and women. SAT is always higher in OWO independently from glucose tolerance and higher in diabetic women than diabetic men, possibly confirming that women start already with larger stores of subcutaneous fat, whereas men present more visceral fat (41). Both SAT and VAT are associated with increased subinflammation and oxidative stress (42). Definitely, VAT has a stronger role compared with SAT in the onset of insulin resistance and diabetes, beyond the increased release of free fatty acid and possibly due to increased levels of proinflammatory cytokines (16, 21, 22, 43). Obesity is in fact the most important risk factor for type 2 diabetes, and they share a number of clinical manifestations, including metabolic syndrome X of insulin resistance, MAFLD, dyslipidemia, and arterial hypertension, which markedly increase morbidity and mortality (18, 24, 25, 44). VAT, SAT, and MAFLD showed similar trends to changes with BMI and fasting blood glucose, confirming their intimate connection (26, 31, 32, 45–54).
Aging is also associated with increased number cardiovascular events and deaths, as they were associated also to biological decline, and, as expected, the mortality became higher with increasing age. Nonetheless, at the same age, there were significant differences between the two sexes, with men having more cardiovascular events and deaths than women; this difference in life expectancy and mortality is in line with data reported in literature (55, 56). In both men and women, the risks associated with CVD increase with age, and these correspond to an overall decline in sex hormones, primarily estrogen and testosterone (57). In either sex, in the same-age groups, higher mortality was seen in the groups with OWO, IFG, and DM than in those with NBW or NFG.
Obesity, even when not associated with metabolic derangements, is a risk factor for the incidence of cardiovascular events and/or premature death (3, 6). In fact, in the NFG/OWO group, there was a statistically higher incidence of events than the NFG/NBW group. Cardiovascular disease risk is increased in populations with overweight and obesity classified as metabolically healthy, even in the absence of metabolic risk factors (58). This discrepancy in the incidence of cardiovascular events in men and women, even at high BMI, had already been reported in the literature: a high BMI has been shown to be more predictive of death from cardiovascular diseases, more in men than in women (3), and in women, a high fat content, regardless of the level of muscle mass, appears to be associated with a lower risk of mortality from cardiovascular diseases (59). Important differences between men and women are present also in the prescription, adherence, and response to cardiovascular drugs (60).
The group of diabetic and normal body weight (DM/NBW) subjects showed a higher incidence of cardiovascular events than the overweight/obese normal glucose-tolerant (NFG/OWO) group, whereas DM/NBW and IFG/OWO had similar incidence of events. Among individuals with or without diabetes, absolute rates of cardiovascular disease are higher in men than in women; however, in reproductive-age women, the presence of type 2 diabetes largely eliminates this protection from cardiovascular disease, and diabetic women are more severely affected than men (61, 62). Interestingly, in males, the DM/NBW group had a higher incidence of events than the IFG/OWO group, whereas in females, it was the opposite. These findings seem to imply that the factors weighing the most on the risk of cardiovascular events and deaths, differed by sex, were hyperglycemia (any value higher that 100 mg/dL) for males and overweight/obesity (any BMI value over 25 kg/m2) for females. Further studies are needed to validate this observation. This observation could also be partially explained by the fact that although IFG and DM subjects generally show a higher BMI than NFG subjects, this increase is more pronounced in men than in women. Pre-DM and undiagnosed T2DM play a more prominent role in the risk of CVD in women than in men. Women must experience a greater overall metabolic deterioration; that is, they must accumulate more fat and experience greater insulin resistance and related risk factors to evolve from normoglycemia to T2DM. Women’s higher diabetic CVD risk than that of men could be ascribed to their greater and more prolonged decline in metabolic homeostasis during the pre-DM stage (63–65).
Regarding anthropometric and clinical parameters changes, subjects in the IFG/OWO and DM/OWO groups showed higher levels of blood pressure, waist circumference, glycemia, cholesterol, and triglycerides as compared with NFG/NBW and NFG/OWO subjects (44, 66, 67). The HOMA index, within the same glucose tolerance class, was higher in OWO subjects than in NBW subjects. Men and women had comparable values, except in diabetic subjects, where NBW women had lower values than NBW men, whereas OWO women had higher values than OWO men. A recent study showed higher HOMA-IR cutoff values for women (68). Differences in insulin sensitivity between men and women may be due to differences in adipose tissue, muscle mass, hormones, and body fat distribution (subcutaneous and visceral fat) (38, 69). High-sensitivity C-reactive protein (hsCRP) increased as glucose intolerance increased, and, within different glucose tolerance classes, it was significantly greater in OWO subjects than in NBW subjects and in women compared with men. Systemic subinflammation has been extensively demonstrated to be strongly related to the patient’s metabolic state and lipid profile. Increased levels of inflammation markers and cytokines have also been associated with a higher risk of developing CVD, especially in women (70). In an analysis of patients from the Framingham Heart Study, the expression of biomarkers representing inflammatory pathways, including CRP, was seen to be higher in women (71). The risk of both macro- and microvascular complications increases before glucose levels reach the diagnostic threshold of diabetes, and 25% of newly diagnosed diabetics already have manifested cardiovascular disease (72–77). Our data underline the possibility of preserving long-term health in people with IFG before type 2 diabetes becomes established. In fact IFG/NBW, DM/NBW, IFG/OWO, and DM/OWO as compared with NFG/NBW display a progressive exponential increase of mortality for all causes, with ORs of 2.7, 2.5, 3.4, and 12.4, respectively, as compared with NFG/NBW.
Therefore, although there is over 10-fold increase in mortality in DM/OWO, consistent with previous studies, also in prediabetic individuals (IFG/NBW and IFG/OWO), there is an approximately threefold increase in mortality for all causes (cancer, cardiovascular, other disease, and unspecified causes). This suggests that aggressively treating IFG and OWO with diet, exercise, and possibly also medications, if necessary, might lower risk of cancer, cardiovascular events, and deaths (78, 79).
The efficacy of lowering blood pressure and blood glucose and lipid-lowering therapies has been shown to reduce subsequent morbidity and mortality in patients with diabetes and without diabetes (80–82), with no different beneficial preventive effects on the incidence of type 2 diabetes and weight gain between men and women (83). Interventions to improve lifestyle are necessary well before the onset of these diseases, and the maintenance of a healthy BMI and normal blood glucose, lipids, and pressure should be recommended in the general population to prolong healthy life.
Limitations
Because the observations referred to the participants’ baseline data (clinical, laboratory, and echography), any changes in parameters during follow-up could not be considered. Approximately 20% of the deaths lacked identifiable causes, and deaths categorized as “unspecified other causes” might not account for potential cardiovascular events contributing to mortality. These limitations could result in an underestimation of deaths for cardiovascular causes or misclassification of events due to changes in participants’ weight or glucose tolerance status over time.
Conclusions
Impaired fasting glucose, diabetes, and overweight/obesity increase the risk of cardiovascular events and all-cause mortality, both independently and more so when present in combination. Any worsening of glucose tolerance has a proportional effect in increasing mortality and the incidence of cardiovascular events. The individual contribution of each condition is at least, in part, sex-dependent, since in women, obesity causes the greatest increase in incidence, whereas in men, this is caused by diabetes.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL MATERIAL
Supplemental Figs. S1–S6 and Supplemental Tables S1–S8: https://doi.org/10.7910/DVN/L7TFFK.
GRANTS
The CA.ME.LI.A project was supported by Regione Lombardia (DG Sanità 08/07/2008 n. 7364), Italian Ministry for Education (MIUR, GR-2011 02350447), and by Dipartimento di Scienze della Salute, Università degli Studi di Milano (LINEA 2 DEL PIANO DI SOSTEGNO ALLA RICERCA PSR2020_DIP_013_PARONI dal titolo: Looking for Novel Biomarkers of Diabetes and Cardiovascular Diseases, “NODICARDIO”). The study was approved by the Ethical Committee of Ospedale San Paolo. L.C. is a PhD student in “Scienze della nutrizione - Università degli Studi di Milano” supported by Project PNC 0000001 D3 4 Health—CUP [B83C22006120001], The National Plan for Complementary Investments to the NRRP, funded by the European Union—NextGenerationEU.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.P., P.M.B., and F.F. conceived and designed research; P.Z. and E. Bertolini performed experiments; M.B., E. Bianco, L.C., A.R., M.D.C., P.Z., C.M., C.B., M.Z., R.P., P.M.B., and F.F. analyzed data; F.B., P.M.B., and F.F. interpreted results of experiments; L.C., M.D.C., C.M., F.S., and P.M.B. prepared figures; M.B., E. Bianco, L.C., M.D.C., R.P., and F.F. drafted manuscript; L.C., R.P., and F.F. edited and revised manuscript; R.P. and F.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Prof. Mario Cozzolino from the Department of Health Sciences, Università degli Studi di Milano, for critical reading of the manuscript and support. The graphical abstract was created with BioRender.com.
REFERENCES
- 1.World Health Organization. Obesity and Overweight (Online). https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight [2024 Mar 1].
- 2. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA 282: 1523–1529, 1999. doi: 10.1001/JAMA.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 3. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW Jr.. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 341: 1097–1105, 1999. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 4. Sarwer DB, Polonsky HM. The psychosocial burden of obesity. Endocrinol Metab Clin North Am 45: 677–688, 2016. doi: 10.1016/J.ECL.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339: 229–234, 1998. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 6. Bobbioni-Harsch E, Pataky Z, Makoundou V, Laville M, Disse E, Anderwald C, Konrad T, Golay A; RISC Investigators. From metabolic normality to cardiometabolic risk factors in subjects with obesity. Obesity (Silver Spring) 20: 2063–2069, 2012. doi: 10.1038/OBY.2012.69. [DOI] [PubMed] [Google Scholar]
- 7. Brun E, Nelson RG, Bennett PH, Imperatore G, Zoppini G, Verlato G, Muggeo M; Verona Diabetes Study. Diabetes duration and cause-specific mortality in the Verona Diabetes Study. Diabetes Care 23: 1119–1123, 2000. doi: 10.2337/DIACARE.23.8.1119. [DOI] [PubMed] [Google Scholar]
- 8. Ferrannini E, Massari M, Nannipieri M, Natali A, Lopez Ridaura R, Gonzales-Villalpando C. Plasma glucose levels as predictors of diabetes: the Mexico City Diabetes Study. Diabetologia 52: 818–824, 2009. doi: 10.1007/S00125-009-1289-8. [DOI] [PubMed] [Google Scholar]
- 9. Bragg F, Kuri-Morales P, Berumen J, Garcilazo-Ávila A, Gonzáles-Carballo C, Ramírez-Reyes R, Santacruz-Benitez R, Aguilar-Ramirez D, Gnatiuc Friedrichs L, Herrington WG, Hill M, Trichia E, Wade R, Collins R, Peto R, Emberson JR, Alegre-Diaz J, Tapia-Conyer R. Diabetes and infectious disease mortality in Mexico City. BMJ Open Diabetes Res Care 11: e003199, 2023. doi: 10.1136/bmjdrc-2022-003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183: 109119, 2022. [Erratum in Diabetes Res Clin Pract 204: 110945, 2023]. doi: 10.1016/J.DIABRES.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elsayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Kosiborod M, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care 46, Suppl 1: S19–S40, 2023. [Erratum in Diabetes Care 46: 1106, 2023 and in Diabetes Care 46: 1715, 2023]. doi: 10.2337/DC23-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Astrup A, Finer N. Redefining type 2 diabetes: “diabesity” or “obesity dependent diabetes mellitus?” Obes Rev 1: 57–59, 2000. doi: 10.1046/J.1467-789X.2000.00013.X. [DOI] [PubMed] [Google Scholar]
- 13. Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, Beguinot F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol 10: 1607, 2019. doi: 10.3389/FPHYS.2019.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Monroy A, Kamath S, Chavez AO, Centonze VE, Veerasamy M, Barrentine A, Wewer JJ, Coletta DK, Jenkinson C, Jhingan RM, Smokler D, Reyna S, Musi N, Khokka R, Federici M, Tripathy D, Defronzo RA, Folli F. Impaired regulation of the TNF-alpha converting enzyme/tissue inhibitor of metalloproteinase 3 proteolytic system in skeletal muscle of obese type 2 diabetic patients: a new mechanism of insulin resistance in humans. Diabetologia 52: 2169–2181, 2009. doi: 10.1007/S00125-009-1451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daniele G, Guardado Mendoza R, Winnier D, Fiorentino TV, Pengou Z, Cornell J, Andreozzi F, Jenkinson C, Cersosimo E, Federici M, Tripathy D, Folli F. The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetol 51: 123–131, 2014. doi: 10.1007/S00592-013-0543-1. [DOI] [PubMed] [Google Scholar]
- 16. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 542: 177–185, 2017. doi: 10.1038/NATURE21363. [DOI] [PubMed] [Google Scholar]
- 17. DeFronzo RA, Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol 108: 3B–24B, 2011. doi: 10.1016/J.AMJCARD.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 18. Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am 95: 875–892, 2011. doi: 10.1016/J.MCNA.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 19. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109: 433–438, 2004. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 20. Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S. Insulin resistance in essential hypertension. N Engl J Med 317: 350–357, 1987. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- 21. Velloso LA, Folli F, Sun XJ, White MF, Saad MJ, Kahn CR. Cross-talk between the insulin and angiotensin signaling systems. Proc Natl Acad Sci USA 93: 12490–12495, 1996. doi: 10.1073/PNAS.93.22.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest 100: 2158–2169, 1997. doi: 10.1172/JCI119752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tutor AW, Lavie CJ, Kachur S, Milani RV, Ventura HO. Updates on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis 78: 2–10, 2023. doi: 10.1016/J.PCAD.2022.11.013. [DOI] [PubMed] [Google Scholar]
- 24. Folli F, Centofanti L, Magnani S, Tagliabue E, Bignotto M, La Sala L, Pontiroli AE. Obesity effect on newly diagnosed and recurrent post-ablation atrial fibrillation: a systematic review and meta-analysis. J Endocrinol Invest 47: 1051–1066, 2024. doi: 10.1007/S40618-023-02225-X. [DOI] [PubMed] [Google Scholar]
- 25. Pontiroli AE, Centofanti L, Le Roux CW, Magnani S, Tagliabue E, Folli F. Effect of prolonged and substantial weight loss on incident atrial fibrillation: a systematic review and meta-analysis. Nutrients 15: 940, 2023. doi: 10.3390/nu15040940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bignotto M, Dei Cas M, Paroni R, Bianco E, Zermiani P, Gangale MG, Zadro V, Maregatti M, Piagnani A, Russo A, Baldassarre D, Folli F, Battezzati PM, Zuin M. CA.ME.LI.A. An epidemiological study on the prevalence of CArdiovascular, MEtabolic, LIver and Autoimmune diseases in Northern Italy. Nutr Metab Cardiovasc Dis 31: 1416–1426, 2021. doi: 10.1016/J.NUMECD.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association Professional Practice Committee. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2024. Diabetes Care 47, Suppl 1: S145–S157, 2024. doi: 10.2337/DC24-S008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Diabetes Association Professional Practice Committee. 1. Improving care and promoting health in populations: standards of medical care in diabetes-2022. Diabetes Care 45, Suppl 1: S8–S16, 2022. doi: 10.2337/DC22-S001. [DOI] [PubMed] [Google Scholar]
- 29. Cuzick J. A Wilcoxon-type test for trend. Stat Med 4: 87–90, 1985. doi: 10.1002/SIM.4780040112. [DOI] [PubMed] [Google Scholar]
- 30. Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med 1: 121–129, 1982. doi: 10.1002/SIM.4780010204. [DOI] [PubMed] [Google Scholar]
- 31. Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158: 1999–2014.e1, 2020. doi: 10.1053/J.GASTRO.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 32. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 73: 202–209, 2020. doi: 10.1016/J.JHEP.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 33.The Lancet Public Health. Obesity prevention: changing perspectives. Lancet Public Health 8: e161, 2023. doi: 10.1016/S2468-2667(23)00033-6. [DOI] [PubMed] [Google Scholar]
- 34. Ferreira SRG, Macotela Y, Velloso LA, Mori MA. Determinants of obesity in Latin America. Nat Metab 6: 409–432, 2024. doi: 10.1038/S42255-024-00977-1. [DOI] [PubMed] [Google Scholar]
- 35.Istat. Fattori di Rischio per la Salute: Fumo, Obesità, Alcol e Sedentarietà (Online). https://www.istat.it/it/archivio/202040 [2024 Mar 12].
- 36.Istat. Il Diabete in Italia, Anni 2000-2016 (Online). https://www.istat.it/it/files//2017/07/REPORT_DIABETE.pdf [2024 Mar 1].
- 37. Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, Gourdy P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 63: 453–461, 2020. doi: 10.1007/S00125-019-05040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav 187: 20–23, 2018. doi: 10.1016/J.PHYSBEH.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goldin C, Lleras-Muney A. XX > XY?: the changing female advantage in life expectancy. J Health Econ 67: 102224, 2019. doi: 10.1016/J.JHEALECO.2019.102224. [DOI] [PubMed] [Google Scholar]
- 40. Sanada H, Yokokawa H, Yoneda M, Yatabe J, Sasaki Yatabe M, Williams SM, Felder RA, Jose PA. High body mass index is an important risk factor for the development of type 2 diabetes. Intern Med 51: 1821–1826, 2012. doi: 10.2169/internalmedicine.51.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Després JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr 58: 463–7, 1993. doi: 10.1093/ajcn/58.4.463. [DOI] [PubMed] [Google Scholar]
- 42. Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O’Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 116: 1234–1241, 2007. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 43. Iacobini C, Pugliese G, Blasetti Fantauzzi C, Federici M, Menini S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism 92: 51–60, 2019. doi: 10.1016/J.METABOL.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 44. Ferrannini E, Nannipieri M, Williams K, Gonzales C, Haffner SM, Stern MP. Mode of onset of type 2 diabetes from normal or impaired glucose tolerance. Diabetes 53: 160–165, 2004. doi: 10.2337/DIABETES.53.1.160. [DOI] [PubMed] [Google Scholar]
- 45. Davis TME. Diabetes and metabolic dysfunction-associated fatty liver disease. Metabolism 123: 154868, 2021. doi: 10.1016/J.METABOL.2021.154868. [DOI] [PubMed] [Google Scholar]
- 46. Shaltout I, Alkandari H, Fouad Y, Hamed AE. Arabic Association for the Study of Diabetes and Metabolism (AASD) endorsing the MAFLD definition of fatty liver disease. J Hepatol 76: 739–740, 2022. doi: 10.1016/J.JHEP.2021.11.027. [DOI] [PubMed] [Google Scholar]
- 47. Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, Orlandoni P, Macarri G, Perego L, Benedetti A, Folli F. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology 29: 1743–1751, 1999. doi: 10.1002/HEP.510290632. [DOI] [PubMed] [Google Scholar]
- 48. Svegliati-Baroni G, Ridolfi F, Caradonna Z, Alvaro D, Marzioni M, Saccomanno S, Candelaresi C, Trozzi L, Macarri G, Benedetti A, Folli F. Regulation of ERK/JNK/p70S6K in two rat models of liver injury and fibrosis. J Hepatol 39: 528–537, 2003. doi: 10.1016/S0168-8278(03)00291-5. [DOI] [PubMed] [Google Scholar]
- 49.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). Tacke F, Horn P, Wai-Sun Wong V, Ratziu V, Bugianesi E, Francque S, Zelber-Sagi S, Valenti L, Roden M, Schick F, Yki-Järvinen H, Gastaldelli A, Vettor R, Frühbeck G, Dicker D. EASL-EASD-EASO clinical practice guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). Obes Facts 17: 374–444, 2024. doi: 10.1159/000539371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD). Am J Gastroenterol 102: 2716–2717, 2007. doi: 10.1111/J.1572-0241.2007.01520.X. [DOI] [PubMed] [Google Scholar]
- 51.Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): updates and future directions. Dig Liver Dis 49: 471–483, 2017. doi: 10.1016/j.dld.2017.01.147. [DOI] [PubMed] [Google Scholar]
- 52. Méndez-Sánchez N, Bugianesi E, Gish RG, Lammert F, Tilg H, Nguyen MH, Sarin SK, Fabrellas N, Zelber-Sagi S, Fan JG, Shiha G, Targher G, Zheng MH, Chan WK, Vinker S, Kawaguchi T, Castera L, Yilmaz Y, Korenjak M, Spearman CW, Ungan M, Palmer M, El-Shabrawi M, Gruss HJ, Dufour JF, Dhawan A, Wedemeyer H, George J, Valenti L, Fouad Y, Romero-Gomez M, Eslam M; Global multi-stakeholder consensus on the redefinition of fatty liver disease. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol 7: 388–390, 2022. doi: 10.1016/S2468-1253(22)00062-0. [DOI] [PubMed] [Google Scholar]
- 53. Saad MJ, Folli F, Kahn CR. Insulin and dexamethasone regulate insulin receptors, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in Fao hepatoma cells. Endocrinology 136: 1579–1588, 1995. doi: 10.1210/ENDO.136.4.7895667. [DOI] [PubMed] [Google Scholar]
- 54. Saad MJ, Folli F, Araki E, Hashimoto N, Csermely P, Kahn CR. Regulation of insulin receptor, insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-F442A adipocytes. Effects of differentiation, insulin, and dexamethasone. Mol Endocrinol 8: 545–557, 1994. doi: 10.1210/MEND.8.5.7520127. [DOI] [PubMed] [Google Scholar]
- 55. Oksuzyan A, Brønnum-Hansen H, Jeune B. Gender gap in health expectancy. Eur J Ageing 7: 213–218, 2010. doi: 10.1007/s10433-010-0170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Preis SR, Hwang SJ, Coady S, Pencina MJ, Agostino RB Sr, Savage PJ, Levy D, Fox CS. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation 119: 1728–1735, 2009. doi: 10.1161/CIRCULATIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, Karia K, Panguluri SK. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis 6: 19, 2019. doi: 10.3390/jcdd6020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Opio J, Croker E, Odongo GS, Attia J, Wynne K, McEvoy M. Metabolically healthy overweight/obesity are associated with increased risk of cardiovascular disease in adults, even in the absence of metabolic risk factors: a systematic review and meta-analysis of prospective cohort studies. Obes Rev 21: e13127, 2020. doi: 10.1111/OBR.13127. [DOI] [PubMed] [Google Scholar]
- 59. Srikanthan P, Horwich TB, Calfon Press C, Gornbein J, Watson KE. Sex differences in the association of body composition and cardiovascular mortality. J Am Heart Assoc 10: e017511, 2021. doi: 10.1161/JAHA.120.017511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tamargo J, Rosano G, Walther T, Duarte J, Niessner A, Kaski JC, Ceconi C, Drexel H, Kjeldsen K, Savarese G, Torp-Pedersen C, Atar D, Lewis BS, Agewall S. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother 3: 163–182, 2017. doi: 10.1093/EHJCVP/PVW042. [DOI] [PubMed] [Google Scholar]
- 61. Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, De Vries GJ, Epperson CN, Govindan R, Klein SL, Lonardo A, Maki PM, McCullough LD, Regitz-Zagrosek V, Regensteiner JG, Rubin JB, Sandberg K, Suzuki A. Sex and gender: modifiers of health, disease, and medicine. Lancet 396: 565–582, 2020. [Erratum in Lancet 396: 668, 2020]. doi: 10.1016/S0140-6736(20)31561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Harreiter J, Kautzky-Willer A. Sex and gender differences in prevention of type 2 diabetes. Front Endocrinol (Lausanne) 9: 220, 2018. doi: 10.3389/FENDO.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yoshida Y, Chen Z, Fonseca VA, Mauvais-Jarvis F. Sex differences in cardiovascular risk associated with prediabetes and undiagnosed diabetes. Am J Prev Med 65: 854–862, 2023. doi: 10.1016/J.AMEPRE.2023.05.011. [DOI] [PubMed] [Google Scholar]
- 64. Mauvais-Jarvis F, Arnold AP, Reue K. A guide for the design of pre-clinical studies on sex differences in metabolism. Cell Metab 25: 1216–1230, 2017. doi: 10.1016/J.CMET.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fuselier T, Mota de Sa P, Qadir MMF, Xu B, Allard C, Meyers MM, Tiano JP, Yang BS, Gelfanov V, Lindsey SH, Dimarchi RD, Mauvais-Jarvis F. Efficacy of glucagon-like peptide-1 and estrogen dual agonist in pancreatic islets protection and pre-clinical models of insulin-deficient diabetes. Cell Rep Med 3: 100598, 2022. doi: 10.1016/J.XCRM.2022.100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ferrannini E, Natali A, Muscelli E, Nilsson PM, Golay A, Laakso M, Beck-Nielsen H, Mari A; RISC Investigators. Natural history and physiological determinants of changes in glucose tolerance in a non-diabetic population: the RISC Study. Diabetologia 54: 1507–1516, 2011. doi: 10.1007/S00125-011-2112-X. [DOI] [PubMed] [Google Scholar]
- 67. Haffner SM. Obesity and the metabolic syndrome: the San Antonio Heart Study. Br J Nutr 83, Suppl 1: S67–S70, 2000. doi: 10.1017/s0007114500000970. [DOI] [PubMed] [Google Scholar]
- 68. Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, Gude F, García F, De Francisco A, Quintela AG. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 13: 47, 2013. doi: 10.1186/1472-6823-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, Rizka A, Tarigan TJE, Harbuwono DS, Purnamasari D, Soewondo P. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and tryglyceride/glucose index. Diabetes Metab Syndr 16: 102581, 2022. doi: 10.1016/J.DSX.2022.102581. [DOI] [PubMed] [Google Scholar]
- 70. Bergami M, Scarpone M, Bugiardini R, Cenko E, Manfrini O. Sex beyond cardiovascular risk factors and clinical biomarkers of cardiovascular disease. Rev Cardiovasc Med 23: 19, 2022. doi: 10.31083/J.RCM2301019. [DOI] [PubMed] [Google Scholar]
- 71. Lau ES, Paniagua SM, Guseh JS, Bhambhani V, Zanni MV, Courchesne P, Lyass A, Larson MG, Levy D, Ho JE. Sex differences in circulating biomarkers of cardiovascular disease. J Am Coll Cardiol 74: 1543–1553, 2019. doi: 10.1016/J.JACC.2019.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gottwald-Hostalek U, Gwilt M. Vascular complications in prediabetes and type 2 diabetes: a continuous process arising from a common pathology. Curr Med Res Opin 38: 1841–1851, 2022. doi: 10.1080/03007995.2022.2101805. [DOI] [PubMed] [Google Scholar]
- 73. Kim C, Catov J, Schreiner PJ, Appiah D, Wellons MF, Siscovick D, Calderon-Margalit R, Huddleston H, Ebong IA, Lewis CE. Women’s reproductive milestones and cardiovascular disease risk: a review of reports and opportunities from the CARDIA Study. J Am Heart Assoc 12: e028132, 2023. doi: 10.1161/JAHA.122.028132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ruiz-Ramie JJ, Barber JL, Lloyd-Jones DM, Gross MD, Rana JS, Sidney S, Jacobs DR Jr, Lane-Cordova AD, Sarzynski MA. Cardiovascular health trajectories and elevated C-reactive protein: the CARDIA Study. J Am Heart Assoc 10: e019725, 2021. doi: 10.1161/JAHA.120.019725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. VanWagner LB, Wilcox JE, Ning H, Lewis CE, Carr JJ, Rinella ME, Shah SJ, Lima JAC, Lloyd-Jones DM. Longitudinal association of non-alcoholic fatty liver disease with changes in myocardial structure and function: the CARDIA Study. J Am Heart Assoc 9: e014279, 2020. doi: 10.1161/JAHA.119.014279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ke Z, Huang R, Xu X, Liu W, Wang S, Zhang X, Guo Y, Zhuang X, Zhen L. Long-term high level of insulin resistance is associated with an increased prevalence of coronary artery calcification: the CARDIA Study. J Am Heart Assoc 12: e028985, 2023. doi: 10.1161/JAHA.122.028985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Murthy VL, Abbasi SA, Siddique J, Colangelo LA, Reis J, Venkatesh BA, Carr JJ, Terry JG, Camhi SM, Jerosch-Herold M, De Ferranti S, Das S, Freedman J, Carnethon MR, Lewis CE, Lima JAC, Shah RV. Transitions in metabolic risk and long-term cardiovascular health: Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAHA 5: e003934, 2016. doi: 10.1161/JAHA.116.003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Hodis HN, Kitabchi AE, Mack WJ, Mudaliar S, Ratner RE, Williams K, Stentz FB, Musi N, Reaven PD; ACT NOW Study. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 364: 1104–1115, 2011[Erratum in N Engl J Med 365: 189, 2011 and in N Engl J Med 365: 869, 2011]. doi: 10.1056/NEJMOA1010949. [DOI] [PubMed] [Google Scholar]
- 79. Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP, Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O’Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder TR; IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 374: 1321–1331, 2016. doi: 10.1056/NEJMOA1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Teo KK, Rafiq T. Cardiovascular risk factors and prevention: a perspective from developing countries. Can J Cardiol 37: 733–743, 2021. doi: 10.1016/J.CJCA.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 81. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 348: 383–393, 2003. doi: 10.1056/NEJMOA021778. [DOI] [PubMed] [Google Scholar]
- 82. Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358: 580–591, 2008. doi: 10.1056/NEJMOA0706245. [DOI] [PubMed] [Google Scholar]
- 83. Glechner A, Harreiter J, Gartlehner G, Rohleder S, Kautzky A, Tuomilehto J, Van Noord M, Kaminski-Hartenthaler A, Kautzky-Willer A. Sex-specific differences in diabetes prevention: a systematic review and meta-analysis. Diabetologia 58: 242–254, 2015. doi: 10.1007/S00125-014-3439-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1–S6 and Supplemental Tables S1–S8: https://doi.org/10.7910/DVN/L7TFFK.
Data Availability Statement
Data will be made available upon reasonable request.