Abstract
It has been reported that fluoxetine, a selective serotonin (5-hydroxytryptamine; 5-HT) reuptake inhibitor, has neuroprotective properties in the lithium–pilocarpine model of status epilepticus (SE) in rats. The aim of the present study was to investigate the effect of 5-HT depletion by short-term administration of p-chlorophenylalanine (PCPA), a specific tryptophan hydroxylase inhibitor, on the brain hypometabolism and neurodegeneration induced in the acute phase of this SE model. Our results show that 5-HT depletion did modify neither the brain basal metabolic activity nor the lithium–pilocarpine-induced hypometabolism when evaluated 3 days after the insult. In addition, hippocampal neurodegeneration and astrogliosis triggered by lithium–pilocarpine were not exacerbated by PCPA treatment. These findings point out that in the early latent phase of epileptogenesis, non-5-HT-mediated actions may contribute, at least in some extent, to the neuroprotective effects of fluoxetine in this model of SE.
Keywords: p-Chlorophenylalanine, [18F]FDG PET, Fluoro-Jade C, Status epilepticus, Lithium–pilocarpine, Serotonin
Introduction
The role of serotonin (5-hydroxytryptamine; 5-HT) and 5-HTergic drugs in the control of epileptogenesis still remains controversial. Thus, antidepressant drugs which increase the availability of monoamine neurotransmitters in the synaptic cleft have been associated both with proconvulsant and anticonvulsant effects (Jobe and Browning 2005; Hernandez et al. 2002).
The temporal lobe epilepsy (TLE) model by administration of pilocarpine (or its variant by previous administration of lithium) is one of the most employed animal models of epilepsy (Turski et al. 1983). It is characterized by a rapid status epilepticus (SE) followed by a latent period in which generalized brain hypometabolism (Goffin et al. 2009; Guo et al. 2009; Lee et al. 2012) and neuronal damage (primarily in hippocampus) appear (Mello et al. 1993; Rossi et al. 2013; Wang et al. 2008). Brain hypometabolism has been described to appear shortly after the SE onset, returning the metabolic activity to baseline values in the subacute period (Lee et al. 2012). Recently, we reported that short-term treatment with the selective 5-HT reuptake inhibitor (SSRI) fluoxetine prevented the acute hypometabolism (measured 3 days after the SE onset) and reduced the medium-term brain damage (evaluated on day 33), induced by lithium–pilocarpine administration in the latent phase of epileptogenesis (Shiha et al. 2015). In particular, at the level of the hippocampus, subacute administration of fluoxetine diminished the number of neurodegenerating neurons (Fluoro-Jade C staining), reactive gliosis (GFAP immunohistochemistry) and apoptosis (caspase-9 immunohistochemistry). In addition, and supporting an antiepileptic effect for fluoxetine, Hernandez et al. (2002) showed that in the long-term, when epilepsy was developed (i.e., several months after the SE onset), this drug reduced the spontaneous-seizure activity in the rat pilocarpine model. Since SSRI drugs increase the extracellular levels of 5-HT, the above-mentioned findings point to brain 5-HT as a neuroprotective factor in this model of SE. Nevertheless, it has also been reported that non-5HTergic mechanisms might participate in some protective actions mediated by fluoxetine (Hellmann-Regen et al. 2015; Vizi et al. 2013; Wang et al. 2003).
Regarding the effects of 5-HT depletion on epilepsy behavior, inconsistent findings have been reported so far (Bercovici et al. 2006; Racine and Coscina 1979; Tagashira et al. 1983). To date and to the best of our knowledge, no studies have evaluated the effects of 5-HT depletion on the metabolic and histochemical disturbances induced by pilocarpine- or lithium–pilocarpine-induced SE. With such a view, and in order to gain knowledge about 5-HTergic control in this experimental model of epilepsy, the current work was aimed to study the effects of p-chlorophenylalanine (PCPA), a specific depletor of brain 5-HT (Koe and Weissman 1966), on the acute metabolic and histochemical alterations triggered by the lithium–pilocarpine SE model in rats.
Materials and Methods
Animals, Seizure Induction, and Drug Protocol
Male adult Sprague–Dawley rats (Charles River Laboratories, Cerdanyola del Vallès, Spain) weighing approx. 250 g were used. The animals were pair-housed under a 12 h light/dark cycle and controlled temperature with ad libitum access to rodent food and water. The Animal Research Ethical Committee of the Universidad Complutense approved the study. All procedures were carried out in accordance with animal regulations of European Union (2010/63/UE) and Spain (RD53/2013).
SE (day 0) was induced by systemic administration of pilocarpine (25 mg/kg, i.p.). Lithium chloride (3 mEq/kg, i.p.) and methylscopolamine (2 mg/kg, i.p.) were administered 24 h and 30 min before pilocarpine injection, respectively. Forty-five min after the onset of the SE (stage 4 or 5 according to Racine scale score; Racine 1972), pentobarbital (25 mg/kg, i.p.) was administered to stop the convulsant activity. Non-pilocarpine injected animals received saline (0.9 % NaCl) as vehicle. PCPA, a specific inhibitor of tryptophan hydroxylase was administered once daily for 7 days (125 mg/kg, i.p., from day −4 to day +2). This drug regimen was chosen based in the high degree of 5-HT depletion (>90 %) yielded by similar treatments (Saadat et al. 2005; Kornum et al. 2006). Thus, 150 mg/kg PCPA for just 2 days was reported to markedly reduce 5-HT tissue concentration in several rat brain regions (e.g., 93 % reduction was found in hippocampus). Control animals were injected with saline instead of PCPA. Only rats scoring above four in the Racine scale were included in the study. All drugs were purchased from Sigma-Aldrich Spain.
[18F]FDG PET Neuroimaging
To evaluate the brain metabolic activity, [18F]FDG PET imaging was performed 3 days after pilocarpine administration, as previously reported (Shiha et al. 2015). Briefly, rats fasted for at least 12 h were injected with the positron emitter radiopharmaceutical [18F]FDG (approx. 18.5 MBq, i.v.; Instituto Tecnológico PET, Madrid, Spain). After an uptake period of 30 min, the rats were scanned with a dedicated small-animal dual PET/CT (computed tomography) device (Albira ARS scanner, Oncovision, Valencia, Spain) under 2 % isoflurane anesthesia. The length of the PET acquisition was 20 min, and it was immediately followed by a CT scanning. Maximum likelihood expectation maximization (MLEM) and filtered back projection (FBP) algorithms were used to reconstruct the PET and CT images, respectively. For the metabolic activity quantification, the procedure used was as follows: first, the CT image of the skull from each animal was co-registered to a magnetic resonance image (MRI) rat brain template in which the regions of interest (ROIs) were previously delineated. Then, the spatial mathematic transformation was applied to its own fused PET image, allowing the correct matching between the PET image and the MRI template as described by Jupp and O’Brien (2007). Unlike other studies that use a volume (such as a spheroid) inside the brain area for calculating the regional brain metabolism, we used the whole structure as a ROI (Fig. 1a). All these steps were carried out with PMOD 3.0 software (PMOD Technologies Ltd., Zurich, Switzerland). As index of regional metabolic activity, the standardized uptake value (SUV) was obtained. The SUV parameter was calculated as a ratio of a ROI radioactivity concentration (kBq/ml) measured by the scanner and the administered dose (kBq) decay-corrected at the time of the injection, divided by the body weight (g).
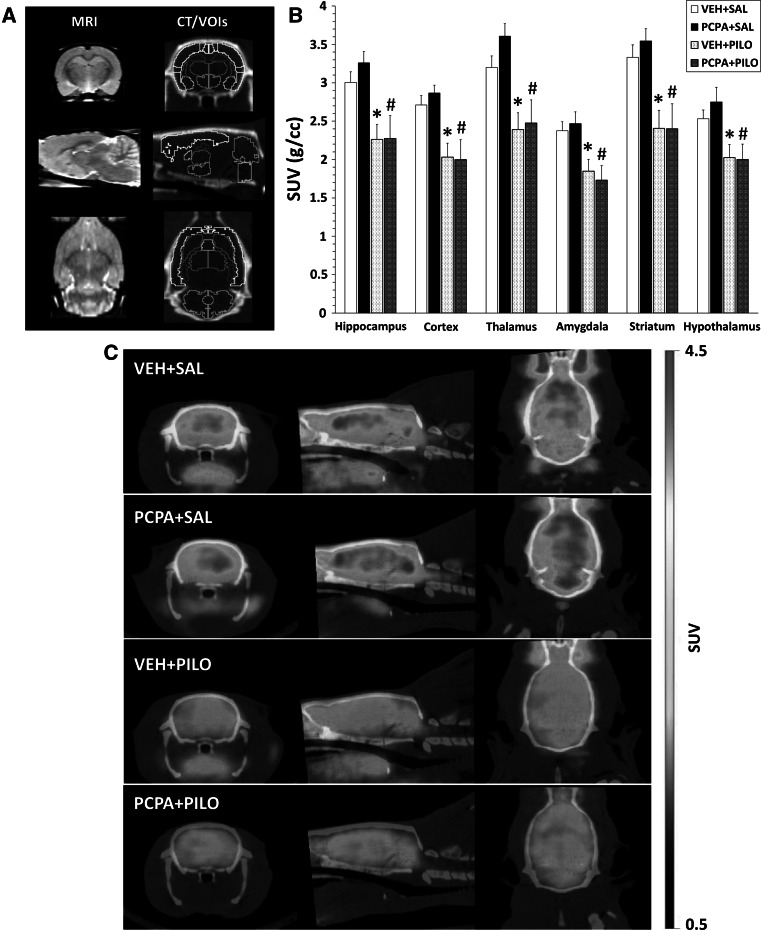
Fig. 1.
Short-term brain hypometabolism triggered by pilocarpine was not modulated by 5-HT depletion when evaluated 3 days after the SE. a The left column depicts the MRI brain template used for coregistration of PET and CT images. The right column shows a typical CT image with the volumes of interest (VOIs) used for quantification of the regional brain metabolic activity by [18F]FDG PET. Coronal, sagittal, and transaxial views are shown in the upper, middle, and bottom rows, respectively. b Regional brain glucose metabolism assessed by [18F]FDG PET (in SUV units) 3 days after the SE onset. Data are expressed as mean ± SEM. VEH + SAL (n = 11), PCPA + SAL (n = 4), VEH + PILO (n = 11), PCPA + PILO (n = 7). *p < 0.05 versus VEH + SAL group, # p < 0.05 versus VEH + PILO group; two-way ANOVA. c Representative [18F]FDG PET/CT merged images (in SUV scale) of the four experimental groups
Histochemical Assessments
Twenty-four hours after scanning, the rats were sacrificed and their brains removed in order to obtain 30-µm coronal brain slices at the level of the dorsal hippocampus (from Bregma −3.60 to −4.20 mm). Six animals per group were used for the histochemical experiments. For Fluoro-Jade C staining, we followed the protocol originally reported by Schmued et al. (2005) with minor modifications (Shiha et al. 2015). Double labeling was achieved by incubating the slices in a 0.0001 % Fluoro-Jade C/0.001 % DAPI solution. GFAP immunohistochemistry was carried out by a one-step technique using a fluorescent-labeled anti-GFAP antibody (Sigma-Aldrich, St. Louis, MO) as described (Shiha et al. 2015). The brain sections were visualized under fluorescence microscopy (Olympus IX51, Olympus Europa Holding, Germany).
Statistical Analyses of Data
Data are presented as mean ± SEM. Two-way analysis of variance (ANOVA) followed by the Tukey test was used for comparing the metabolic and histochemical data. The comparison of the mortality rates between VEH + PILO and PCPA + PILO groups was assessed by the z-test for rates and proportions. Differences at p < 0.05 were considered statistically significant. Sigmaplot v 11.0 software (Systat Software Inc., Chicago, IL) was used to perform the statistical analyses.
Results
Brain Metabolic Activity by [18F]FDG PET Neuroimaging
As shown in Fig. 1b, 3 days after SE onset, pilocarpine significantly reduced the glucose metabolism (VEH + PILO group; n = 6; p < 0.05) when compared to control rats (VEH + SAL group; n = 11). The hypometabolism was detected in all brain regions analyzed, ranging from 19.9 % in hypothalamus (SUVVEH+SAL = 2.53 ± 0.11 g/ml vs. SUVVEH+PILO = 2.03 ± 0.17 g/ml) to 27.8 % in striatum (SUVVEH+SAL = 3.34 ± 0.16 g/ml vs. SUVVEH+PILO = 2.41 ± 0.23 g/ml). Specifically, at the level of the hippocampus a reduction of 24.7 % was observed (SUVVEH+SAL = 3.00 ± 0.14 g/ml vs. SUVVEH+PILO = 2.26 ± 0.19 g/ml). On the other hand, administration of PCPA for 7 days (PCPA + SAL group; n = 4) did not modify the basal [18F]FDG uptake (p > 0.05, Fig. 1b). In addition, PCPA treatment in lithium–pilocarpine injected rats (PCPA + PILO group; n = 7) resulted in similar metabolic responses to those obtained in non-5-HT depleted rats that underwent to SE (VEH + PILO group; p > 0.05, Fig. 1b). The latter demonstrates that under our experimental conditions, 5-HT depletion had no effect on pilocarpine-induced hypometabolism. Figure 1c depicts representative PET/CT images.
Histochemical Assessments
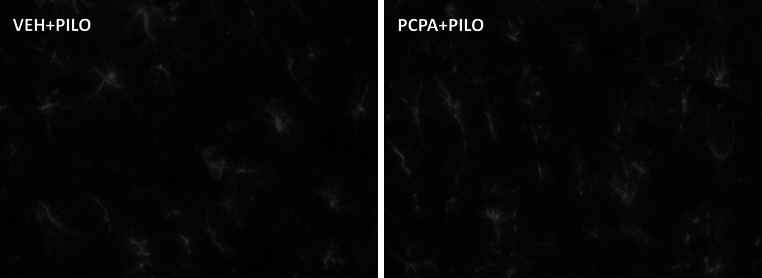
Four days after the SE, lithium–pilocarpine treatment produced a noticeable neurodegeneration in the hippocampal hilar region as assessed by Fluoro-Jade C labeling (Fig. 2a, b). As expected, no Fluoro-Jade C labeling was observed in the hilus of control rats (VEH + SAL group). Besides, PCPA administration did not affect this neurodegeneration marker (Fig. 2a, b).
Fig. 2.
Effect of PCPA treatment on the neurodegeneration induced by lithium–pilocarpine 4 days after the insult. a Quantification of hilar neurodegeneration induced by pilocarpine assessed by counting Fluoro-Jade C labeled neurons. Data are expressed as percentage of mean ± SEM (n = 6 rats/group) from VEH + PILO group. No statistically significant differences between both experimental groups were found (p > 0.05; two-way ANOVA). b Representative DAPI/Fluoro-Jade C fluorescence micrographs at the level of the dentate gyrus/hilus of the hippocampus of the four experimental groups. The images show neurodegenerating neurons in the hilus 4 days after SE onset after pilocarpine injection, not being modulated by PCPA administration. hil hilus, dg dentate gyrus. Scale bar 250 µm
GFAP immunohistochemistry was performed to evidence signs of astrogliosis as consequence of brain damage triggered by the pilocarpine-induced SE. Our results revealed that pilocarpine administration induced a marked gliosis in the hilus area (VEH + PILO group; Fig. 3, left panel). In the same way as the Fluoro-Jade/neurodegeneration results, 5-HT depletion did not statistically modify the SE-induced gliosis (Fig. 3, right panel). Accordingly, the number GFAP labeled cells in this area, expressed as percentage of VEH + PILO group, was 100.0 ± 6.17 for the VEH + PILO group and 100.8 ± 6.5 for the PCPA + PILO group. On the other hand, non-pilocarpine-insulted rats (with or without PCPA treatment) showed very low number of GFAP labeled glial cells (data not shown).
Fig. 3.
Astrogliosis induced by the lithium–pilocarpine model of SE at the level of hippocampus was not affected by PCPA treatment. The images show representative GFAP immunohistochemistry micrographs (n = 6 rats/group) at the level of the hilus of VEH + PILO (left panel) and PCPA + PILO (right panel) groups. Magnification ×40
Mortality Rate and SE Latency Time
The acute mortality rate was calculated at 24 h after SE (n = 12 rats/experimental group). Interestingly, although there were no a statistically significant difference between VEH + PILO and PCPA + PILO groups, a lower death rate tendency was observed in the PCPA + PILO group. Thus, the number of rats surviving the first 24 h after the insult was 6 of 12 for the VEH + PILO group, whereas the survival rate was 8 of 12 for the PCPA + PILO group. Therefore, the mortality rate 24 h after the SE was 50 % for the VEH + PILO group and 33 % for the PCPA + PILO group (p > 0.05; z-test for rates and proportions).
Similarly, SE latency time was not significantly affected by 5-HT depletion either, although an increasing tendency in the latency time of PCPA + PILO group was observed (VEH + PILO = 25.2 ± 2.8 min vs. PCPA + PILO = 33.5 ± 5.5 min; mean ± SEM; p > 0.05).
Discussion
Herein, we have studied the effect of brain 5-HT depletion on the short-term brain impairment triggered by the lithium–pilocarpine model of SE in rats. Dose and duration of PCPA (125 mg/kg/day × 7 days; from day −4 to day +2) were chosen in order to achieve an intense brain 5-HT depletion during the SE and the acute phase of epileptogenesis. The expected depletion should be >90 % according to reports by Saadat et al. (2005) and Kornum et al. (2006). Although a 15-day PCPA treatment has been associated with a plethora of brain alterations, including reactive gliosis (Ramos et al. 2000), neither neurodegeneration, astrogliosis nor modification of basal brain glucose consumption were detected in our study, pointing that the different length of PCPA treatment may account for this discrepancy.
When evaluated by [18F]FDG PET, lithium–pilocarpine triggered an intense and widespread brain hypometabolism not just restricted to hippocampus or temporal lobes, as reported by our group and others (Goffin et al. 2009; Guo et al. 2009; Lee et al. 2012; Shiha et al. 2015). Despite the superb sensitivity of PET, the latter can be explained by the physical and technical limits of the current PET cameras (Moses 2011). Besides, the metabolic disturbance induced by pilocarpine (alone or combined with lithium) is transient, since 7 days after the insult, the brain metabolic activity returned to baseline levels (Lee et al. 2012).
In the current study, the metabolic impairment induced by lithium–pilocarpine was accompanied with signs of hippocampal damage. Thus, neurodegeneration (Fig. 2) and astrogliosis (Fig. 3) was observed in the hippocampus shortly after (on day 4) pilocarpine-induced SE. These signs of hippocampal damage (neurodegeneration and reactive gliosis) have also been reported to show up a few hours after SE (Rossi et al. 2013; Wang et al. 2008). Unlike glucose metabolism, these brain damage markers remain longer, as they were even detectable 33 days following the SE (Shiha et al. 2015). On the other hand, pilocarpine-induced brain damage is not restricted to hippocampus, since neurodegeneration and gliosis have been reported to take place in other brain areas, including cerebral cortex, amygdala, striatum, and septum (Rossi et al. 2013; Wang et al. 2008).
Subacute fluoxetine administration prevented the brain metabolic impairment and reduced damage signs induced by lithium–pilocarpine (Shiha et al. 2015). As a prototypical SSRI drug, the neuroprotective effects of fluoxetine are thought to be mediated by increasing extracellular 5-HT (Mahar et al. 2014; Malberg et al. 2000). Nonetheless, some protective actions of fluoxetine have been associated with direct actions through 5-HT independent mechanisms by interacting with multiple targets, including receptors, voltage-gated ion channels and enzymes. Thus, fluoxetine selectively inhibits GluN2B-containing NMDA receptors, showing neuroprotective effects against excitotoxicity (Vizi et al. 2013). In addition, it has been reported to inhibit P/Q-type calcium channels, reducing glutamate exocytosis evoked by 4-aminopyridine (Wang et al. 2003) a well-known depolarizing and proconvulsant agent (Lévesque et al. 2013). Other direct action of fluoxetine that may participate in such beneficial effects is the neuronal excitability reduction by activity-dependent inhibition of sodium channels (Lenkey et al. 2006). Finally, direct actions on enzyme systems may also contribute to the antidepressant and neuroprotective effects of fluoxetine. In this way, direct actions on the acid sphingomyelinase–ceramide system, lowering the ceramide abundance (Gulbins et al. 2013) and inhibition of the retinoic acid metabolism (Hellmann-Regen et al. 2015) have recently been described. Surprisingly, detrimental effects of fluoxetine in the rat pilocarpine model have also been reported. Thus, a single dose of fluoxetine administered 30 min before pilocarpine injection reduced SE latency time and increased the mortality rate measured 24 h following SE (Freitas et al. 2006). Instead, in the current study, and in line with the metabolic and histochemical results, PCPA administration did not significantly alter the acute death rate and SE latency time triggered by lithium–pilocarpine.
The present study show that, in the early phase after SE induction by lithium–pilocarpine, 5-HT depletion does not deteriorate brain hypometabolism, neurodegeneration, and gliosis. Accordingly, it is likely that fluoxetine does not exert its neuroprotective effects via 5-HT-mediated actions in this phase of epileptogenesis. Due to the limited duration of this study, the present results are not suitable to make a general statement of 5-HT involvement in neuroprotection in the lithium–pilocarpine SE model.
Acknowledgments
This work was financially supported by grants from the Spanish Ministerio de Ciencia e Innovación (SAF2009-09020) and Comunidad de Madrid (I2M2, S2010/BMD-2349). We are grateful to Dr. José Luis López-Lacomba, director of the Instituto de Estudios Biofuncionales UCM, and Dr. Enrique Martínez-Campos for kindly allowing us to use the fluorescence microscope.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Bercovici E, Cortez MA, Wang X, Snead OC 3rd (2006) Serotonin depletion attenuates AY-9944-mediated atypical absence seizures. Epilepsia 47:240–246 [DOI] [PubMed] [Google Scholar]
- Freitas RM, Sousa FC, Viana GS, Fonteles MM (2006) Effect of gabaergic, glutamatergic, antipsychotic and antidepressant drugs on pilocarpine-induced seizures and status epilepticus. Neurosci Lett 408:79–83 [DOI] [PubMed] [Google Scholar]
- Goffin K, Van Paesschen W, Dupont P, Van Laere K (2009) Longitudinal microPET imaging of brain glucose metabolism in rat lithium–pilocarpine model of epilepsy. Exp Neurol 217:205–209 [DOI] [PubMed] [Google Scholar]
- Gulbins E, Palmada M, Reichel M, Lüth A, Böhmer C, Amato D, Müller CP, Tischbirek CH, Groemer TW, Tabatabai G, Becker KA, Tripal P, Staedtler S, Ackermann TF, van Brederode J, Alzheimer C, Weller M, Lang UE, Kleuser B, Grassmé H, Kornhuber J (2013) Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs. Nat Med 19:934–938 [DOI] [PubMed] [Google Scholar]
- Guo Y, Gao F, Wang S, Ding Y, Zhang H, Wang J, Ding MP (2009) In vivo mapping of temporospatial changes in glucose utilization in rat brain during epileptogenesis: an (18)F-fluorodeoxyglucose-small animal positron emission tomography study. Neuroscience 162:972–979 [DOI] [PubMed] [Google Scholar]
- Hellmann-Regen J, Uhlemann R, Regen F, Heuser I, Otte C, Endres M, Gertz K, Kronenberg G (2015) Direct inhibition of retinoic acid catabolism by fluoxetine. J Neural Transm. doi:10.1007/s00702-015-1407-3 [DOI] [PubMed] [Google Scholar]
- Hernandez EJ, Williams PA, Dudek FE (2002) Effects of fluoxetine and TFMPP on spontaneous seizures in rats with pilocarpine-induced epilepsy. Epilepsia 43:1337–1345 [DOI] [PubMed] [Google Scholar]
- Jobe PC, Browning RA (2005) The serotonergic and noradrenergic effects of antidepressant drugs are anticonvulsant, not proconvulsant. Epilepsy Behav 7:602–619 [DOI] [PubMed] [Google Scholar]
- Jupp B, O’Brien TJ (2007) Application of coregistration for imaging of animal models of epilepsy. Epilepsia 48(Suppl. 4):82–89 [DOI] [PubMed] [Google Scholar]
- Koe BK, Weissman A (1966) p-Chlorophenylalanine, a specific depletor of brain serotonin. J Pharmacol Exp Ther 154:499–516 [PubMed] [Google Scholar]
- Kornum BR, Licht CL, Weikop P, Knudsen GM, Aznar S (2006) Central serotonin depletion affects rat brain areas differently: a qualitative and quantitative comparison between different treatment schemes. Neurosci Lett 392:129–134 [DOI] [PubMed] [Google Scholar]
- Lee EM, Park GY, Im KC, Kim ST, Woo CW, Chung JH, Kim KS, Kim JS, Shon YM, Kim YI, Kang JK (2012) Changes in glucose metabolism and metabolites during the epileptogenic process in the lithium–pilocarpine model of epilepsy. Epilepsia 53:860–969 [DOI] [PubMed] [Google Scholar]
- Lenkey N, Karoly R, Kiss JP, Szasz BK, Vizi ES, Mike A (2006) The mechanism of activity-dependent sodium channel inhibition by the antidepressants fluoxetine and desipramine. Mol Pharmacol 70:2052–2063 [DOI] [PubMed] [Google Scholar]
- Lévesque M, Salami P, Behr C, Avoli M (2013) Temporal lobe epileptiform activity following systemic administration of 4-aminopyridine in rats. Epilepsia 54:596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar I, Bambico FR, Mechawar N, Nobrega JN (2014) Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev 38:173–192 [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello LE, Cavalheiro EA, Tan AM, Kupfe WR, Pretorius JK, Babb TL, Finch DM (1993) Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia 34:985–995 [DOI] [PubMed] [Google Scholar]
- Moses WW (2011) Fundamental limits of spatial resolution in PET. Nucl Instrum Methods Phys Res A 648(Supplement 1):S236–S240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294 [DOI] [PubMed] [Google Scholar]
- Racine R, Coscina DV (1979) Effects of midbrain raphe lesions or systemic p-chlorophenylalanine on the development of kindled seizures in rats. Brain Res Bull 4:1–7 [DOI] [PubMed] [Google Scholar]
- Ramos AJ, Tagliaferro P, López EM, Pecci Saavedra J, Brusco A (2000) Neuroglial interactions in a model of para-chlorophenylalanine-induced serotonin depletion. Brain Res 883:1–14 [DOI] [PubMed] [Google Scholar]
- Rossi AR, Angelo MF, Villarreal A, Lukin J, Ramos AJ (2013) Gabapentin administration reduces reactive gliosis and neurodegeneration after pilocarpine-induced status epilepticus. PLoS ONE 8:e78516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat KS, O’shea E, Colado MI, Elliott JM, Green AR (2005) The role of 5-HT in the impairment of thermoregulation observed in rats administered MDMA (‘ecstasy’) when housed at high ambient temperature. Psychopharmacology 179:884–890 [DOI] [PubMed] [Google Scholar]
- Schmued LC, Stowers CC, Scallet AC, Xu L (2005) Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res 1035:24–31 [DOI] [PubMed] [Google Scholar]
- Shiha AA, de Cristóbal J, Delgado M, Fernández de la Rosa R, Bascuñana P, Pozo MA, García-García L (2015) Subacute administration of fluoxetine prevents short-term brain hypometabolism and reduces brain damage markers induced by the lithium–pilocarpine model of epilepsy in rats. Brain Res Bull 111:36–47 [DOI] [PubMed] [Google Scholar]
- Tagashira E, Hiramori T, Nakao K, Urano T, Yanaura S (1983) Effects of central monoamine compounds on tranylcypromine-induced barbital-withdrawal convulsions. Life Sci 32:1599–1606 [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L (1983) Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res 9:315–335 [DOI] [PubMed] [Google Scholar]
- Vizi ES, Kisfali M, Lőrincz T (2013) Role of nonsynaptic GluN2B-containing NMDA receptors in excitotoxicity: evidence that fluoxetine selectively inhibits these receptors and may have neuroprotective effects. Brain Res Bull 93:32–38 [DOI] [PubMed] [Google Scholar]
- Wang SJ, Su CF, Kuo YH (2003) Fluoxetine depresses glutamate exocytosis in the rat cerebrocortical nerve terminals (synaptosomes) via inhibition of P/Q-type Ca2+ channels. Synapse 48:170–177 [DOI] [PubMed] [Google Scholar]
- Wang L, Liu YH, Huang YG, Chen LW (2008) Time-course of neuronal death in the mouse pilocarpine model of chronic epilepsy using Fluoro-Jade C staining. Brain Res 1241:157–167 [DOI] [PubMed] [Google Scholar]