SUMMARY
Objective:
Thiazide diuretic use is associated with higher bone mineral density (BMD) and possibly lower serum magnesium levels than loop diuretic use, and both high BMD and low serum magnesium have been linked to high prevalent knee osteoarthritis. This study aimed to compare the risk of a clinically relevant endpoint, knee replacement (KR) surgery, among initiators of thiazide and loop diuretics.
Design:
Among patients aged ≥50 years with a diagnosis of knee osteoarthritis in The Health Improvement Network (THIN) in United Kingdom, we conducted a propensity score-matched cohort study to examine the relation of thiazide diuretic initiation vs loop diuretic initiation to the risk of KR over 5 years.
Results:
Among thiazide and loop diuretic initiators (n = 3,488 for each group; mean age: 73 years; female ratio: 59%), 359 (28.6/1,000 person-years) and 283 (24.1/1,000 person-years) KRs occurred during the follow-up period, respectively. The hazard ratio (HR) of KR for thiazide diuretic initiation vs loop diuretic initiation was 1.26 (95% confidence interval [CI]: 1.08–1.47). The adherence-adjusted HR of KR for continuous use of thiazide diuretics was 1.44 (95% CI: 1.21–1.72).
Conclusions:
In this population-based cohort of patients with knee osteoarthritis, thiazide diuretic use was associated with a higher risk of KR than loop diuretic use. This association may potentially be due to thiazide diuretics’ effect on BMD and serum magnesium.
Keywords: Diuretics, Osteoarthritis, Knee replacement, Cohort
Introduction
High bone mineral density (BMD) and low serum magnesium levels are both associated with a high prevalence of knee osteoarthritis (OA)1-3. High BMD is also associated with an increased risk of incident knee OA but may protect against knee OA progression4. Such a paradoxical phenomenon is not fully understood, though selection bias due to conditioning on an intermediate stage of knee OA has been postulated as one potential explanation. Identifying risk factors of knee OA progression would provide insightful guidance for secondary prevention of this disabling disease; however, owing to methodological (e.g., confounding by indication and index event bias) and logistic (e.g., repeated assessment of BMD or serum magnesium level) challenges, few, if any, studies have examined the effect of changes in either BMD or serum magnesium on the risk of knee OA progression due to exposure to incident extraneous factors (e.g., medication use) occurring after knee OA diagnosis.
Diuretics are commonly-used medications with an acceptable side–effect profile4. There is increasing evidence for a beneficial effect of thiazide diuretic therapy in preserving BMD5. In contrast, loop diuretics have been found to decrease BMD6. In addition, there is increasing recognition of the potential effect of diuretic use on lowering serum magnesium levels4,7,8; the effect appears to be greater for thiazide than loop diuretics9,10. Of relevance, the Rotterdam Study recently reported that serum magnesium levels were lower among thiazide users but higher among loop diuretic users than nonusers of either medications9.
Since diuretics are often used for relatively long periods of time, and thiazide and loop diuretics may have different impact on BMD and serum magnesium levels, initiation of these two medications could serve as a potential indicator for changes in BMD and serum magnesium levels4,7-9. Assessment of their relation to the risk of end stage knee OA should shed light on our understanding of the role of both BMD and magnesium in knee OA progression. We conducted a sequential propensity score-matched cohort study to compare the risk of the clinically relevant endpoint of knee replacement (KR) surgery among patients with knee OA who initiated thiazide vs those who initiated loop diuretics.
Methods
Data source
We used The Health Improvement Network (THIN), an electronic medical record database from general practitioners in United Kingdom10. THIN contains health information on approximately 17 million patients from 770 general practices in the UK, and previous study has shown that THIN is representative of the UK population in terms of patient demographics and the prevalence of common illnesses11. During consultation with patients, health information is recorded by general practitioners using a computerized system. The information includes socio-demographics, anthropometrics, lifestyle factors, details from general practice visits, diagnoses from specialists’ referrals as well as hospital admissions, and results of laboratory tests. The Read classification system is used to code specific diagnoses. Prescription medications are coded based on a drug dictionary in BNF code and ATC code formats from the Multilex classification system12. Scientific Review Committee for the THIN database and the Institutional Review Board at Xiangya Hospital approved this study, with waiver of informed consent.
Study population
We identified knee OA based on Read codes (i.e., a coded thesaurus of clinical terms) that have been used in the National Health Service in the UK since 1985. Read codes provide a standard vocabulary for clinicians to record findings and procedures of the patients when they receive health and social care. We identified knee OA based on the following Read codes: N053611 (patellofe-moral osteoarthritis), N05z611 (knee osteoarthritis not otherwise specified), N05zL00 (osteoarthritis not otherwise specified, of knee), and N05zM00 (osteoarthritis not otherwise specified, of tibio-fibular joint). We included individuals (≥50 years) who had a diagnosis of knee OA by Read codes between January 2000 and December 2016. Patients with OA coded only as general, without specifying the knee, were ineligible for the current analysis. We also excluded subjects who had KR prior to knee OA diagnosis, or who were deemed unlikely to be a candidate for KR (i.e., history of joint infection or comorbidities with poor prognosis [end-stage renal disease on dialysis, severe pulmonary disease requiring supplemental oxygen, or any cancer])10. In addition, we introduced a 3-month exposure lag period to exclude subjects with KR within 3 months after entering the study cohort because KRs that occur right after the initiation of diuretic were likely to have been scheduled before initiation of the medication.
Assessment of exposure and active comparator
We identified individuals who initiated thiazide or loop diuretics through the following ATC codes: C03AA01 (Bendro-flumethiazide), C03AA04 (Chlorothiazide), C03BA04 (Chlortalidone), C03BA12 (Clorexolone), C03AA03 (Hydrochloro-thiazide), C03AA02 (Hydroflumethiazide), C03BA11 (Indapamide), C03BA05 (Mefruside), C03AA08 (Methyclothiazide), C03BA08 (Metolazone), C03AA05 (Polythiazide), and C03BA10 (Xipamide) for thiazide diuretics; C03CA02 (Bumetanide), C03CC01 (Etacrynic), C03CA01 (Furosemide), C03CA03 (Piretanide), and C03CA04 (Tor-asemide) for loop diuretics, and BNF (thiazide diuretics: 2.2.1; loop diuretics: 2.2.2). The “initiation” of thiazide or loop diuretic was defined as the first prescription of thiazide or loop diuretic after the knee OA diagnosis during the study period in THIN database. Subjects with a history of prescription of thiazide or loop diuretic before entering the study were considered prevalent users and excluded from the study. In addition, subjects were required to be continuously enrolled with the general practice for ≥ 1 year in THIN database before the date of first prescription date of either thiazide or loop diuretic (i.e., index date).
Assessment of outcome
The outcome was the first (i.e., incident) primary KR that occurred 3 months after initiation of either thiazide or loop diuretics during the follow-up period. Primary KR included both total and partial KR, identified by Read codes. Previous studies have used this approach to identify KRs in THIN and Clinical Practice Research Datalink (a similar database to THIN)10,13-15.
Sequential propensity score-matched cohorts
We conducted a sequential propensity score-matched cohort study to compare the risk of KR among thiazide diuretic initiators with that among loop diuretic initiators. Propensity score is the probability of treatment assignment (e.g., thiazide initiation) conditional on observed baseline characteristics. Propensity score matching is used to mitigate the effects of confounding by indication, especially in the presence of a large number of covariates, in epidemiological studies16. We divided calendar time into 1-year blocks from 2000 to 2016 (i.e., 17 blocks). Specifically, subjects were allocated into 17 blocks based on their index date, which was based on the date of initiation of either their thiazide or loop diuretic. For example, subjects whose initiation date fell between January 1, 2000 and December 31, 2000 would be allocated into the first (year-2000) time block. Within each time block, we assembled a cohort of thiazide initiators, defined as patients who started thiazide during that time block, and a comparator cohort of matched loop diuretic initiators, who started loop diuretic during the same time block. We conducted propensity score matching within each time block using a greedy matching algorithm17, i.e., for each thiazide initiator, an initiator of a loop diuretic with the closest propensity score was selected as a comparator from the same time block. Propensity scores (i.e., predicted probability of thiazide initiation) were estimated using logistic regression separately for each time block. The variables included in the model consisted of sociodemographic factors (age at index date, sex, the Townsend Deprivation Index score18), body mass index (BMI), duration of osteoarthritis prior to the index date, lifestyle factors (smoking status, and alcohol use), comorbidities and medication use recorded in THIN at any time prior to the index date, and healthcare utilization during the 1 year before the index date (see Table I). Comorbidities and medication use were assessed from the date subjects entered into THIN until their index date.
Table I.
Baseline Characteristics of Propensity Score-matched Knee Osteoarthritis Patients (≥50 years) Initiating Thiazide or Loop Diuretics
| Variable list | Thiazide diuretics (n = 3,488) |
Loop diuretics (n = 3,488) |
Standard difference |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD), y | 72.6 (9.0) | 72.5 (9.6) | 0.010 |
| Socioeconomic deprivation index score*, mean (SD) | 2.8 (1.3) | 2.8 (1.3) | 0.018 |
| Female (%) | 59.1 | 58.4 | 0.015 |
| OA duration, mean (SD), y | 7.4 (6.6) | 7.5 (6.7) | 0.010 |
| BMI, mean (SD), kg/m2 | 29.9 (6.0) | 29.8 (6.3) | 0.010 |
| Lifestyle factors | |||
| Drinking (%) | 0.025 | ||
| None | 24.6 | 23.9 | |
| Past | 3.6 | 3.2 | |
| Current | 71.8 | 72.8 | |
| Smoking (%) | 0.006 | ||
| None | 54.6 | 54.9 | |
| Past | 33.3 | 33.1 | |
| Current | 12.0 | 12.0 | |
| Comorbidity (%) | |||
| Chronic kidney diseases | 8.2 | 8.3 | 0.003 |
| Congestive heart failure | 2.4 | 2.7 | 0.018 |
| Hypertension | 59.5 | 59.8 | 0.007 |
| Atrial fibrillation | 7.9 | 8.3 | 0.014 |
| Chronic obstructive pulmonary disease | 6.7 | 7.0 | 0.011 |
| Myocardial infarction | 6.5 | 6.5 | <0.001 |
| Peripheral vascular disease | 2.4 | 2.7 | 0.015 |
| Angina | 14.4 | 14.5 | 0.003 |
| Diabetes | 19.0 | 19.3 | 0.007 |
| Venous thromboembolism | 5.1 | 5.3 | 0.012 |
| Hyperlipidemia | 17.2 | 17.8 | 0.016 |
| Ischemic heart disease | 20.1 | 20.2 | 0.004 |
| Liver disease | 2.6 | 2.8 | 0.012 |
| Pneumonia or infection | 8.5 | 7.7 | 0.026 |
| Stroke | 5.8 | 5.3 | 0.021 |
| Transient ischemic attack | 5.9 | 5.4 | 0.021 |
| Varicose veins | 15.2 | 15.8 | 0.017 |
| Depression | 14.6 | 14.2 | 0.012 |
| Dementia | 1.4 | 1.5 | 0.002 |
| Fall | 17.4 | 17.9 | 0.012 |
| Fracture | 11.5 | 11.2 | 0.010 |
| Peptic ulcer | 8.7 | 9.3 | 0.021 |
| Osteoporosis | 10.0 | 10.1 | 0.004 |
| Rheumatic arthritis | 2.2 | 2.2 | 0.004 |
| Medication (%) | |||
| ACE inhibitors | 38.0 | 38.4 | 0.007 |
| Beta receptor inhibitors | 36.0 | 36.3 | 0.006 |
| Calcium channel blockers | 39.9 | 39.6 | 0.006 |
| Angiotensin receptor blockers | 11.4 | 11.4 | <0.001 |
| Statins | 42.6 | 42.8 | 0.005 |
| Anticoagulants | 8.7 | 9.5 | 0.031 |
| Antidiabetic medicine | 14.6 | 14.9 | 0.009 |
| Insulin | 3.5 | 3.6 | 0.005 |
| Aspirin | 44.2 | 43.2 | 0.020 |
| Glucocorticoids | 25.7 | 25.0 | 0.017 |
| Nitrates | 17.9 | 18.4 | 0.013 |
| NSAIDs | 86.0 | 85.7 | 0.008 |
| Opioids | 45.4 | 44.5 | 0.020 |
| Estrogen | 11.9 | 12.7 | 0.025 |
| Bisphosphonates | 8.6 | 8.4 | 0.005 |
| PPIs | 50.1 | 49.7 | 0.007 |
| H2 blockers | 27.7 | 28.2 | 0.010 |
| Healthcare utilization, mean (SD) | |||
| Hospitalizations† | 0.4 (0.9) | 0.4 (0.9) | 0.010 |
| General practice visits† | 7.8 (5.8) | 7.9 (6.4) | 0.025 |
| Specialist referrals† | 0.6 (1.1) | 0.6 (1.1) | 0.003 |
BMI, body mass index; n, number; y, years; SD, standard deviation; NSAIDs, non-steroidal anti-inflammatory drugs; ACE, angiotensin converting enzyme; OA, osteoarthritis; PPIs, proton pump inhibitors; H2, histamine-2.
The Socio-Economic Deprivation Index was measured by the Townsend Deprivation Index, which was grouped into quintiles from 1 (least deprived) to 5 (most deprived).
Frequency during the past 1 year.
Statistical analysis
We compared the baseline characteristics of the two cohorts (i.e., thiazide diuretic initiators and loop diuretic initiators). The follow-up time for each subject began from the index date until the date of KR, death, age of 90, date of disenrollment in THIN, or the end of the fifth year of follow-up (because approximately 90% of subjects’ diuretics prescriptions were for 5 years or less), whichever occurred first. We calculated the cumulative incidence rate of KR to depict the risk of KR for each cohort accounting for the competing risk of death19. The absolute rate difference (RD) in KR was estimated between the thiazide cohort and loop diuretic cohort. Since the two compared cohorts were balanced at baseline, we were able to calculate the crude RD between two groups. The following formula was used for RD (95% confidence interval [CI]) calculation: RD = rate (exposed) - rate (non-exposed); , where and refer to the number of events in each cohort, PTa and PTb refer to the total person-time accumulated in each cohort, and 95% CI: RD ± 1.96*SERD. We fitted cause-specific Cox proportional hazard models to examine the relation of thiazide vs loop diuretic initiation to the hazard of KR while accounting for the competing risk of death19.
To account for time-varying exposures and confounders, we performed a marginal structural model to estimate the average adherence-adjusted hazard ratio (HR) of KR for continuous use of thiazide vs loop diuretic use20. Specifically, time-varying exposures and confounders were updated each year. We fit pooled logistic regression models to obtain their predicted values for each person-year remaining off thiazide diuretic prescription and uncensored. We then used a SAS data step to calculate stabilized inverse-probability weights for each person-year from the predicted values of the previous models. Last, we used generalized estimating equations to fit the final weighted pooled logistic model that estimated the causal parameter and its robust standard error. Variables in the calculation of the propensity score were included in these models.
We performed several sensitivity analyses. First, to further minimize potential confounding by indication, we conducted the analyses by excluding patients with chronic kidney disease. Second, we used asymmetric trimming to exclude patients whose propensity score was <2.5th percentile of the propensity score of the exposure (thiazide) cohort and >97.5th percentile of the propensity score of the comparison cohort (loop diuretics); thus, patients who were treated with the agent most contrary to prediction were excluded from the analyses to minimize potential unmeasured confounders. Third, we performed an analysis among subjects who were enrolled in THIN for at least 1 year without a diagnosis of OA prior to inclusion in the study sample (i.e., incident OA10) to minimize potential misclassification of the duration of OA. Fourth, since the analyses may not fully adjust for potential confounders we performed quantitative sensitivity analyses to assess the minimum unmeasured confounding effect that would need to explain away an association observed in the main analyses conditional on the included covariates21.
In addition, we conducted a nested case–control study to assess the dose–response relationship between number of prescriptions of thiazides and risk of KR using risk set sampling22. Specifically, for each case of KR, we created a risk set that included up to 10 controls who were alive and free of KR when a KR case occurred and matched by sex, year of entry into study, age of entry into study (within ±1 year), and propensity-score (within a caliper of ±0.1). The number of prescriptions of thiazides was calculated from the date of thiazide initiation to the date the case (i.e., KR) and matched controls (assigned the same date as their matched case) were identified. We divided number of prescriptions of thiazides into three categories: non-use of thiazides, 1–5, and ≥six prescriptions of thiazides. We estimated the relation of each thiazide prescription category to the risk of KR using conditional logistic regression and tested a dose–response relationship by number of thiazide prescriptions.
All P-values were two-sided. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
In total,200,317 subjects with knee OA met our inclusion criteria. Of them 16,431 initiated a thiazide diuretic and 15,069 initiated a loop diuretic. We excluded 9,334 subjects who were deemed unlikely to be a candidate for KR, 269 subjects who had KR within 3 months after initiation of the diuretic because KRs that occur during this period were likely to have been scheduled before initiation of the medication, and 5,091 subjects who had missing information on BMI, Townsend Deprivation Index Score, smoking status and alcohol drinking. Of the remaining (n = 16,806) 3,488 initiators of thiazide were successfully propensity score-matched to the same number of initiators of loop diuretic (Fig. 1). The baseline characteristics of the two propensity score-matched cohorts are shown in Table I. The mean agewas 73 years, and slightly more than 40% were men. The characteristics of the thiazide cohort and its matched comparison loop diuretic cohort were well-balanced, with all standardized differences being < 0.123.
Fig. 1.
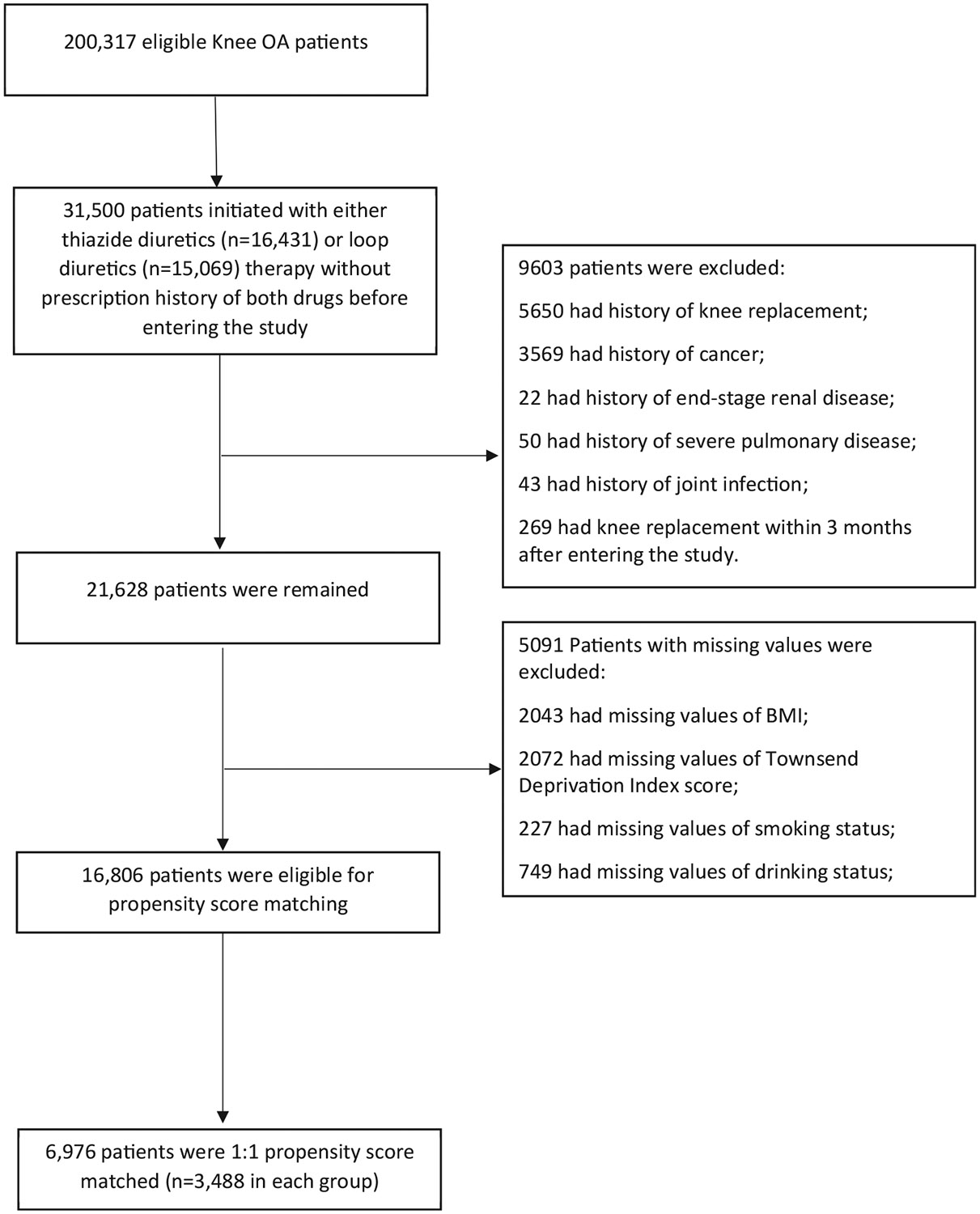
Selection process of included subjects.
The cumulative incidence of KR was higher in the thiazide cohort than in the loop diuretic cohort (Fig. 2). As shown in Table II, 359 kRs (28.6/1,000 person-years) occurred in the thiazide cohort and 283 (24.1/1,000 person-years) occurred in the loop diuretic cohort over 5 years of follow-up. The RD of incident KR in the thiazide cohort vs that in the loop diuretic cohort was 4.5 (95% CI: 1.08 to 1.47) per 1,000 person-years and the corresponding HR was 1.26 (95% CI: 1.08 to 1.47). The adherence-adjusted HR of KR for continuous use of thiazide was 1.44 (95% CI: 1.21 to 1.72).
Fig. 2.
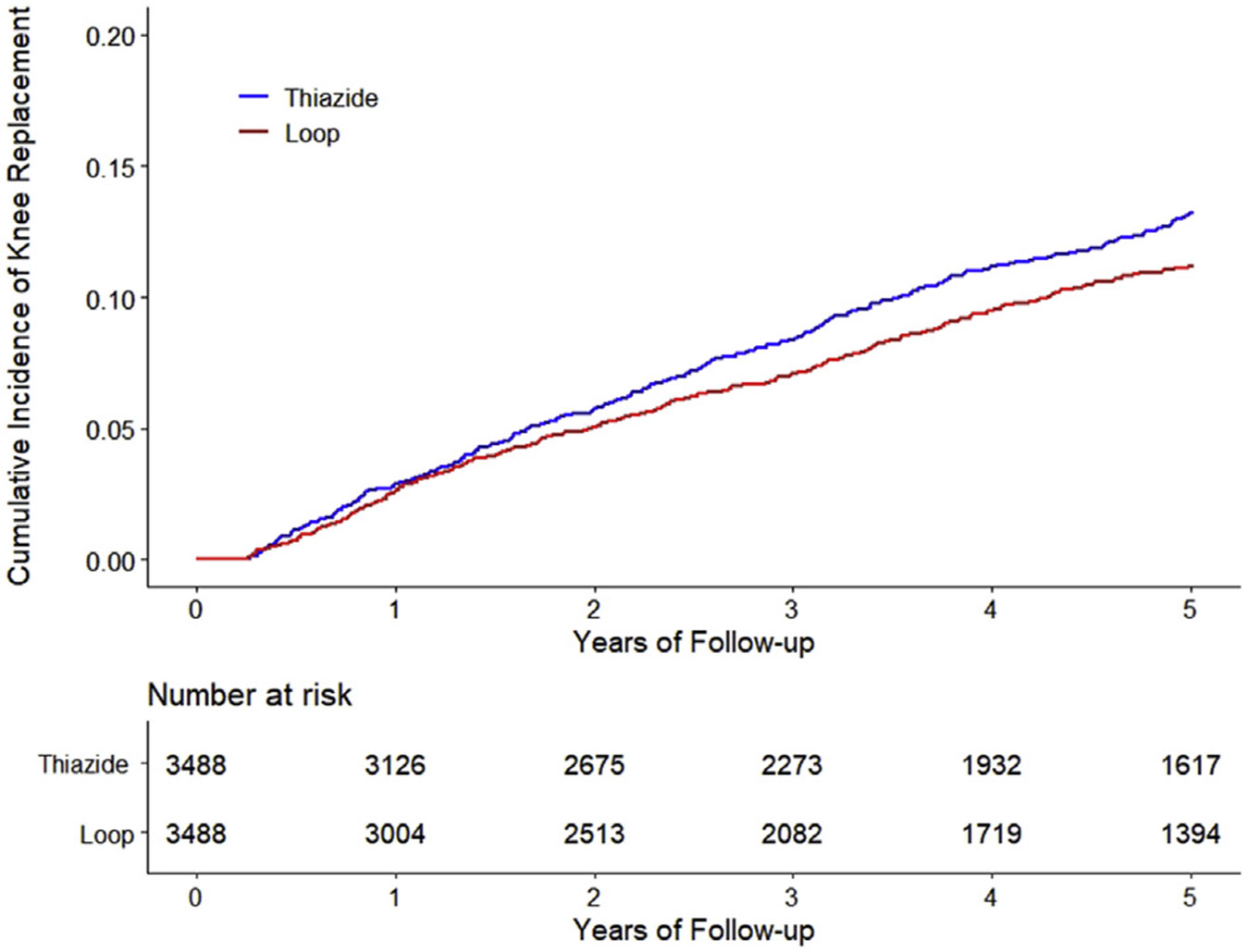
Time to Knee Replacement over Five Years for the Propensity Score-Matched Cohorts of Knee Osteoarthritis Patients with Thiazide Diuretic Initiation compared with Initiation of Loop Diuretic, adjusting for Competing Risk of Death.
Table II.
Association between thiazide diuretic initiation and incidence of knee replacement surgery comparing with loop diuretic initiation
| Thiazide diuretics | Loop diuretics | |
|---|---|---|
| Total population* | ||
| Subject (n) | 3,488 | 3,488 |
| Incident knee replacement (n) | 359 | 283 |
| Mean follow-up (year) | 3.6 | 3.4 |
| Rate (1,000 person-years) | 28.6 | 24.1 |
| RD (1,000 person-years, 95% CI) | 4.5 (0.4, 8.5) | 0.00 (reference) |
| HR (95% CI)† | 1.26 (1.08, 1.47) | 1.00 (reference) |
| Adherence-adjusted, HR (95% CI)† | 1.44 (1.21, 1.72) | 1.00 (reference) |
| Excluding CKD, HR (95% CI)† | 1.27 (1.08, 1.49) | 1.00 (reference) |
| PS trimming, HR (95% CI)† | 1.30 (1.11, 1.52) | 1.00 (reference) |
| Incident OA, HR (95% CI)† | 1.23 (1.02, 1.48) | 1.00 (reference) |
HR, hazard ratio; n, number; RD, rate difference; PS, propensity score; 95% CI, 95% confidence interval; CKD, chronic kidney disease.
Thiazide initiation also showed a higher risk of knee replacement compared with initiation of loop diuretics (hazard ratio = 1.26, 95% confidence interval: 1.10 to 1.44) without restricting to 5-year follow-up.
Hazard ratios were adjusted for competing event (death).
The results from various sensitivity analyses are presented in Table II. Exclusion of subjects with chronic kidney disease (HR = 1.27, 95% CI: 1.08 to 1.49), extreme propensity scores (HR = 1.30, 95% CI: 1.11 to 1.52), or restricting to subjects with incident knee OA (HR = 1.23, 95% CI: 1.02 to 1.48) did not change the association materially. Furthermore, to completely nullify the observed associations (e.g., HR = 1.23 for the smallest effect estimate), the association of residual confounder(s) with either thiazide or with KR should be ≥ an odds ratio of 1.76. Such a strong residual confounder(s) seems unlikely given that many known confounders have been accounted for in the propensity score-matched design.
The odds of KR increased with longer duration of thiazide use (Table III). Compared with non-use of thiazide, multivariable-adjusted odds ratios (ORs) of KR were 1.16 (95% CI: 0.90 to 1.48) and 1.28 (95% CI: 1.03 to 1.58) for 1–5 and ≥six prescriptions of thiazide, respectively (P for trend = 0.04).
Table III.
Dose–response association between thiazide diuretic initiation and incidence of knee replacement surgery
| Cases* | Controls | Odds Ratio (95% CI) |
|
|---|---|---|---|
| Total number | 599 | 2,446 | – |
| Non-use of thiazide (n) | 227 | 1,039 | 1.00 (reference) |
| 1-5 prescriptions of thiazide (n) | 140 | 560 | 1.16 (0.90, 1.48) |
| 6 or more prescriptions of thiazide (n) | 232 | 847 | 1.28 (1.03, 1.58) |
95% CI, 95% confidence interval; n, number.
Incident knee replacement.
Discussion
We found that the risk of KR was higher among thiazide initiators than those of loop diuretic initiators, and long-term use of thiazide was associated with even higher risk of KR than loop diuretics. Our findings were independent of the major confounders and remained stable in various sensitivity analyses, suggesting that the observed associations were robust.
Possible explanations
While the biological mechanisms linking thiazide use to the risk of KR are not fully understood, differential impacts of thiazide vs loop diuretics on changes in both BMD and serum magnesium levels may partly explain these findings. First, randomized controlled trial (RCT) demonstrated that thiazide preserved BMD5, loop diuretics decreased BMD6. Many studies have shown that high BMD is associated with prevalent and incident knee OA; however, its association with knee OA progression remains controversial. One explanation for such paradoxical phenomena is related to the fact that BMD is a chronic risk factor. Observational studies of the association of BMD with the risk of knee OA progression among subjects with mild-to-moderate knee OA are in effect adjusting for an intermediate stage of disease (i.e., mild-to-moderate knee OA). Such studies are affected by collider bias (index event bias) and are susceptible to potential selection bias.
Second, previous studies have shown that chronic use of either thiazide or loop diuretics increases magnesium excretion; however, the degree of magnesium depletion from thiazide use appears to be greater than loop diuretic use4,7-9. Recently, the Rotterdam Study reported that thiazide use was associated with lower whereas loop diuretic use with higher serum magnesium levels than nonuse, respectively7. An animal study demonstrated that intra-articular magnesium sulfate attenuated the development of OA24, and several cross-sectional clinical studies have found that low levels of serum magnesium were associated with high prevalence of knee OA2,3. In addition, magnesium is an antagonist of N-methyl-D-aspartate receptors, which plays an important role in nociceptive transmission, modulation and sensitization of pain25. Indeed, results from a meta-analysis demonstrated that systemic administration of magnesium was effective in minimizing postoperative pain26. Thus, thiazide diuretics could increase the risk of KR by decreasing serum magnesium levels.
Strengths and limitations
Several characteristics of our study are worth noting. First, BMD or serum magnesium are both chronic factors; thus, they likely occur prior to the occurrence of knee OA. Observational studies of the effects of a prevalent exposure on disease progression are susceptible to potential selection bias. To mitigate this kind of bias, we examined initiation of diuretics after knee OA diagnosis, whereby both BMD and serum magnesium levels may be altered by these medications after knee OA diagnosis. Second, in contrast to observational studies that compared users of anti-osteoporotic drugs or magnesium-supplement with non-users, we assembled two comparative cohorts who initiated different types of diuretics to minimize confounding by indication.
Third, we postulated that an increased risk of KR among thiazide users may be through its impact on BMD and/or serum magnesium; however, we can't verify these mechanisms owing to lack of BMD or serum magnesium data in THIN. Fourth, although previous studies have used Read codes to define symptomatic knee OA in THIN27,28 and KR has been generally accepted as a “hard” outcome in cohort studies of knee OA10,14,29,30, we were unable to confirm a diagnosis of radiographic knee OA and to assess the radiographic progression of knee OA since knee image data were not available in THIN. Nonetheless, 96% of primary KRs are performed for knee OA31, though we acknowledge that there is potential for individuals qualifying for KR but not undergoing the procedure due to other factors such as personal preference. In addition, we excluded patients with rheumatoid arthritis (2.2% in thiazide diuretics group and 2.2% in loop diuretics group according to Table I), the result did not change materially (HR = 1.25, 95% CI: 1.07 to 1.47). Fifth, though we took the propensity score-matching method including many comorbidities as covariates to control for potential confounding bias, as in any observational study we cannot rule out residual confounding. Finally, the current study was conducted using UK data. The differences in health care systems between UK and other countries may limit the generalizability of our study findings. Thus, future studies conducted outside UK are warranted to verify the findings.
Clinical and research implications
The present findings may have clinical implications. If replicated and determined to be causal, these findings suggest that thiazide diuretics use may have an unfavorable effect on knee OA progression. In addition, this study may shed light on our understanding of the biological mechanisms linking thiazide use to the risk of knee OA progression. If future studies could collect data on thiazide use, BMD, serum levels of magnesium, as well as changes in knee structures and symptoms, we could assess to what extend the effect of thiazide use on the risk of knee OA progression is mediated via effects on BMD or magnesium levels; such insight could help guide the development of targeted treatment strategies for knee OA prevention and progression.
Conclusions
In this population-based cohort of patients with knee OA, thiazide diuretic use was associated with a higher risk of KR than loop diuretic use. Such an association may potentially be due to thiazide diuretics’ effect on BMD and serum magnesium.
Acknowledgement
Everyone who contributed significantly to the work has been listed.
Funding
This work was supported by the National Natural Science Foundation of China, China (grant number 81772413, 81702207, 81702206), National Institutes of Health, United States (grant number R21 AR47785), Department of Veterans Affairs Merit Review Grant, United States (grant number I01BX001660 [RT]), and National Institutes of Health, United States (grant number K24 AR070892).
Role of the funder/sponsor
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; praparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Conflict of interest
None.
Ethical approval
The Institutional Review Board approved this study, with waiver of informed consent.
Scientific approval
This study was approved by the THIN Scientific Review Committee (18THIN073).
Transparency
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joca.2019.05.020.
Reference
- 1.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med 2000;133:635–46. [DOI] [PubMed] [Google Scholar]
- 2.Hunter DJ, Hart D, Snieder H, Bettica P, Swaminathan R, Spector TD. Evidence of altered bone turnover, vitamin D and calcium regulation with knee osteoarthritis in female twins. Rheumatology 2003;42:1311–6. [DOI] [PubMed] [Google Scholar]
- 3.Zeng C, Wei J, Li H, Yang T, Zhang FJ, Pan D, et al. Relationship between serum magnesium concentration and radiographic knee osteoarthritis. J Rheumatol 2015;42:1231–6. [DOI] [PubMed] [Google Scholar]
- 4.Ernst ME, Moser M. Use of diuretics in patients with hypertension. N Engl J Med 2009;361:2153–64. [DOI] [PubMed] [Google Scholar]
- 5.LaCroix AZ, Ott SM, Ichikawa L, Scholes D, Barlow WE. Low-dose hydrochlorothiazide and preservation of bone mineral density in older adults. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2000;133:516–26. [DOI] [PubMed] [Google Scholar]
- 6.Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Loop diuretics increase bone turnover and decrease BMD in osteopenic postmenopausal women: results from a randomized controlled study with bumetanide. J Bone Miner Res 2006;21:163–70. [DOI] [PubMed] [Google Scholar]
- 7.Kieboom BCT, Zietse R, Ikram MA, Hoorn EJ, Stricker BH. Thiazide but not loop diuretics is associated with hypo-magnesaemia in the general population. Pharmacoepidemiol Drug Saf 2018;27:1166–73. [DOI] [PubMed] [Google Scholar]
- 8.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Investig 2005;115:1651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agus ZS. Mechanisms and causes of hypomagnesemia. Curr Opin Nephrol Hypertens 2016;25:301–7. [DOI] [PubMed] [Google Scholar]
- 10.Neogi T, Li S, Peloquin C, Misra D, Zhang Y. Effect of bisphosphonates on knee replacement surgery. Ann Rheum Dis 2018;77:92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf 2007;16:393–401. [DOI] [PubMed] [Google Scholar]
- 12.Stuart-Buttle CD, Read JD, Sanderson HF, Sutton YM. A language of health in action: read Codes, classifications and groupings. Proc AMIA Annu Fall Symp 1996:75–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo CF, Chou IJ, See LC, Chen JS, Yu KH, Luo SF, et al. Urate-lowering treatment and risk of total joint replacement in patients with gout. Rheumatology 2018;57:2129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu D, Jordan KP, Snell KIE, Riley RD, Bedson J, Edwards JJ, et al. Development and validation of prediction models to estimate risk of primary total hip and knee replacements using data from the UK: two prospective open cohorts using the UK Clinical Practice Research Datalink. Ann Rheum Dis 2019;78:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misra D, Lu N, Felson D, Choi HK, Seeger J, Einhorn T, et al. Does knee replacement surgery for osteoarthritis improve survival? The jury is still out. Ann Rheum Dis 2017;76:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf 2005;14:465–76. [DOI] [PubMed] [Google Scholar]
- 17.Parsons L. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. In: Proceedings of the Twenty-Sixth Annual SAS Users Group International Conference 2001. SAS Institute Inc.; 2001. [Google Scholar]
- 18.Morris R, Carstairs V. Which deprivation? A comparison of selected deprivation indexes. J Public Health Med 1991;13:318–26. [PubMed] [Google Scholar]
- 19.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–70. [DOI] [PubMed] [Google Scholar]
- 21.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268–74. [DOI] [PubMed] [Google Scholar]
- 22.Breslow NE, Day NE. Statistical methods in cancer research. Volume II-The design and analysis of cohort studies. IARC Sci Publ 1987:178–229. [PubMed] [Google Scholar]
- 23.Franklin JM, Rassen JA, Ackermann D, Bartels DB, Schneeweiss S. Metrics for covariate balance in cohort studies of causal effects. Stat Med 2014;33:1685–99. [DOI] [PubMed] [Google Scholar]
- 24.Lee CH, Wen ZH, Chang YC, Huang SY, Tang CC, Chen WF, et al. Intra-articular magnesium sulfate (MgSO4) reduces experimental osteoarthritis and nociception: association with attenuation of N-methyl-D-aspartate (NMDA) receptor sub-unit 1 phosphorylation and apoptosis in rat chondrocytes. Osteoarthritis Cartilage 2009;17:1485–93. [DOI] [PubMed] [Google Scholar]
- 25.Herroeder S, Schonherr ME, De Hert SG, Hollmann MW. Magnesium-essentials for anesthesiologists. Anesthesiology 2011;114:971–93. [DOI] [PubMed] [Google Scholar]
- 26.De Oliveira GS Jr, Castro-Alves LJ, Khan JH, McCarthy RJ. Peri-operative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 2013;119:178–90. [DOI] [PubMed] [Google Scholar]
- 27.Gore M, Sadosky AB, Leslie DL, Tai KS, Emery P. Therapy switching, augmentation, and discontinuation in patients with osteoarthritis and chronic low back pain. Pain Pract 2012;12:457–68. [DOI] [PubMed] [Google Scholar]
- 28.Zeng C, Dubreuil M, LaRochelle MR, Lu N, Wei J, Choi HK, et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. J Am Med Assoc 2019;321:969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan T, Alvand A, Prieto-Alhambra D, Culliford DJ, Judge A, Jackson WF, et al. ACL and meniscal injuries increase the risk of primary total knee replacement for osteoarthritis: a matched case-control study using the Clinical Practice Research Datalink (CPRD). Br J Sports Med 13 January 2018, 10.1136/bjsports-2017-097762. [DOI] [PubMed] [Google Scholar]
- 30.Nielen JT, de Vries F, Dagnelie PC, van den Bemt BJ, Emans PJ, Lalmohamed A, et al. Use of thiazolidinediones and the risk of elective hip or knee replacement: a population based case- control study. Br J Clin Pharmacol 2016;81:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Joint Registry. 14th Annual Report. Wales, Northern Ireland and the Isle of Man: National Joint Registry for England; 2017. [Google Scholar]


