Abstract
The blood–brain barrier (BBB) is formed by brain endothelial cells, and decreased BBB integrity contributes to vasogenic cerebral edema and increased mortality after stroke. In the present study, we investigated the protective effect of perampanel, an orally active noncompetitive AMPA receptor antagonist, on BBB permeability in an in vitro ischemia model in murine brain endothelial cells (mBECs). The results showed that perampanel significantly attenuated oxygen glucose deprivation (OGD)-induced loss of cell viability, release of lactate dehydrogenase, and apoptotic cell death in a dose-dependent manner. Perampanel treatment did not alter the expression and surface distribution of various glutamate receptors. Furthermore, the results of calcium imaging showed that perampanel had no effect on OGD-induced increase in intracellular Ca2+ concentrations. Treatment with perampanel markedly reduced the paracellular permeability of mBECs after OGD in different time points, as measured by transepithelial electrical resistance assay. In addition, the expression of claudin-5 at protein level, but not at mRNA level, was increased by perampanel treatment after OGD. Knockdown of claudin-5 partially prevented perampanel-induced protection in cell viability and BBB integrity in OGD-injured mBECs. These data show that the noncompetitive AMPA receptor antagonist perampanel affords protection against ischemic stroke through caludin-5 mediated regulation of BBB permeability.
Keywords: AMPA receptor, Perampanel, Blood brain barrier, Claudin-5
Introduction
Stroke is one of the leading causes of mortality in adults, and an estimated 6.8 million Americans over 20 years of age have had a stroke (Go et al. 2014). It triggers a complex series of biochemical cascades that impairs the neurological functions via breakdown of cellular integrity mediated by oxidative stress, ionic imbalance, oxcitotoxic signaling, and inflammation (Mehta et al. 2007; Manzanero et al. 2013). The blood–brain barrier (BBB) is a highly selective permeability barrier that segregates the central nervous system from the systemic circulation. It is formed by brain endothelial cells (BECs) and serves to prevent uncontrolled flux of ions, amino acids, and peptides into the brain (Brown and Davis 2002). Under ischemic conditions, the decreased BBB integrity contributes to vasogenic cerebral edema, hemorrhagic transformation, and increased mortality, making it an ideal therapeutic target for stroke treatment (Sandoval and Witt 2008).
Excessive release of glutamate can lead directly to excitotoxicity in neuronal cells via the activation of various glutamate receptors, including a-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor (AMPAR), which is considered to be involved in many neurological diseases, such as brain trauma and stroke (Bell et al. 2009; Soundarapandian et al. 2005). The AMPAR is composed of various combinations of four possible subunits, GluR1, GluR2, GluR3, and GluR4, and its Ca2+ conductance differs markedly according to whether the GluR2 subunit is present or not (Seeburg et al. 2001). The Ca2+ permeable AMPAR mediates neuronal injury by serving as entry routes for the divalent cations, such as Zn2+ and Ca2+, whereas the Ca2+ unpermeable AMPAR channels associated injury may result from indirect Ca2+ entry through voltage-gated Ca2+ channels or transient receptor potential (TRP) channels and other downstream signaling cascades (Soundarapandian et al. 2005). Blocking AMPAR activation by antagonists has been investigated for anti-ischemia activity in both in vitro and in vivo experiments with mixed success. The selective competitive AMPAR antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline (NBQX) was shown to exert robust neuroprotective effects in models of focal and global ischemia (Smith and Meldrum 1993; Sheardown et al. 1990). However, its clinical use was prevented by its poor solubility, resulting in precipitation in the kidneys (Weiser 2002). In this study, perampanel, a novel non-competitive AMPAR antagonist, was used in an in vitro ischemia model in primary murine brain endothelial cells (mBECs) to determine its protective activity and effects on brain endothelial cell permeability with focus on claudin-5 associated mechanism.
Experimental Procedures
Primary Brain Endothelial Cell Cultures
The primary mBECs were obtained from 2-month-old C57 BL/6 mice as described previously (Hawkins et al. 2015). Briefly, the brains were removed from mice, stripped of meninges and finely minced. Tissues were dissociated in a solution containing 20 U/ml papain and 250 U/ml DNase I type IV in MEM-HEPES. The dissociated brain tissues were triturated, added into a 10-ml tube containing 22 % bovine serum albumin and centrifuged at 1000×g for 20 min. The isolated cells were then washed and resuspended in endothelial cell growth media consisting of Hams F12, supplemented with 10 % fetal bovine serum, heparin, ascorbic acid, l-glutamine, and endothelial cell growth supplement. The mBECs were maintained at 37 °C in a humidified 5 % CO2 incubator and half of the culture medium was changed every other day.
Oxygen Glucose Deprivation (OGD)
The mBECs were treated with OGD to mimic ischemia in vitro. In briefly, serum containing media were removed from the cell cultures by washing with phosphate buffered saline (PBS) for three times. The mBECs were placed into a specialized, humidified chamber containing 5 % CO2, 95 % N2 at 37 °C with low-glucose medium (1 g/l, supplemented DMEM), which was pre-gassed with N2/CO2 (95 %/5 %) to remove residual oxygen. After 2 h challenge, the mBECs were removed from the anaerobic chamber, and the culture medium was replaced with normal culture media to generate reperfusion insult. Perampanel was purchased from Santa Cruz (sc-477647, CA, USA), and the mBECs were treated with perampanel at the beginning of OGD insult.
Cell Viability Assay
The cell viability assay was performed using the WST-1 assay kit following the manufacture’s protocol (Roche, Basel, Switzerland). Briefly, mBECs were cultured at a concentration of 3 × 105 in microplates in a final volume of 100 μl culture medium. After OGD and perampanel treatments, 10 μl WST-1 was added into each well and incubated for 4 h at 37 °C. Then, 100 μl/well culture medium and 10 μl WST-1 were added into one well in the absence of cells, and its absorbance was used as a blank value. Cells were shaken thoroughly for 1 min on a shaker and the absorbance of the samples was measured using a microplate (ELISA) reader.
Lactate Dehydrogenase (LDH) Release Assay
LDH release into the culture medium was detected using a diagnostic kit following the manufacturer’s instructions (Jiancheng Bioengineering Institute, Nanjing, China). Briefly, 50 µl of supernatant from each well was collected, incubated with reduced form of nicotinamide-adenine dinucleotid (NADH) and pyruvate for 15 min at 37 °C, and the reaction was stopped by adding 0.4 M NaOH. The activity of LDH was calculated from the absorbance at 440 nm and background absorbance from culture medium that was not used for any cell cultures was subtracted from all absorbance measurements.
Flow Cytometry
The mBECs were harvested 24 h after OGD and perampanel treatment, washed with ice-cold Ca2+ free PBS, and resuspended in binding buffer. Cell suspension was transferred into a tube and double-stained for 15 min with the Alexa Fluor 488-conjugated annexin V (AV) and propidium iodide (PI) at room temperature in the dark. After addition of 400 µl binding buffer, the stained cells were analyzed by an FC500 flow cytometer with the fluorescence emission at 530 and >575 nm. The CXP cell quest software (Beckman-Coulter, USA) was used to count the number of AV+/PI− and AV+/PI+ cells, and analyzed the results.
Surface Biotinylation Assay
The surface biotinylation assay was performed with the Pierce Cell Surface Isolation Kit according to the manufacturer’s instructions. Briefly, the mBECs were washed twice with ice-cold NaCl/Pi, and then incubated with Sulfo-NHS-SSBiotin solution for 30 min at 4 °C. After quenching the unreacted biotin, the cell pellet was collected and then resuspended in RIPA buffer. After sonication and incubation, the cell lysate was centrifuged at 10,000×g for 2 min at 4 °C. Twenty percent of the supernatant was reserved as the total protein, and the remaining 80 % was rotated with NeutrAvidin Agarose for 1 h at 4 °C. Gels were washed with wash buffer, and centrifuged at 1000×g for 1 min at 4 °C for three times. Sample buffer containing dithiothreitol was added to the gels, which was followed by centrifugation at 1000×g for 2 min at 4 °C. After addition of bromophenol blue, samples were used to perform western blot analysis.
Calcium Imaging
Intracellular Ca2+ concentration ([Ca2+]cyt) was measured using the ratiometric calcium indicator Fura-2-AM (Chen et al. 2012). The mBECs grown on glass slides were loaded with 5 μM fura-2 AM for 45 min in Hanks Balanced Salt Solution (HBSS), and equilibrated for 30 min in the dark at room temperature. Cells were then placed in the open-bath imaging chamber containing HBSS. Using the Nikon inverted epifluorescence microscope, mBECs were excited at 345 and 385 nm and the emission fluorescence at 510 nm was recorded. Images were collected and analyzed with the MetaFluor image-processing software, and the results were calculated and shown as fold of baseline.
Transepithelial Electrical Resistance (TEER) Measurement
TEER was measured as a measure of paracellular permeability using previously published protocol (Hind et al. 2015). The resistance across the membrane was measured using STX2 electrodes linked to an EVOM2 resistance meter. Three readings were taken per insert and the average value used. A baseline TEER reading was taken and the percentage change from this value was calculated for subsequent readings. To assess the impact on permeability, mBECs were treated with OGD in the presence or absence of perampanel and TEER was measured at various time points over 24 h.
RT-PCR
Briefly, a 2-mg template RNA was used to synthesize the first strand of cDNA using a reverse transcription kit (Takara, Dalian, China). The mRNA levels of claudin-5 were quantitated using a Bio-Rad iQ5 Gradient Real-Time PCR system (Bio-Rad Laboratories), and GAPDH was used as an endogenous control. Primers for all Real-Time PCR experiments were listed as follows: claudin-5: forward: 5′-CTG GAC CAC AAC ATC GTG AC-3′, reverse: 5′-GTA CTT GAC CGG GAA GCT GA-3′; GAPDH: forward: 5′-AAG GTG AAG GTC GGA GTC AA-3′, reverse: 5′-AAT GAA GGG GTC ATT GAT GG-3′. Samples were tested in triplicates and data from four independent experiments were used for analysis.
Short Interfering RNA (siRNA) and Transfection
The specific siRNA targeted caludin-5 (Si-claudin-5, sc-43045) and control siRNA (Si-Control, sc-37007), which should not knock down any known proteins, were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The above siRNA molecules were transfected with Lipofectamine 2000 (Invitrogen, CA, USA) for 48 h, and the mBECs were treated with OGD and perampanel and subjected to various measurements.
Immunocytochemistry (ICC)
ICC was performed following the previously published methods (Chen et al. 2013). The mBECs were fixed for 30 min with 4 % paraformaldehyde, rinsed twice with PBS, and subsequently incubated with 1 % hydrogen peroxide for 10 min. Following two PBS rinses, cells were incubated with blocking solution (PBS containing 1 % bovine serum albumin, 0.4 % Triton X-100 and 4 % normal goat serum) for 20 min. Next, cells were incubated with primary anti-claudin-5 antibody (1:100) at 4 °C overnight. Cells were then rinsed twice with PBS and incubated with fluorescein isothiocyanate (FITC) labeled secondary antibody (1:500) for 1 h at room temperature. Coverslips were mounted in mounting medium and visualized by a fluorescence microscope. DAPI (10 μg/ml) was used to stain the nucleus.
Western Blot Analysis
Equivalent amounts of protein (40 μg per lane) were loaded and separated by 10 % SDS-PAGE gels, and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 5 % nonfat milk solution in tris-buffered saline with 0.1 % Triton X-100 (TBST) for 1 h, and then incubated overnight at 4 °C with the primary mGluR1/5 (1:300, NeuroMab, P31424, CA, USA), NR1 (1:200, NeuroMab, P35439-2, CA, USA), GluR1 (1:300, NeuroMab, P19490, CA, USA), GluR2 (1:300, NeuroMab, P19491, CA, USA), claudin-5 (1:100, Santa Cruz, sc-28670, CA, USA) or β-actin (1:500, Santa Cruz, sc-47778, CA, USA) antibody dilutions in TBST. After that the membranes were washed and incubated with secondary antibody for 1 h at room temperature. Immunoreactivity was detected with Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA).
Statistical Analysis
Statistical analysis was performed using SPSS 16.0, a statistical software package. Statistical evaluation of the data was performed by one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparisons or unpaired t test (two groups). A value of p < 0.05 was considered statistically significant.
Results
Perampanel Protects Against OGD-Induced Cell Injury in mBECs
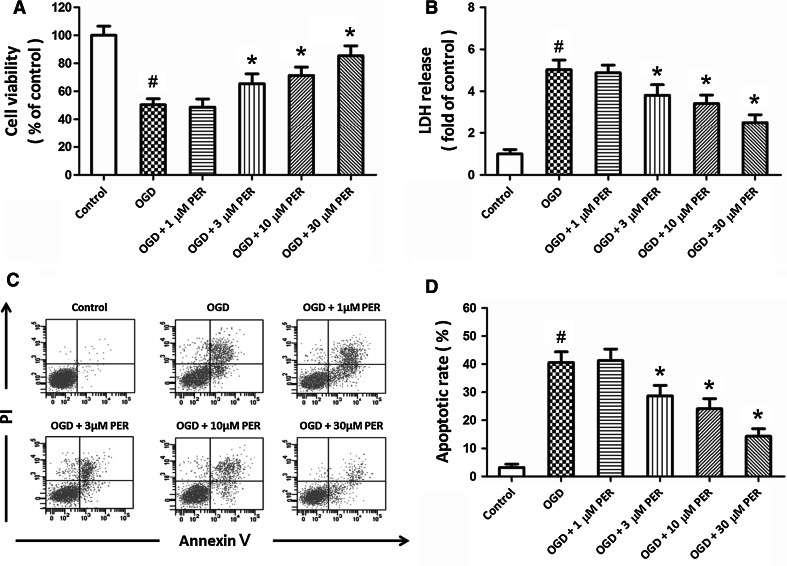
To investigate the potential protective effect of perampanel against ischemic injury, mBECs were treated with perampanel at different concentrations and exposed to OGD. The results of WST assay showed that perampanel significantly increased the cell viability after OGD in a dose-dependent manner, although 1 μM perampanel was not effective (Fig. 1a). As shown in Fig. 1b, perampanel treatment also decreased the LDH release induced by OGD in mBECs. In addition, flow cytometry was used to detect apoptotic cell death after OGD injury (Fig. 1c). Perampanel at 3 μM and higher markedly inhibited apoptosis in mBECs, whereas the apoptotic rate of 1 μM perampanel group was not altered as compared with OGD group (Fig. 1d).
Fig. 1.
Perampanel protects against OGD-induced cell injury in mBECs. mBECs were exposed to OGD with or without perampanel (PER) at different concentrations. The cell viability (a) and LDH release (b) were measured at 24 h after injury. Apoptotic cell death was detected by flow cytometry (c), and the apoptotic rate was calculated (d). Data are shown as mean ± SD of five experiments. # p < 0.05 versus control. *p < 0.05 versus OGD
Effects of Perampanel on Glutamate Receptors Expression and Intracellular Ca2+
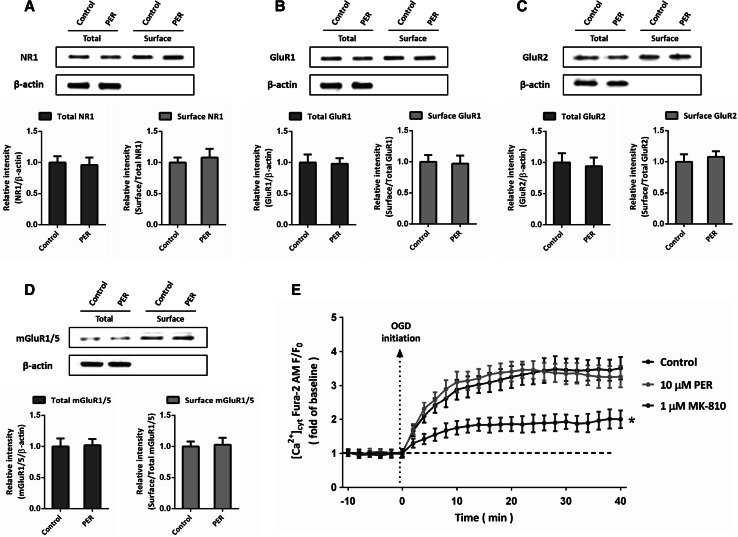
To determine the effect of perampanel on the expression of glutamate receptors, mBECs were treated with 10 μM perampanel for 24 h. The results of western blot demonstrated that neither the total expression nor the surface expression of NR1 (Fig. 2a), GluR1 (Fig. 2b), GluR2 (Fig. 2c), and mGluR1/5 (Fig. 2d) significantly changed in perampanel treated mBECs, as compared with control cells. AMPA receptors are demonstrated to be involved in glutamate-associated excitotoxicity via regulating cation homeostasis, thus we detected changes of intracellular Ca2+ concentrations after OGD and perampanel treatment. As shown in Fig. 2e, the Ca2+ concentration was detected by calcium imaging, and the NMDAR antagonist MK-810 was used as a positive control. The OGD-induced increase in intracellular Ca2+ was significantly suppressed by MK-810, but not perampanel, suggesting that perampanel does not affect the Ca2+ influx after OGD insult in mBECs.
Fig. 2.
Effects of perampanel on glutamate receptors expression and intracellular Ca2+. mBECs were treated with 10 μM perampanel (PER) for 24 h, and the total and surface expression of NMDAR NR1 (a), AMPAR GluR1 (b), AMPAR GluR2 (b), and mGluR1/5 (d) were detected by western blot, respectively. mBECs were subjected to OGD with or without 10 μM perampanel or 1 μM NMDAR inhibitor MK-810, and the intracellular Ca2+ was monitored by Ca2+ imaging up to 40 min after OGD initiation (e). Data are shown as mean ± SD of five experiments. *p < 0.05 versus control (Color figure online)
Perampanel Abrogates Brain Endothelial Cell Permeability in Response to OGD
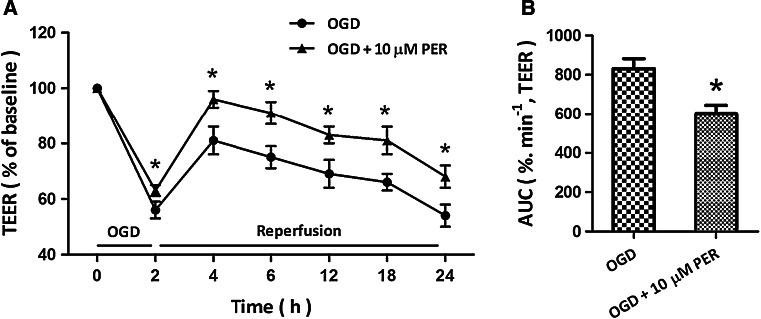
To assess the effect of perampanel on BBB permeability in our in vitro conditions, TEER was measured as a marker of culture integrity and as a measure of paracellular permeability (Fig. 3a). Exposing the mBECs to 2 h OGD increased permeability as shown by a reduction in TEER of approximately 65 %. A time-response curve showed that an obvious recovery of permeability and followed decrease in TEER were observed after reperfusion. As shown in Fig. 3b, perampanel treatment significantly reduced the increase in permeability induced by the OGD protocol, as presented by decreased AUC values.
Fig. 3.
Perampanel abrogates brain endothelial cell permeability in response to OGD. mBECs were treated with OGD with or without 10 μM perampanel (PER). The brain endothelial cell permeability was measured by TEER (a), and the corresponding area under curve (AUC) was calculated (b). Data are shown as mean ± SD of five experiments. *p < 0.05 versus OGD
Involvement of Claudin-5 in Perampanel-Induced Protection in mBECs
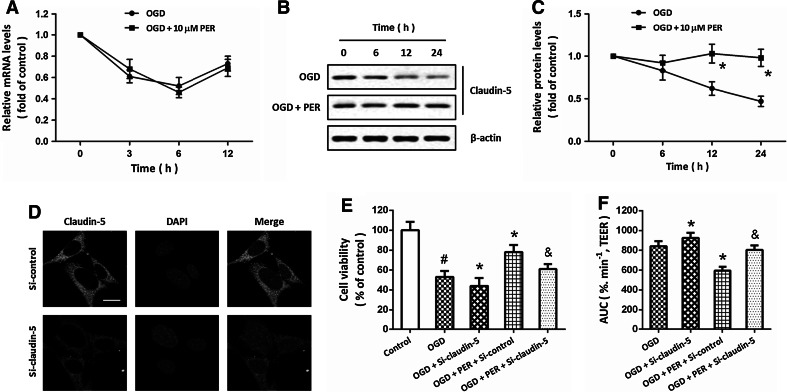
To clarify the involvement of claudin-5 in perampanel-induced protection against OGD, the expression of claudin-5 mRNA was detected by RT-PCR at 3, 6, and 12 h after OGD initiation (Fig. 4a). OGD treatment resulted in significant decreases in claudin-5 mRNA at 3, 6, and 12 h, and perampanel had no effect on the expression of claudin-5 mRNA. We also detected the expression of claudin-5 at protein levels using western blot (Fig. 4b). The results showed that OGD significantly decreased the expression of claudin-5 protein in a time-dependent manner, which was prevented by perampanel treatment (Fig. 4c). As shown in Fig. 4d, the expression of claudin-5 in mBECs was downregulated by transfection with claudin-5 specific targeted siNRA (Si-claudin-5) to further confirm the involvement of claudin-5 in perampanel-induced protection. The results of WST assay showed that perampanel-induced protection increased in cell viability after OGD was partially reversed by claudin-5 (Fig. 4e). A similar result in brain endothelial cell permeability, as presented by AUC values in TEER measurement, was also observed (Fig. 4f).
Fig. 4.
Involvement of claudin-5 in perampanel-induced protection in mBECs. mBECs were exposed to OGD with or without 10 μM perampanel (PER). The expression of claudin-5 mRNA was measured by RT-PCR at different time points (a). The expression of claudin-5 protein was detected by western blot (b) and calculated (c). mBECs were transfected with Si-claudin-5 or Si-control for 48 h, and the expression of claudin-5 was detected by fluorescence staining (d). After siRNA transfection, mBECs were exposed to OGD with or without 10 μM PER. The cell viability (e) and cell permeability (f) were measured. Scale bar 20 μm. Data are shown as mean ± SD of five experiments. # p < 0.05 versus control. *p < 0.05 versus OGD. & p < 0.05 versus Si-control
Discussion
In mammalian central nervous system, overactivation of the APMAR can induce severe neuronal damage, as is evident for brain ischemia, and thus blockade of this receptors is shown to be neuroprotective (Gill 1994). Extensive investigations with APMAR antagonists have demonstrated that the ideal compound was postulated to have the following properties: the blockade of AMPAR should be non-competitive and independent from the membrane potential (Weiser 2002). Perampanel is a recently developed noncompetitive AMPAR antagonist, which has been evaluated in many preclinical experiments and clinical trials (Rugg-Gunn 2014). It is structurally dissimilar to other AMPAR ligands and has been shown to exert broad spectrum anticonvulsant activity in animal models of epilepsy (Hanada 2014). Our present study showed that perampanel protects mBECs against OGD-induced neuronal injury in a dose-dependent manner, which was associated with its anti-apoptotic activity.
In primary neuronal cultures, perampanel was shown to inhibit AMPAR-mediated responses during neurotransmission with an IC50 of 93–230 nM (Ceolin et al. 2012; Hanada et al. 2011). Perampanel did not displace [3H]AMPA binding activity at the concentration of 1.25 μM, and [3H]perampanel binding activity to rat forebrain membranes was only slightly altered by high concentrations of AMPA treatment, suggesting a noncompetitive interaction of perampanel with the AMPAR (Hanada et al. 2011). Although the potential benefits of noncompetitive antagonists over competitive antagonists are not fully understood, noncompetitive antagonists are shown to be effective in the presence of high agonist concentrations, allowing them to be more effective under conditions of increased excitation (Yamaguchi et al. 1993). In addition, perampanel did not inhibit kainate- and NMDAR-mediated responses, and, therefore, could exert a low risk of the psychotomimetic side effects that are known to be elicited by inhibition of NMDAR (Olney et al. 1991). Our results using TEER assay showed that perampanel was effective to reduce the increased brain endothelial cell permeability under ischemic conditions, which was consistent with previous study showing the effective BBB penetration of perampanel (Hibi et al. 2012). These data, for the first time, extended the protective effect of perampanel into brain ischemia in vitro, and the results observed here should be further confirmed in in vivo models.
Under ischemic conditions, excitotoxicity induced by excessive glutamate is mediated by activation of various glutamate receptors, including NMDAR, AMPAR, and metabotropic glutamate receptors (mGluRs). The expression of these receptors, especially the trafficking of these receptors from the surface to the cytoplasm, directly affects their functions in excitotoxicity and related neurological diseases (Lau and Tymianski 2010; Wyllie et al. 2013). To confirm the selectivity of perampanel for AMPAR over other glutamate receptors, we detected the total and surface expression of glutamate receptors after perampanel treatment. However, perampanel neither changed the expression of the various subunits constituting NMDAR, AMPAR, and mGluRs, nor influenced the surface distribution of these glutamate receptors. These results indicated that perampanel may not be related to the expression and trafficking of these receptors, and its effects on excitotoxicity are presumably mediated by downstream signaling of AMPAR. As the change in intracellular Ca2+ level is one of the critical initiators of ischemia-induced neurotoxicity and apoptosis (Stanika et al. 2009), we also monitored intracellular Ca2+ after perampanel treatment and OGD. In previous in vitro studies, perampanel was shown to potently inhibit AMPA-induced increase in intracellular Ca2+, with no obvious effects NMDA-induced Ca2+ responses (Ceolin et al. 2012; Hanada et al. 2011). A similar phenomenon was observed in our in vitro ischemic models, suggesting that perampanel protects mBECs against ischemia through a Ca2+ independent mechanism.
The BBB provides anatomical and physiological protection for the brain via filtering harmful compounds from the brain back to the bloodstream and shielding the brain from toxic substances in the blood. It relies on the tight junctions (TJs) that are present between the endothelial cells to provide a closed environment for the brain (Jia et al. 2014). The effects of brain ischemia on the BBB permeability have been extensively studied, and altered barrier function has been demonstrated in many in vitro model systems, including immortalized mBEC, immortalized human BEC, and rat primary BECs (Brown et al. 2008; Hawkins et al. 2015). In the present study, OGD itself caused increased permeability, as evidenced by decreased TEER values, which was partially reversed by perampanel treatment, indicating that alterations in TJ proteins might be involved in perampanel-induced protection.
Claudin-5, a member of the integral membrane proteins claudins, is a key component of the TJ strand, especially in brain endothelial cells (Morita et al. 1999). It was shown to mediate the changes in endothelial or epithelial permeability in a number of pathological disorders, including ischemia (Jia et al. 2014). In claudin-5 deficient mice, the ability of the BBB to act against small molecules was selectively reduced (Nitta et al. 2003). Our results of western blot showed that OGD increased BBB permeability in vitro in parallel with reduced expression levels of claudin-5 in a time-dependent manner, which was consistent with a previous study using high glucose models (Liu et al. 2012), confirming that claudin-5 is a key determinant of BBB permeability. The function of claudin-5 can be regulated by many factors, such as cyclic AMP (cAMP), protein kinase A (PKA), and transforming growth factor-β1 (TGF-β1) (Ishizaki et al. 2003; Shen et al. 2011). In a previous study, claudin-5 expression was shown to be regulated by HIV-1 Tat protein via activation of vascular endothelial growth factor receptor-2 (VEGFR-2) and multiple redox-regulated signal transduction pathways (Andras and Toborek 2011). Our results showed that the expression of claudin-5 after OGD was preserved by perampanel treatment in mBECs. To the best of our knowledge, this is the first report of perampanel-induced regulation of claudin-5 expression in mBECs, as well as first such study to investigate the effects of AMPAR antagonist on claudin-5 regulation under ischemia conditions. Activation of AMPAR can induce a variety of downstream kinases, including mitogen-activated protein kinase (MAPKs), Src family kinases, and calcium calmodulin-dependent protein kinase II (Kalia et al. 2004; Wang et al. 2005; Neale et al. 2014), which alone or in concert, can result in alterations of tight junction phosphorylation in response to ischemia. A previous study showed that inhibition of AMPAR attenuated glutamate-induced changes in occluding redistribution but not in the total protein levels in brain endothelial cells (Andras et al. 2007). Treatment with the AMPAR inhibitor 6,7-dinitroquinoxaline-2,3-dione (DNQX) was shown to protect against hypophosphorylation of threonine residues of occluding with no effect on disruption of endothelial integrity. It is well known that both occludin and claudin-5 belong to tight junction-associated proteins, which play important roles in BBB integrity (Haseloff et al. 2015). Thus, these data strongly supported that the AMPAR activity is involved in the regulation of tight junction-associated proteins. Intriguingly, the results of RT-PCR showed that perampanel had no effect on decreased claudin-5 mRNA after OGD. Thus, the perampanel-induced regulation of claudin-5 expression at protein level might be mediated by degradation-associated mechanism, which has been investigated in previous studies and needs to be further determined in in vivo models (Yang and Rosenberg 2011).
In conclusion, we have shown that perampanel, an orally active noncompetitive AMPA receptor antagonist, exerts a protective effect on an in vitro model of BBB permeability in the setting of experimental ischemia (OGD) in mBECs. The protective effect does not appear to involve any alteration in the glutamate receptor expression and intracellular Ca2+ regulation. The perampanel-induced effects on the BBB permeability after OGD might be mediated by preservation of claudin-5 protein expression, possibly through protein degradation-associated mechanisms.
Acknowledgments
This work was financially supported by the Shaanxi Province Scientific and Technological Research and Development Program (Nos. 2004K14-G; 2010, K01-154) and Shaanxi Province Natural Science Foundation Research Program (No. 2014JM4131).
Compliance with Ethical Standards
Conflict of interest
There is no conflict of interest.
References
- Andras IE, Toborek M (2011) HIV-1-induced alterations of claudin-5 expression at the blood–brain barrier level. Methods Mol Biol 762:355–370. doi:10.1007/978-1-61779-185-7_26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andras IE, Deli MA, Veszelka S, Hayashi K, Hennig B, Toborek M (2007) The NMDA and AMPA/KA receptors are involved in glutamate-induced alterations of occludin expression and phosphorylation in brain endothelial cells. J Cereb Blood Flow Metab 27(8):1431–1443. doi:10.1038/sj.jcbfm.9600445 [DOI] [PubMed] [Google Scholar]
- Bell JD, Park E, Ai J, Baker AJ (2009) PICK1-mediated GluR2 endocytosis contributes to cellular injury after neuronal trauma. Cell Death Differ 16(12):1665–1680. doi:10.1038/cdd.2009.106 [DOI] [PubMed] [Google Scholar]
- Brown RC, Davis TP (2002) Calcium modulation of adherens and tight junction function: a potential mechanism for blood–brain barrier disruption after stroke. Stroke 33(6):1706–1711 [DOI] [PubMed] [Google Scholar]
- Brown RC, Wu L, Hicks K, O’Neil RG (2008) Regulation of blood–brain barrier permeability by transient receptor potential type C and type v calcium-permeable channels. Microcirculation 15(4):359–371. doi:10.1080/10739680701762656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceolin L, Bortolotto ZA, Bannister N, Collingridge GL, Lodge D, Volianskis A (2012) A novel anti-epileptic agent, perampanel, selectively inhibits AMPA receptor-mediated synaptic transmission in the hippocampus. Neurochem Int 61(4):517–522. doi:10.1016/j.neuint.2012.02.035 [DOI] [PubMed] [Google Scholar]
- Chen T, Fei F, Jiang XF, Zhang L, Qu Y, Huo K, Fei Z (2012) Down-regulation of Homer1b/c attenuates glutamate-mediated excitotoxicity through endoplasmic reticulum and mitochondria pathways in rat cortical neurons. Free Radic Biol Med 52(1):208–217. doi:10.1016/j.freeradbiomed.2011.10.451 [DOI] [PubMed] [Google Scholar]
- Chen T, Yang YF, Luo P, Liu W, Dai SH, Zheng XR, Fei Z, Jiang XF (2013) Homer1 knockdown protects dopamine neurons through regulating calcium homeostasis in an in vitro model of Parkinson’s disease. Cell Signal 25(12):2863–2870. doi:10.1016/j.cellsig.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Gill R (1994) The pharmacology of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate antagonists and their role in cerebral ischaemia. Cerebrovasc Brain Metab Rev 6(3):225–256 [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB (2014) Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 129(3):e28–e292. doi:10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada T (2014) The discovery and development of perampanel for the treatment of epilepsy. Expert Opin Drug Discov 9(4):449–458. doi:10.1517/17460441.2014.891580 [DOI] [PubMed] [Google Scholar]
- Hanada T, Hashizume Y, Tokuhara N, Takenaka O, Kohmura N, Ogasawara A, Hatakeyama S, Ohgoh M, Ueno M, Nishizawa Y (2011) Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia 52(7):1331–1340. doi:10.1111/j.1528-1167.2011.03109.x [DOI] [PubMed] [Google Scholar]
- Haseloff RF, Dithmer S, Winkler L, Wolburg H, Blasig IE (2015) Transmembrane proteins of the tight junctions at the blood–brain barrier: structural and functional aspects. Semin Cell Dev Biol 38:16–25. doi:10.1016/j.semcdb.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Gu YH, Izawa Y, Del Zoppo GJ (2015) Dabigatran abrogates brain endothelial cell permeability in response to thrombin. J Cereb Blood Flow Metab 35(6):985–992. doi:10.1038/jcbfm.2015.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi S, Ueno K, Nagato S, Kawano K, Ito K, Norimine Y, Takenaka O, Hanada T, Yonaga M (2012) Discovery of 2-(2-oxo-1-phenyl-5-pyridin-2-yl-1,2-dihydropyridin-3-yl)benzonitrile (perampanel): a novel, noncompetitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropanoic acid (AMPA) receptor antagonist. J Med Chem 55(23):10584–10600. doi:10.1021/jm301268u [DOI] [PubMed] [Google Scholar]
- Hind WH, Tufarelli C, Neophytou M, Anderson SI, England TJ, O’Sullivan SE (2015) Endocannabinoids modulate human blood–brain barrier permeability in vitro. Br J Pharmacol 172(12):3015–3027. doi:10.1111/bph.13106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Chiba H, Kojima T, Fujibe M, Soma T, Miyajima H, Nagasawa K, Wada I, Sawada N (2003) Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood–brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp Cell Res 290(2):275–288 [DOI] [PubMed] [Google Scholar]
- Jia W, Lu R, Martin TA, Jiang WG (2014) The role of claudin-5 in blood–brain barrier (BBB) and brain metastases (review). Mol Med Rep 9(3):779–785. doi:10.3892/mmr.2013.1875 [DOI] [PubMed] [Google Scholar]
- Kalia LV, Gingrich JR, Salter MW (2004) Src in synaptic transmission and plasticity. Oncogene 23(48):8007–8016. doi:10.1038/sj.onc.1208158 [DOI] [PubMed] [Google Scholar]
- Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460(2):525–542. doi:10.1007/s00424-010-0809-1 [DOI] [PubMed] [Google Scholar]
- Liu C, Wu J, Zou MH (2012) Activation of AMP-activated protein kinase alleviates high-glucose-induced dysfunction of brain microvascular endothelial cell tight-junction dynamics. Free Radic Biol Med 53(6):1213–1221. doi:10.1016/j.freeradbiomed.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanero S, Santro T, Arumugam TV (2013) Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int 62(5):712–718. doi:10.1016/j.neuint.2012.11.009 [DOI] [PubMed] [Google Scholar]
- Mehta SL, Manhas N, Raghubir R (2007) Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 54(1):34–66. doi:10.1016/j.brainresrev.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita S (1999) Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 147(1):185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale SA, Copeland CS, Salt TE (2014) Effect of VGLUT inhibitors on glutamatergic synaptic transmission in the rodent hippocampus and prefrontal cortex. Neurochem Int 73:159–165. doi:10.1016/j.neuint.2013.10.001 [DOI] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. J Cell Biol 161(3):653–660. doi:10.1083/jcb.200302070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA (1991) NMDA antagonist neurotoxicity: mechanism and prevention. Science 254(5037):1515–1518 [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn F (2014) Adverse effects and safety profile of perampanel: a review of pooled data. Epilepsia 55(Suppl 1):13–15. doi:10.1111/epi.12504 [DOI] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA (2008) Blood–brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis 32(2):200–219. doi:10.1016/j.nbd.2008.08.005 [DOI] [PubMed] [Google Scholar]
- Seeburg PH, Single F, Kuner T, Higuchi M, Sprengel R (2001) Genetic manipulation of key determinants of ion flow in glutamate receptor channels in the mouse. Brain Res 907(1–2):233–243 [DOI] [PubMed] [Google Scholar]
- Sheardown MJ, Nielsen EO, Hansen AJ, Jacobsen P, Honore T (1990) 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science 247(4942):571–574 [DOI] [PubMed] [Google Scholar]
- Shen W, Li S, Chung SH, Zhu L, Stayt J, Su T, Couraud PO, Romero IA, Weksler B, Gillies MC (2011) Tyrosine phosphorylation of VE-cadherin and claudin-5 is associated with TGF-beta1-induced permeability of centrally derived vascular endothelium. Eur J Cell Biol 90(4):323–332. doi:10.1016/j.ejcb.2010.10.013 [DOI] [PubMed] [Google Scholar]
- Smith SE, Meldrum BS (1993) Cerebroprotective effect of a non-N-methyl-d-aspartate antagonist, NBQX, after focal ischaemia in the rat. Funct Neurol 8(1):43–48 [PubMed] [Google Scholar]
- Soundarapandian MM, Tu WH, Peng PL, Zervos AS, Lu Y (2005) AMPA receptor subunit GluR2 gates injurious signals in ischemic stroke. Mol Neurobiol 32(2):145–155. doi:10.1385/MN:32:2:145 [DOI] [PubMed] [Google Scholar]
- Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB (2009) Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc Natl Acad Sci USA 106(24):9854–9859. doi:10.1073/pnas.0903546106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Arora A, Yang L, Parelkar NK, Zhang G, Liu X, Choe ES, Mao L (2005) Phosphorylation of AMPA receptors: mechanisms and synaptic plasticity. Mol Neurobiol 32(3):237–249. doi:10.1385/MN:32:3:237 [DOI] [PubMed] [Google Scholar]
- Weiser T (2002) AMPA receptor antagonists with additional mechanisms of action: new opportunities for neuroprotective drugs? Curr Pharm Des 8(10):941–951 [DOI] [PubMed] [Google Scholar]
- Wyllie DJ, Livesey MR, Hardingham GE (2013) Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology 74:4–17. doi:10.1016/j.neuropharm.2013.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Donevan SD, Rogawski MA (1993) Anticonvulsant activity of AMPA/kainate antagonists: comparison of GYKI 52466 and NBOX in maximal electroshock and chemoconvulsant seizure models. Epilepsy Res 15(3):179–184 [DOI] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA (2011) MMP-mediated disruption of claudin-5 in the blood–brain barrier of rat brain after cerebral ischemia. Methods Mol Biol 762:333–345. doi:10.1007/978-1-61779-185-7_24 [DOI] [PMC free article] [PubMed] [Google Scholar]