Abstract
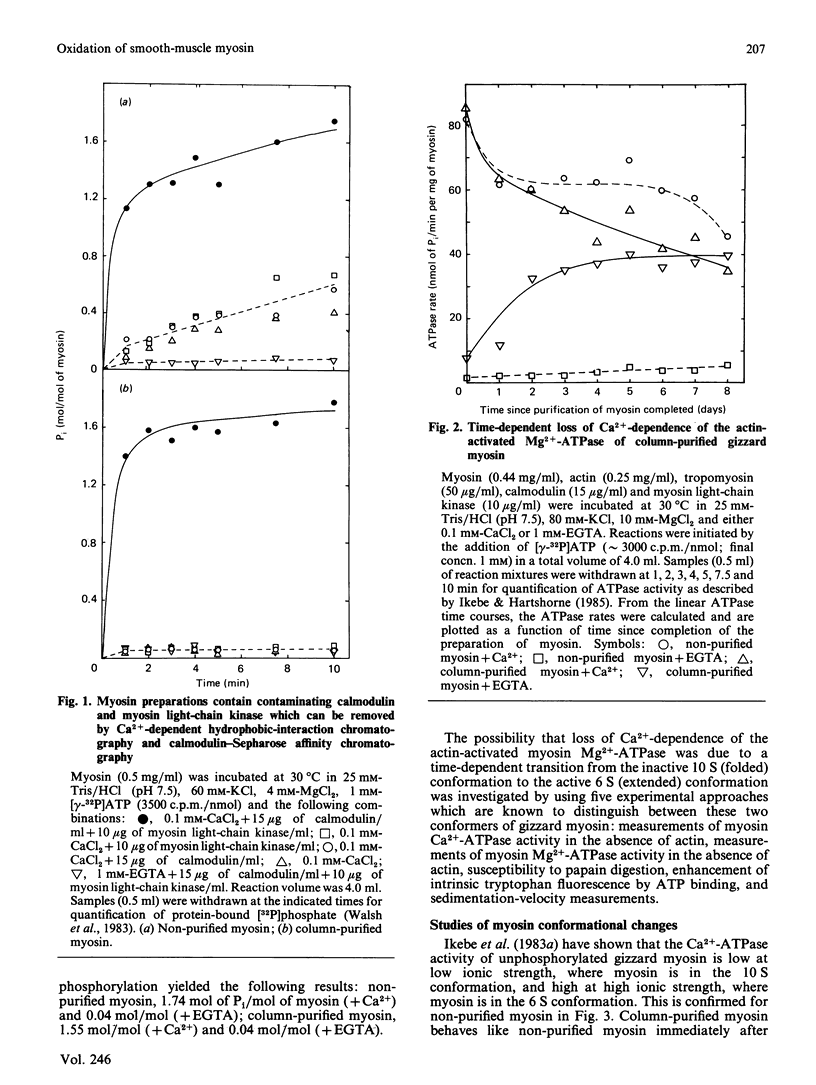
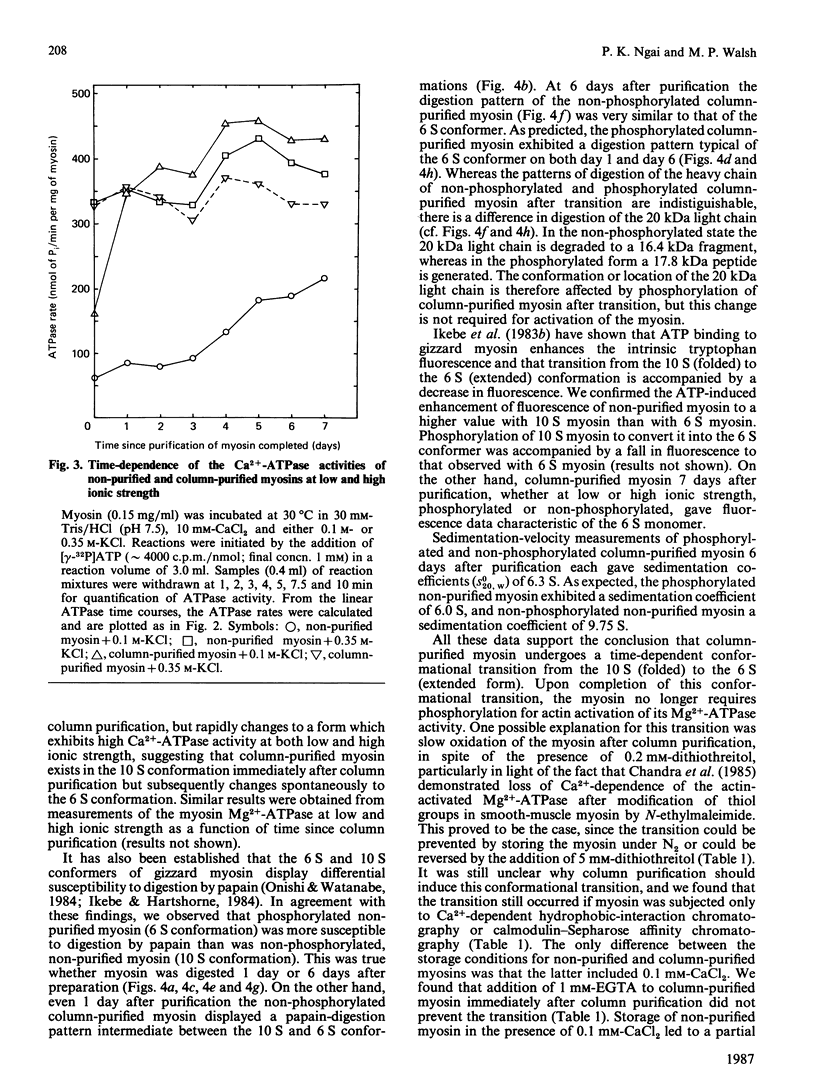
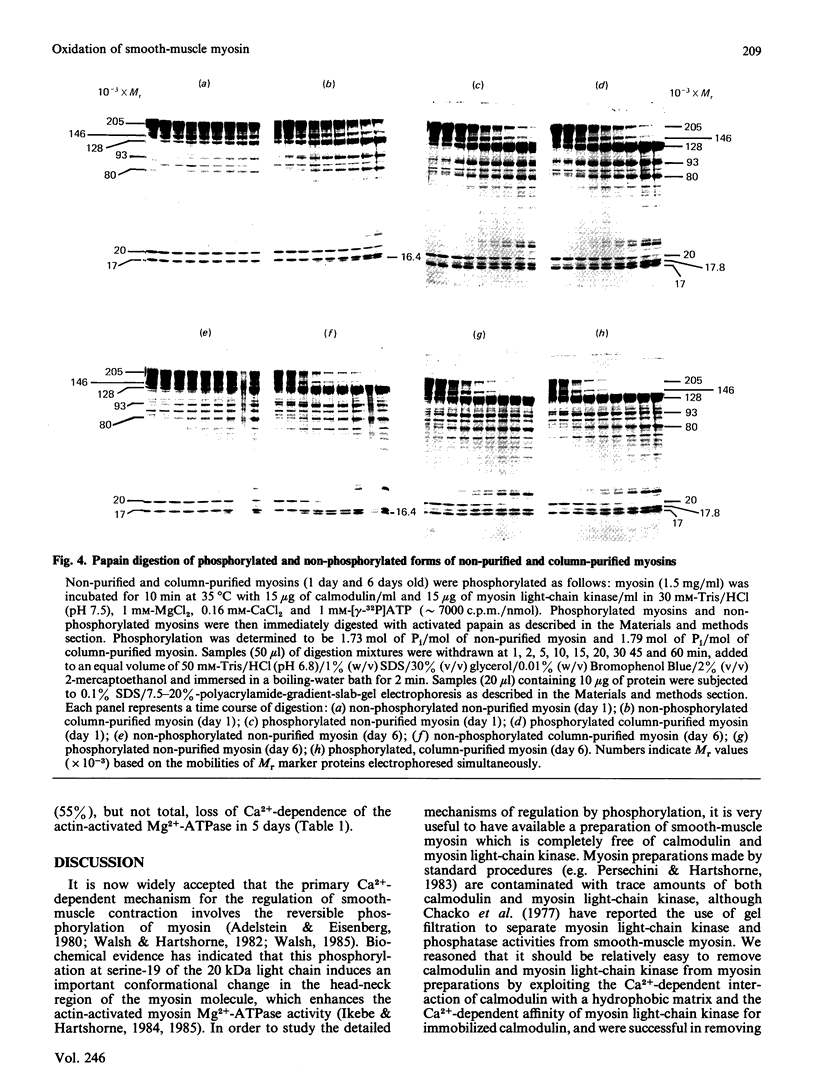
Smooth-muscle myosin purified as described by Persechini & Hartshorne [(1983) Biochemistry 22, 470-476] contains trace amounts of calmodulin and myosin light-chain kinase, which can be removed by Ca2+-dependent hydrophobic-interaction chromatography followed by calmodulin-Sepharose affinity chromatography. The resultant column-purified myosin exhibits properties similar to those of the non-purified myosin, e.g. actin activation of the Mg2+-ATPase requires Ca2+/calmodulin-dependent phosphorylation of the two 20 kDa light chains. However, unlike the non-purified myosin, the column-purified myosin undergoes a time-dependent transition to a form which no longer requires phosphorylation for actin activation of the myosin Mg2+-ATPase. This transition is identified as a time-dependent change in conformation of the column-purified myosin from a 10 S to 6 S form and is caused by slow oxidation of the column-purified myosin, since it could be prevented by storage under N2 and reversed by 5 mM-dithiothreitol.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Chacko S., Conti M. A., Adelstein R. S. Effect of phosphorylation of smooth muscle myosin on actin activation and Ca2+ regulation. Proc Natl Acad Sci U S A. 1977 Jan;74(1):129–133. doi: 10.1073/pnas.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra T. S., Nath N., Suzuki H., Seidel J. C. Modification of thiols of gizzard myosin alters ATPase activity, stability of myosin filaments, and the 6-10 S conformational transition. J Biol Chem. 1985 Jan 10;260(1):202–207. [PubMed] [Google Scholar]
- Clark T., Ngai P. K., Sutherland C., Gröschel-Stewart U., Walsh M. P. Vascular smooth muscle caldesmon. J Biol Chem. 1986 Jun 15;261(17):8028–8035. [PubMed] [Google Scholar]
- Craig R., Smith R., Kendrick-Jones J. Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. 1983 Mar 31-Apr 6Nature. 302(5907):436–439. doi: 10.1038/302436a0. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Barsotti R. J., Hinkins S., Hartshorne D. J. Effects of magnesium chloride on smooth muscle actomyosin adenosine-5'-triphosphatase activity, myosin conformation, and tension development in glycerinated smooth muscle fibers. Biochemistry. 1984 Oct 9;23(21):5062–5068. doi: 10.1021/bi00316a036. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D. J. Conformation-dependent proteolysis of smooth-muscle myosin. J Biol Chem. 1984 Oct 10;259(19):11639–11642. [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D. J. Proteolysis of smooth muscle myosin by Staphylococcus aureus protease: preparation of heavy meromyosin and subfragment 1 with intact 20 000-dalton light chains. Biochemistry. 1985 Apr 23;24(9):2380–2387. doi: 10.1021/bi00330a038. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Hinkins S., Hartshorne D. J. Correlation of enzymatic properties and conformation of smooth muscle myosin. Biochemistry. 1983 Sep 13;22(19):4580–4587. doi: 10.1021/bi00288a036. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Hinkins S., Hartshorne D. J. Correlation of intrinsic fluorescence and conformation of smooth muscle myosin. J Biol Chem. 1983 Dec 25;258(24):14770–14773. [PubMed] [Google Scholar]
- Klee C. B. Conformational transition accompanying the binding of Ca2+ to the protein activator of 3',5'-cyclic adenosine monophosphate phosphodiesterase. Biochemistry. 1977 Mar 8;16(5):1017–1024. doi: 10.1021/bi00624a033. [DOI] [PubMed] [Google Scholar]
- Ngai P. K., Carruthers C. A., Walsh M. P. Isolation of the native form of chicken gizzard myosin light-chain kinase. Biochem J. 1984 Mar 15;218(3):863–870. doi: 10.1042/bj2180863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Inhibition of smooth muscle actin-activated myosin Mg2+-ATPase activity by caldesmon. J Biol Chem. 1984 Nov 25;259(22):13656–13659. [PubMed] [Google Scholar]
- Okamoto Y., Sekine T. Effects of tryptic digestion on the enzymatic activites of chicken gizzard myosin. J Biochem. 1978 May;83(5):1375–1379. doi: 10.1093/oxfordjournals.jbchem.a132046. [DOI] [PubMed] [Google Scholar]
- Onishi H. N-Iodoacetyl-N'-(5-sulfo-1-naphthyl)ethylenediamine modification of myosin from chicken gizzard. J Biochem. 1985 Jul;98(1):81–86. doi: 10.1093/oxfordjournals.jbchem.a135276. [DOI] [PubMed] [Google Scholar]
- Onishi H., Watanabe S. Correlation between the papain digestibility and the conformation of 10s-myosin from chicken gizzard. J Biochem. 1984 Mar;95(3):899–902. doi: 10.1093/oxfordjournals.jbchem.a134685. [DOI] [PubMed] [Google Scholar]
- Persechini A., Hartshorne D. J. Ordered phosphorylation of the two 20 000 molecular weight light chains of smooth muscle myosin. Biochemistry. 1983 Jan 18;22(2):470–476. doi: 10.1021/bi00271a033. [DOI] [PubMed] [Google Scholar]
- Seidel J. C. Activation by actin of ATPase activity of chemically modified gizzard myosin without phosphorylation. Biochem Biophys Res Commun. 1979 Aug 13;89(3):958–964. doi: 10.1016/0006-291x(79)91871-0. [DOI] [PubMed] [Google Scholar]
- Sobieszek A. Phosphorylation reaction of vertebrate smooth muscle myosin: an enzyme kinetic analysis. Biochemistry. 1985 Feb 26;24(5):1266–1274. doi: 10.1021/bi00326a032. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Butler T. M., Bond M., Somlyo A. P. Myosin filaments have non-phosphorylated light chains in relaxed smooth muscle. Nature. 1981 Dec 10;294(5841):567–569. doi: 10.1038/294567a0. [DOI] [PubMed] [Google Scholar]
- Sparrow M. P., Maxwell L. C., Ruegg J. C., Bohr D. F. Preparation and properties of a calcium ion-sensitive actomyosin from arteries. Am J Physiol. 1970 Nov;219(5):1366–1372. doi: 10.1152/ajplegacy.1970.219.5.1366. [DOI] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Ikebe M., Hartshorne D. J. Trinitrophenylation of smooth muscle myosin. Biochem Biophys Res Commun. 1985 Jan 31;126(2):748–755. doi: 10.1016/0006-291x(85)90248-7. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kamata T., Onishi H., Watanabe S. Adenosine triphosphate-induced reversible change in the conformation of chicken gizzard myosin and heavy meromyosin. J Biochem. 1982 May;91(5):1699–1705. doi: 10.1093/oxfordjournals.jbchem.a133861. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Onishi H., Takahashi K., Watanabe S. Structure and function of chicken gizzard myosin. J Biochem. 1978 Dec;84(6):1529–1542. doi: 10.1093/oxfordjournals.jbchem.a132278. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Huiatt T. W., Lowey S. A bent monomeric conformation of myosin from smooth muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6151–6155. doi: 10.1073/pnas.79.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M. P., Hinkins S., Dabrowska R., Hartshorne D. J. Smooth muscle myosin light chain kinase. Methods Enzymol. 1983;99:279–288. doi: 10.1016/0076-6879(83)99063-8. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Hinkins S., Flink I. L., Hartshorne D. J. Bovine stomach myosin light chain kinase: purification, characterization, and comparison with the turkey gizzard enzyme. Biochemistry. 1982 Dec 21;21(26):6890–6896. doi: 10.1021/bi00269a041. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Valentine K. A., Ngai P. K., Carruthers C. A., Hollenberg M. D. Ca2+-dependent hydrophobic-interaction chromatography. Isolation of a novel Ca2+-binding protein and protein kinase C from bovine brain. Biochem J. 1984 Nov 15;224(1):117–127. doi: 10.1042/bj2240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zot H. G., Potter J. D. Purification of actin from cardiac muscle. Prep Biochem. 1981;11(4):381–395. doi: 10.1080/00327488108065530. [DOI] [PubMed] [Google Scholar]



