Abstract
Simple Summary
Nutritional composition of rapeseed cake varies considerably due to differences in cultivars, growing conditions, harvesting time, and processing techniques, making it difficult to formulate precise diets. Therefore, we conducted a study to determine the chemical composition and standardized ileal digestibility (SID) of the amino acids (AA) in rapeseed cake and to test the hypothesis that their nutritional value is influenced by their origin. At the same time, we attempted to establish a predictive equation for SID based on the chemical composition of AA in RSC fed to pigs.
Abstract
Objective: The experiment was conducted to determine the apparent or standardized ileal digestibility (AID or SID) of crude protein (CP) and amino acids (AA) in 10 rapeseed cake samples fed to pigs, and to construct predictive models for the SID of CP and AA based on the chemical composition of rapeseed cakes. Methods: Twenty-two cannulated pigs (initial body weight: 39.8 ± 1.2 kg) were assigned to two 11 × 3 incomplete Latin square designs, including an N-free diet and 10 diets containing rapeseed cake. Each experimental period included 5 days of adaptation and 2 days of ileal digesta collection. Titanium dioxide (TiO2) was added at 0.3% to all the diets as an indigestible marker for calculating the ileal CP and AA digestibility. Results: The coefficients of variation (CV) of the content of crude fat (EE), crude fiber (CF), neutral detergent fiber (NDF), acid detergent fiber (ADF), and total glucosinolates (TGS) in 10 samples of rapeseed cake were greater than 10%. The standardized ileal digestibility (SID) of crude protein (CP), lysine (Lys), methionine (Met), threonine (Thr), and tryptophan (Trp) in rapeseed cake was 73.34% (61.49 to 81.12%), 63.01% (41.41 to 73.10%), 69.47% (50.55 to 88.16%), 79.61% (74.41 to 87.58%), and 94.43% (91.34 to 97.20%), respectively. The best prediction equations for SIDCP, SIDLys, and SIDVal were as follows: SIDCP = 90.124 − 0.54NDF (R2 = 0.58), SIDLys = 100.107 − 1.229NDF (R2 = 0.94), and SIDVal = 151.012 − 2.990TGS (R2 = 0.57). Conclusion: Overall, great variation exists among the 10 rapeseed cakes, and the NDF, TGS, and heating temperature can be used as the key predictors for the SID of CP and AA.
Keywords: rapeseed cake, amino acids digestibility, prediction model
1. Introduction
The demand for protein feed resources is expected to rise due to global population growth and the increasing need for animal-based food [1,2]. Data from the National Bureau of Statistics show that animal production in China is highly dependent on the imported soybean, which significantly affects national food security. The Ministry of Agriculture and Rural Affairs of China has drawn up several strategies to reduce the utilization of soybean meal in animal feed. Rapeseed is one of the major global sources of vegetable oil and protein feed, ranking second only to soybeans in trade volume [2]. Rapeseed cake (RSC), a by-product after oil extraction, contains around 36% of crude protein (CP) but offers an unbalanced amino acid profile [2,3,4,5,6,7]. Rapeseed cake has lower lysine (Lys) contents than soybean meal, and it is rich in sulfur-containing amino acids and more cost-effective [2,3,4,5,6,7]. Studies have shown that rapeseed cake can effectively replace soybean meal in the diets of adult pigs [5,7,8]. However, greater variations in the nutrient composition of RSC exist due to the differences in cultivars, growing conditions, harvest time, and processing technology, making precision formulation difficult [2]. Accurate estimation of amino acid availability in diets is essential for low-protein diet systems [9], and the standardized ileal digestibility (SID) of animo acids is the gold standard for characterizing the amino acid availability in feed ingredients [9,10].
Therefore, we conducted research to determine the chemical composition and standardized ileal digestibility (SID) of amino acids (AA) in the RSC, and tested the hypothesis that its nutritional value is affected by its origin. Meanwhile, we tried to establish predicted equations for the SID of AA in the RSC fed to pigs based on its chemical composition.
2. Materials and Methods
2.1. Sources of Rapeseed Cake Samples
Ten RSC samples were selected from Guizhou (n = 1), Sichuan (n = 1), Anhui (n = 1), Shaanxi (n = 1), Henan (n = 1), Hubei (n = 2), and Hunan (n = 2) provinces and from Chongqing municipality (n = 1) (Table 1). The main production areas of rapeseed in China are in the Yangtze River Basin and its surrounding areas [11]. In our research, the selection of rapeseed cake samples was mainly concentrated in the Yangtze River Basin of China, but there was also a sample RSC5 from the Huaihe River Basin. The RSC ingredients were ground in a feed mill and used to prepare experimental diets. The samples were sieved through a 40-mesh screen, obtained using stratified sampling and the quadrant method, and stored at −20 °C until use [12].
Table 1.
Sources of rapeseed cake.
| Sample Numbers | Colors * | Source of Variety | Heating Temperature and Time | Sources | Storage Conditions |
|---|---|---|---|---|---|
| RSC1 | brown | Brassica napus L. | 130 ± 10 °C, 30 min | Liupanshui city, Guizhou | Normal temperature, <3 months |
| RSC2 | reddish brown | Brassica napus L. | 165 ± 15 °C, 25 min | Neijiang city, Sichuang | Normal temperature, <3 months |
| RSC3 | yellowish green | Brassica napus L. | 115 ± 10 °C, 30 min | Huainan city, Anhui | Normal temperature, <3 months |
| RSC4 | greener | Brassica napus L. | 40 ± 20 °C, 60~120 min | Hanzhong city, Shaanxi | Normal temperature, <3 months |
| RSC5 | yellowish green | Brassica napus L. | 95 ± 10 °C, 25 min | Xuchang city, Henan | Normal temperature, <3 months |
| RSC6 | yellowish green | Brassica napus L. | 115 ± 10 °C, 30 min | Xiantao city, Hubei | Normal temperature, <3 months |
| RSC7 | reddish brown | Brassica napus L. | 165 ± 15 °C, 25 min | Huaihua city, Hunan | Normal temperature, <3 months |
| RSC8 | brown | Brassica napus L. | 130 ± 10 °C, 30 min | Chongqing Municipality | Normal temperature, <3 months |
| RSC9 | brown | Brassica napus L. | 130 ± 10 °C, 30 min | Xiangxiang city, Hunan | Normal temperature, <3 months |
| RSC10 | yellowish brown | Brassica napus L. | 95 ± 10 °C, 60 min | Hanchuan city, Hubei | Normal temperature, <3 months |
RSC, rapeseed cake; * The color information was derived from the color of the entire powder after comminution.
2.2. Animals, Diets, and Experimental Design
A total of 22 castrated pigs [Duroc × (Yorkshire × Landrace), initial BW: (39.8 ± 1.7) kg], with a distal ileum T-cannula were assigned to two incomplete 11 × 3 Latin square designs and fed with 10 different diets containing RSC as the sole nitrogen source and one nitrogen-free diet for determining the basal endogenous losses of CP and AA (Table 2) [3,12,13]. All diets contained 0.30% of titanium dioxide (TiO2) as an indigestible marker for calculating the digestibility of CP and AA. Adequate amounts of vitamins and minerals were added to all diets according to the NRC (2012) [6] recommendations. The experiment consisted of three consecutive periods. Every experimental cycle included 5 days of adaptation followed by 2 days of ileal digesta collection. Six replicate samples were obtained for each diet treatment. All pigs were individually housed in metabolic cages (1.4 m × 0.7 m × 0.5 m) and maintained at a temperature of (23 ± 1) °C. Before each experimental cycle, the pigs were individually weighed, fed equal amounts at 0800 and 1700 h, totaling 4% of the average initial body weight, and had free access to water.
Table 2.
Ingredient composition of experimental diet and nitrogen-free diet (air-dry basis, %).
| Items | Experimental Diet | Nitrogen-Free Diet |
|---|---|---|
| Maize starch | 42.90 | 78.90 |
| Rapeseed cake | 40.00 | |
| Soybean oil | 3.00 | 3.00 |
| Cellulose acetate | 4.00 | |
| Sucrose | 10.00 | 10.00 |
| Limestone | 0.5 | 0.5 |
| Calcium phosphite (Ca(H2PO4)2) | 1.9 | 1.9 |
| Titanium dioxide (TiO2) | 0.3 | 0.3 |
| Sodium chloride (NaCl) | 0.4 | 0.4 |
| Potassium carbonate (K2CO3) | 0.4 | 0.4 |
| Magnesium oxide (MgO) | 0.1 | 0.1 |
| Vitamin and mineral premix | 0.5 | 0.5 |
| Total | 100.00 | 100.00 |
The vitamin and mineral premix provided the following per kg of diets: VA 4200 IU, VD3 400 IU, VE 36 IU, VK3 1.2 mg, VB12 23 ug, VB2 5.63 mg, VB5 20.5 mg, VB3 28 mg, choline chloride 1.00 g, folic acid 0.8 mg, VB1 3.4 mg, VB6 2.7 mg, VH 0.18 mg, Mn (as manganese sulfate) 40.0 mg, Fe (as ferrous sulfate) 70.0 mg, Zn (as copper sulfate) 70 mg, I (as potassium iodide) 0.3 mg, Se (as sodium selenite) 0.3 mg.
2.3. Sample Collection and Preparation
During the 2 days of ileal digesta collection, ileal digesta was collected for 8 h daily from 8:00 a.m. to 4:00 p.m. each day. The procedure for collecting ileal digesta was as follows: the plug cap and inner sleeve were removed, and a plastic bag was attached to the T-shaped cannula port and secured with a rubber band to collect the ileal digesta. After collecting for a maximum of 30 min or obtaining approximately more than one-third of the volume of the plastic bag, the plastic bag was removed and immediately frozen at −20 °C to minimize bacterial fermentation [9,12]. At the end of each cycle, the frozen ileal digesta samples were combined and placed in a Vacuum-Freeze Dryer (SCIENTZ-50F/A, Ningbo Xinzhi Lyophilization Equipment Co., Ltd., Ningbo, China) for drying. After drying, the resulting samples were finely ground to pass through a 1 mm sieve.
2.4. Sample Analysis and Calculation
The levels of dry matter (DM), ether extract (EE), crude protein (CP), ash, calcium (Ca), and total phosphorus (TP) in the RSC were measured following AOAC (2006) [14] procedures [(DM,930.15), (EE, 920.39), (CP, 984.13), (ash, 942.05), (Ca, 968.08), (TP, 964.06)]. The amounts of crude fiber (CF), neutral detergent fiber (NDF), and acid detergent fiber (ADF) were determined using a fiber analyzer (ANKOM A200i Fiber Analyzer, Beijing ANKOM Technology Co., Ltd., Beijing, China) with fiber bags, as per the method outlined by Van Soest et al. (1991) [15]. The content of total glucosinolates (TGS) was analyzed using an Elisa kit (Fankew, Shanghai Kexing Trading Co., Ltd., Shanghai, China). The gross energy (GE) content of the RSC was determined using an automatic oxygen bomb calorimeter (HXR-6000 calorimeter, Hunan Huaxing Energy Instrument Co. Ltd., Changsha, China).
We used the same method to detect the CP levels in the feed and in the freeze-dried ileal digesta samples as we did for the RSC. The analysis of the AA content in the rapeseed cake, feed, and ileal digesta samples followed these steps: First, we conducted acid hydrolysis using 6 M HCl, then measured the content of 15 amino acids using high-performance liquid chromatography (Agilent 1200, Agilent Technologies, Santa Clara, CA, USA). Methionine (Met) and cysteine levels were determined through the oxidative hydrolysis (method 982.30 E(a); AOAC, 2006) [14]. Tryptophan (Trp) was hydrolyzed with 10% KOH at 40 °C for 16–18 h, and its content was determined using spectrophotometry according to GB/T 15400-2018 [16]. The content of TiO2 in the diets and ileal digesta was analyzed using an inductively coupled plasma optical emission spectrometer (ICP-OES 5110, Agilent Technologies) according to the method of GB/T 5009.246-2016 [17]. The apparent ileal digestibility (AID) and SID of amino acids (%), in the RSC samples, were determined based on the method described by Stein et al. (2007) [10] using the following equation:
| AID = [1 − (AAd × Tr)/(AAr × Td)] × 100% |
AAd and Td represent the concentrations of AA and TiO2 in the ileal digesta (g/kg of DM), respectively, while AAr and Tr are the concentrations of AA and TiO2 in the RSC diets (g/kg of DM), respectively. The same equation was used to calculate the AID of CP:
| IAAend = [AAd × (Tr/Td)] |
In the equation, IAAend represents the basal endogenous loss of each AA (g/kg of DM intake, DMI), and AAd and Td represent the concentrations of AA and TiO2 in the ileal digesta from the growing-finishing pigs fed the N-free diet, respectively. The Tr represents the concentration of TiO2 in the N-free diet. The same equation was used to calculate the endogenous loss of CP:
| SID = [AID + (IAAend/AAd) × 100%] |
2.5. Statistical Analysis
We used SPSS 27.0 (SPSS Inc., Chicago, IL, USA) to assess the normality and equal variance of the data, and Z-scores were analyzed to identify outliers. The CORR procedure was employed to examine the correlation coefficients among the chemical composition and the AA digestibility (AID and SID of Lys, Met, Trp, and Thr) of the RSC samples. Stepwise regression was used to establish prediction equations for the SID of Lys, Met, Trp, and Thr of the RSC samples based on their chemical compositions. The best-fit equations were selected based on their relative standard deviation (RSD), R2, and p-value. p < 0.05 means significant difference and p < 0.01 means extremely significant difference; when R2 is closer to 1 and p-value represents a significant difference, the equation is considered more accurate.
3. Results
3.1. Chemical Composition and AA Profile of Rapeseed Cake and Its Diet
On the air-dry basis, the mean contents of GE, DM, CP, EE, ash, CF, NDF, ADF, Ca, TP, and TGS of the 10 RSC samples were 19.25 MJ/kg (18.45 to 20.84 MJ/kg), 91.62% (87.95% to 94.68%), 39.15% (35.15% to 43.61%), 8.11% (5.48% to 11.76%), 6.77% (5.99% to 7.75%), 9.34% (8.26% to 11.32%), 30.19% (21.89% to 48.61%), 17.68% (13.13% to 22.87%), 0.58% (0.50% to 0.62%), 1.16% (0.92% to 1.30%), and 22.35 μmol/g (20.25 to 25.02 μmol/g), respectively. The coefficients of variation (CV) of EE, CF, NDF, ADF, and TGS in the RSC samples were greater than 10% (Table 3).
Table 3.
Analyzed chemical composition, physical characteristics of 10 rapeseed cake samples (air-dry basis, %).
| Items | Rapeseed Cake Number | Mean | CV (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RSC1 | RSC2 | RSC3 | RSC4 | RSC5 | RSC6 | RSC7 | RSC8 | RSC9 | RSC10 | |||
| DM | 93.02 | 87.95 | 90 | 91.67 | 92.36 | 91.57 | 94.68 | 92.06 | 91.08 | 91.85 | 91.62 | 1.94 |
| GE, MJ/kg | 19.08 | 19.07 | 18.6 | 19.47 | 18.45 | 20.11 | 20.84 | 18.94 | 18.72 | 19.18 | 19.25 | 3.8 |
| CP | 43.61 | 38.66 | 38.48 | 39.33 | 42.78 | 35.15 | 38.37 | 41.73 | 36.67 | 36.76 | 39.15 | 7.07 |
| EE | 7.96 | 5.81 | 7.98 | 7.76 | 6.46 | 11.76 | 9.73 | 7.54 | 7.92 | 8.13 | 8.11 | 32.79 |
| Ash | 7.07 | 6.62 | 6.11 | 5.99 | 7.75 | 6.42 | 7.09 | 7.02 | 6.78 | 6.89 | 6.77 | 7.65 |
| CF | 8.26 | 10.25 | 9.34 | 8.81 | 8.4 | 9.72 | 11.32 | 8.55 | 9.29 | 9.5 | 9.34 | 10.01 |
| NDF | 30.87 | 44.25 | 24.19 | 23.27 | 21.89 | 26.61 | 48.61 | 27.77 | 29.3 | 25.12 | 30.19 | 29.94 |
| ADF | 15.83 | 22.25 | 16.89 | 16.28 | 14.71 | 15.91 | 22.87 | 13.13 | 19.59 | 19.39 | 17.68 | 18.19 |
| Ca | 0.58 | 0.58 | 0.5 | 0.57 | 0.62 | 0.56 | 0.59 | 0.57 | 0.62 | 0.6 | 0.58 | 5.92 |
| TP | 1.25 | 1.08 | 0.92 | 1.19 | 1.19 | 1.05 | 1.3 | 1.27 | 1.17 | 1.18 | 1.16 | 9.67 |
| TGS/(μmol/g) | 21.84 | 22.81 | 23.82 | 20.27 | 21.39 | 22.97 | 25.02 | 20.25 | 20.86 | 24.27 | 22.35 | 15.19 |
| Essential amino acids, % | ||||||||||||
| Arginine | 2.86 | 2.03 | 2.54 | 2.55 | 2.48 | 2.18 | 2.32 | 2.45 | 2.45 | 1.74 | 2.36 | 13.18 |
| Histidine | 1.78 | 1.52 | 1.65 | 1.59 | 1.58 | 1.60 | 1.56 | 1.60 | 1.63 | 1.13 | 1.56 | 10.79 |
| Isoleucine | 2.63 | 1.54 | 1.58 | 2.14 | 1.56 | 2.06 | 2.07 | 1.99 | 2.02 | 1.16 | 1.87 | 22.13 |
| Leucine | 2.29 | 2.29 | 2.39 | 2.40 | 2.34 | 2.38 | 2.24 | 2.24 | 2.33 | 1.98 | 2.29 | 5.35 |
| Lysine | 2.20 | 1.22 | 1.91 | 2.08 | 2.14 | 2.04 | 1.23 | 1.42 | 1.73 | 2.08 | 1.80 | 21.13 |
| Methionine | 0.90 | 0.73 | 0.77 | 0.74 | 0.76 | 0.95 | 0.82 | 1.29 | 0.71 | 0.82 | 0.85 | 20.30 |
| Phenylalanine | 1.96 | 1.61 | 1.69 | 1.21 | 1.66 | 1.25 | 1.14 | 1.16 | 1.10 | 1.48 | 1.43 | 20.55 |
| Threonine | 1.97 | 1.65 | 1.64 | 1.60 | 1.73 | 1.66 | 1.45 | 1.58 | 1.59 | 1.20 | 1.61 | 12.14 |
| Tryptophan | 0.36 | 0.30 | 0.25 | 0.28 | 0.29 | 0.29 | 0.37 | 0.36 | 0.27 | 0.33 | 0.31 | 13.41 |
| Valine | 2.68 | 2.25 | 2.34 | 2.14 | 2.31 | 2.28 | 1.81 | 2.84 | 2.32 | 1.88 | 2.29 | 13.72 |
| Non-essential amino acids, % | ||||||||||||
| Alanine | 2.12 | 1.64 | 1.66 | 1.69 | 1.62 | 1.76 | 1.57 | 1.60 | 1.60 | 1.28 | 1.65 | 12.57 |
| Aspartate | 3.17 | 2.39 | 2.67 | 2.49 | 2.49 | 2.53 | 2.36 | 2.47 | 2.48 | 1.99 | 2.50 | 11.73 |
| Cystine | 1.14 | 1.05 | 1.11 | 1.03 | 1.23 | 1.69 | 1.10 | 1.14 | 1.13 | 1.01 | 1.16 | 16.67 |
| Glutamine | 8.55 | 5.83 | 6.70 | 6.40 | 6.57 | 6.11 | 5.94 | 5.92 | 6.22 | 5.40 | 6.36 | 13.44 |
| Glycine | 2.25 | 1.65 | 1.71 | 1.70 | 1.69 | 1.74 | 1.61 | 1.63 | 1.67 | 1.45 | 1.71 | 12.06 |
| Proline | 2.28 | 1.83 | 1.87 | 3.91 | 2.07 | 3.17 | 3.44 | 3.71 | 2.24 | 1.95 | 2.65 | 30.92 |
| Serine | 2.23 | 1.55 | 1.63 | 1.60 | 1.62 | 1.63 | 1.42 | 1.61 | 1.63 | 2.31 | 1.72 | 17.15 |
| Tyrosine | 1.84 | 1.29 | 1.43 | 1.30 | 1.41 | 1.38 | 1.27 | 1.35 | 1.33 | 0.74 | 1.33 | 20.00 |
DM, dry matter; GE, gross energy; CP, crude protein; EE, ether extract; Ash, crude ash; CF, crude fiber; NDF, neutral detergent fiber; ADF, acid detergent fiber; Ca, calcium; TP, total phosphorus; TGS, total glucosinolate; CV, coefficient of variation.
Except for leucine, the CV of other amino acids exceeded 10%. The concentrations of Lys, Met, Thr, and Trp in the 10 RSC samples were 1.80% (1.22% to 2.20%), 0.82% (0.71% to 1.29%), 1.61% (1.20% to 1.97%), and 0.31% (0.25% to 0.37%), respectively.
The chemical composition and AA profile of rapeseed cake diet are shown in Table 4.
Table 4.
Analyzed chemical composition of experiment diets (air-dry basis, %).
| Rapeseed Cake Diet | Mean | CV (%) | N-Free Diet | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Items | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| DM,% | 90.85 | 90.20 | 90.61 | 90.36 | 92.34 | 91.11 | 92.89 | 92.46 | 90.89 | 91.30 | 91.30 | 1.03 | 90.28 |
| CP,% | 17.71 | 12.94 | 14.04 | 14.19 | 13.88 | 14.18 | 12.82 | 14.21 | 13.52 | 11.59 | 13.91 | 11.33 | 1.45 |
| Essential amino acids, % | |||||||||||||
| Arginine | 1.15 | 0.80 | 1.00 | 0.99 | 0.97 | 0.84 | 0.89 | 0.96 | 0.96 | 0.67 | 0.92 | 14.00 | - |
| Histidine | 0.72 | 0.60 | 0.65 | 0.62 | 0.62 | 0.61 | 0.59 | 0.62 | 0.64 | 0.44 | 0.61 | 11.52 | - |
| Isoleucine | 1.05 | 0.61 | 0.62 | 0.83 | 0.61 | 0.79 | 0.79 | 0.78 | 0.78 | 0.45 | 0.73 | 22.69 | - |
| Leucine | 0.92 | 0.90 | 0.94 | 0.93 | 0.92 | 0.91 | 0.86 | 0.88 | 0.91 | 0.77 | 0.89 | 5.68 | - |
| Lysine | 0.89 | 0.48 | 0.75 | 0.81 | 0.84 | 0.78 | 0.47 | 0.56 | 0.67 | 0.80 | 0.70 | 21.43 | - |
| Methionine | 0.36 | 0.29 | 0.30 | 0.29 | 0.30 | 0.36 | 0.32 | 0.51 | 0.28 | 0.32 | 0.33 | 20.46 | - |
| Phenylalanine | 0.78 | 0.63 | 0.67 | 0.47 | 0.65 | 0.48 | 0.44 | 0.45 | 0.43 | 0.57 | 0.56 | 21.60 | - |
| Threonine | 0.79 | 0.65 | 0.65 | 0.63 | 0.68 | 0.64 | 0.56 | 0.62 | 0.62 | 0.46 | 0.63 | 13.13 | - |
| Tryptophan | 0.15 | 0.12 | 0.10 | 0.11 | 0.11 | 0.11 | 0.14 | 0.14 | 0.10 | 0.13 | 0.12 | 13.57 | - |
| Valine | 1.08 | 0.89 | 0.92 | 0.83 | 0.90 | 0.88 | 0.69 | 1.11 | 0.90 | 0.72 | 0.89 | 14.72 | - |
| Non-essential amino acids, % | - | ||||||||||||
| Alanine | 0.86 | 0.65 | 0.65 | 0.66 | 0.63 | 0.68 | 0.60 | 0.63 | 0.62 | 0.49 | 0.65 | 14.02 | - |
| Aspartate | 1.28 | 0.94 | 1.04 | 0.97 | 0.97 | 0.97 | 0.90 | 0.96 | 0.96 | 0.76 | 0.98 | 13.05 | - |
| Cystine | 0.46 | 0.41 | 0.43 | 0.40 | 0.48 | 0.65 | 0.42 | 0.45 | 0.44 | 0.39 | 0.45 | 16.38 | 0.08 |
| Glutamine | 3.40 | 2.30 | 2.64 | 2.48 | 2.59 | 2.36 | 2.26 | 2.31 | 2.41 | 2.08 | 2.48 | 14.48 | - |
| Glycine | 0.91 | 0.65 | 0.68 | 0.66 | 0.67 | 0.67 | 0.61 | 0.64 | 0.65 | 0.56 | 0.67 | 13.47 | - |
| Proline | 0.91 | 0.72 | 0.74 | 1.51 | 0.82 | 1.22 | 1.32 | 1.44 | 0.87 | 0.75 | 1.03 | 30.20 | 0.51 |
| Serine | 0.89 | 0.61 | 0.64 | 0.62 | 0.63 | 0.63 | 0.54 | 0.63 | 0.63 | 0.89 | 0.67 | 17.53 | - |
| Tyrosine | 0.74 | 0.51 | 0.56 | 0.50 | 0.55 | 0.53 | 0.48 | 0.53 | 0.52 | 0.29 | 0.52 | 21.18 | - |
DM, dry matter; CP, crude protein; CV, coefficient of variation.
3.2. AID and SID of CP and AA
The AID values for CP, Lys, Met, Thr, and Trp in the 10 RSC samples were 59.72% (46.54% to 70.29%), 61.97% (39.94% to 72.23%), 65.31% (46.08% to 83.92%), 67.88% (62.81% to 75.86%), and 79.91% (73.45% to 84.12%), respectively (Table 5). Significant differences (p < 0.01) were observed in the AID values for CP, Lys, Met, and Trp.
Table 5.
Apparent ileal digestibility (AID) of crude protein (CP) and amino acids (AA) in rapeseed cake fed to growing-finishing pigs (%).
| Rapeseed Cake | Mean | SEM | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Items | RSC1 | RSC2 | RSC3 | RSC4 | RSC5 | RSC6 | RSC7 | RSC8 | RSC9 | RSC10 | |||
| CP, % | 70.29 a | 52.19 de | 62.36 abc | 63.38 abc | 66.29 ab | 66.09 ab | 46.54 e | 54.76 cde | 60.46 bcd | 54.81 cde | 59.72 | 1.29 | <0.01 |
| Essential amino acids, % | |||||||||||||
| Arginine | 75.56 ab | 70.61 ab | 80.29 a | 77.19 ab | 75.38 ab | 78.09 ab | 75.59 ab | 73.39 ab | 79.82 ab | 68.94 b | 75.49 | 1.07 | 0.28 |
| Histidine | 73.22 cd | 72.62 cd | 76.80 bc | 68.01 d | 67.27 d | 73.71 cd | 81.92 ab | 86.27 a | 88.41 a | 83.20 ab | 77.14 | 1.26 | <0.01 |
| Isoleucine | 81.20 ab | 71.92 d | 72.45 cd | 80.09 ab | 76.50 bcd | 84.81 a | 78.38 abc | 78.73 ab | 81.65 ab | 66.13 e | 77.19 | 0.91 | <0.01 |
| Leucine | 72.30 ab | 73.35 ab | 76.54 ab | 75.59 ab | 79.96 a | 81.19 a | 77.42 a | 60.59 bc | 51.72 c | 53.25 c | 70.19 | 2.04 | <0.01 |
| Lysine | 64.34 a | 41.12 b | 65.21 a | 67.90 a | 71.61 a | 68.56 a | 39.94 b | 64.70 a | 64.12 a | 72.23 a | 61.97 | 2.16 | <0.01 |
| Methionine | 64.76 b | 53.52 bc | 46.08 c | 58.70 bc | 49.67 c | 52.17 c | 83.92 a | 81.18 a | 81.99 a | 81.05 a | 65.31 | 2.39 | <0.01 |
| Phenylalanine | 76.87 b | 74.26 b | 76.44 b | 67.30 c | 76.58 b | 75.29 a | 83.22 a | 88.51 a | 88.89 a | 85.34 a | 79.27 | 1.08 | <0.01 |
| Threonine | 68.20 ab | 64.24 ab | 66.55 ab | 62.81 b | 65.36 ab | 71.63 ab | 66.37 ab | 68.24 ab | 75.86 a | 69.58 ab | 67.88 | 1.16 | 0.37 |
| Tryptophan | 84.12 a | 80.20 ab | 73.75 c | 78.11 abc | 80.59 ab | 81.69 ab | 81.40 ab | 83.36 a | 75.91 bc | 79.98 ab | 79.91 | 0.68 | <0.01 |
| Valine | 81.62 a | 77.76 ab | 77.99 ab | 80.57 ab | 83.36 a | 79.91 ab | 68.88 bc | 82.89 a | 82.55 a | 59.86 c | 77.54 | 1.48 | <0.01 |
| Non-essential amino acids, % | |||||||||||||
| Alanine | 73.80 ab | 68.54 ab | 70.70 ab | 69.53 ab | 73.43 ab | 74.34 a | 64.28 b | 68.89 ab | 75.35 a | 67.39 ab | 70.62 | 0.96 | 0.20 |
| Aspartate | 73.74 | 67.17 | 71.78 | 69.05 | 72.67 | 73.33 | 66.47 | 67.98 | 73.88 | 66.22 | 70.23 | 0.88 | 0.21 |
| Cystine | 67.90 bc | 66.84 bc | 71.11 abc | 64.24 c | 76.15 abc | 60.58 c | 80.28 ab | 83.27 a | 82.67 a | 69.21 bc | 72.23 | 1.56 | <0.01 |
| Glutamine | 86.46 ab | 80.33 bc | 83.02 abc | 81.26 bc | 83.79 abc | 84.37 abc | 77.10 c | 82.10 bc | 89.62 a | 83.73 abc | 83.18 | 0.79 | <0.05 |
| Glycine | 74.65 a | 63.50 ab | 66.55 ab | 62.44 ab | 60.50 ab | 62.30 ab | 32.28 c | 35.01 c | 56.10 b | 57.49 b | 57.08 | 2.17 | <0.01 |
| Proline | 55.06 a | 47.90 ab | 52.08 ab | 63.70 a | 24.90 b | 50.26 ab | 44.90 ab | 63.77 a | 61.72 a | 46.77 ab | 51.11 | 2.83 | 0.33 |
| Serine | 78.99 abc | 69.58 cd | 75.54 abc | 71.68 bcd | 75.14 abcd | 69.31 cd | 65.26 d | 74.37 bcd | 80.84 ab | 84.47 a | 74.52 | 1.16 | <0.01 |
| Tyrosine | 79.09 a | 73.49 ab | 75.87 ab | 75.02 ab | 78.86 a | 79.40 a | 74.98 ab | 70.97 ab | 76.49 ab | 67.82 b | 75.20 | 0.92 | 0.09 |
CP, crude protein; a,b,c,d,e Means that values in the same row with no letter or the same letter are not different at p < 0.05.
The SID values of CP, Lys, Met, Thr, and Trp in the 10 RSC samples were 73.81% (61.92% to 81.19%), 63.02% (41.45% to 73.11%), 69.51% (50.56% to 88.28%), 79.75% (74.42% to 87.66%), and 94.63% (91.41% to 97.76%), respectively (Table 6 and Table 7). Significant differences (p < 0.01) were observed in the SID of CP, Lys, and Met.
Table 6.
Analysis of basal endogenous loss of crude protein and amino acids (g/kg DM intake).
| IAAend | Mathai [18] | Espinosa et al. [19] | Son et al. [20] | Zhang et al. [21] | Li et al. [9] | Min | Max | Mean | Our Study |
|---|---|---|---|---|---|---|---|---|---|
| Arg | 1.01 | 0.89 | 2.14 | 0.14 | 0.43 | 0.14 | 2.14 | 0.92 | 1.96 |
| His | 0.31 | 0.19 | 0.25 | 0.11 | 0.09 | 0.09 | 0.31 | 0.19 | 0.23 |
| Ile | 0.57 | 0.32 | 0.37 | 0.18 | 0.28 | 0.18 | 0.57 | 0.34 | 0.56 |
| Leu | 0.9 | 0.48 | 0.6 | 0.21 | 0.38 | 0.21 | 0.90 | 0.51 | 0.43 |
| Lys | 0.91 | 0.62 | 0.54 | 0.09 | 0.36 | 0.09 | 0.91 | 0.50 | 0.08 |
| Met | 0.16 | 0.08 | 0.11 | 0.04 | 0.08 | 0.04 | 0.16 | 0.09 | 0.15 |
| Met + Cys | - | - | 0.32 | - | - | 0.32 | 0.32 | 0.32 | - |
| Phe | 0.55 | 0.29 | 0.36 | 0.28 | 0.21 | 0.21 | 0.55 | 0.34 | 0.48 |
| Thr | 0.86 | 0.55 | 0.67 | 0.41 | 0.48 | 0.41 | 0.86 | 0.59 | 0.8 |
| Trp | 0.2 | 0.09 | 0.19 | - | 0.1 | 0.09 | 0.20 | 0.15 | 0.19 |
| Val | 0.93 | 0.41 | 0.53 | 0.51 | 0.35 | 0.35 | 0.93 | 0.55 | 0.6 |
| Ala | 1.01 | 0.68 | 1.43 | 0.26 | 0.61 | 0.26 | 1.43 | 0.80 | 1.01 |
| Asp | 1.32 | 0.78 | 1.09 | 0.49 | 0.8 | 0.49 | 1.32 | 0.90 | 0.77 |
| Cys | 0.29 | 0.2 | 0.2 | 0.14 | 0.07 | 0.07 | 0.29 | 0.18 | 0.04 |
| Glu | 1.58 | 0.94 | 1.3 | 0.63 | 1.06 | 0.63 | 1.58 | 1.10 | 1.03 |
| Gly | 2.57 | 1.94 | 3.77 | 0.61 | 1 | 0.61 | 3.77 | 1.98 | 1.02 |
| Pro | - | - | 20.1 | 2.47 | 1.26 | 1.26 | 20.10 | 7.94 | 6.39 |
| Ser | 0.8 | 0.55 | 0.84 | 0.35 | 0.49 | 0.35 | 0.84 | 0.61 | 0.64 |
| Tyr | 0.45 | 0.25 | - | 0.38 | 0.14 | 0.14 | 0.45 | 0.31 | 0.63 |
| CP | - | 20.27 | 36.3 | - | 10.71 | 10.71 | 36.30 | 22.43 | 21.24 |
Table 7.
Standardized ileal digestibility (SID) of crude protein (CP) and amino acids (AA) in rapeseed cake fed to growing-finishing pigs (%).
| Rapeseed Cake | Mean | SEM | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Items | RSC1 | RSC2 | RSC3 | RSC4 | RSC5 | RSC6 | RSC7 | RSC8 | RSC9 | RSC10 | |||
| CP, % | 81.19 a | 67.00 de | 76.07 abc | 76.9 abc | 80.42 ab | 79.73 ab | 61.92 e | 68.59 cde | 74.74 abcd | 71.54 bcd | 73.81 | 1.18 | <0.001 |
| Essential amino acids, % | |||||||||||||
| Arginine | 91.04 | 92.55 | 98.09 | 95.04 | 93.9 | 99.27 | 96.06 | 92.23 | 98.4 | 95.48 | 95.21 | 1.02 | 0.72 |
| Histidine | 76.17 cd | 76.14 cd | 80.05 bc | 71.42 d | 70.73 d | 77.16 cd | 85.56 ab | 89.71 a | 91.73 a | 88.07 ab | 80.68 | 1.27 | <0.01 |
| Isoleucine | 86.05 ab | 80.22 bc | 80.72 bc | 86.21 ab | 85.04 ab | 91.28 a | 85 ab | 85.44 ab | 88.19 a | 77.55 c | 84.57 | 0.77 | <0.01 |
| Leucine | 76.53 ab | 77.65 ab | 80.67 ab | 79.75 ab | 84.27 a | 85.48 a | 82.06 a | 65.11 bc | 56.01 c | 58.35 c | 74.59 | 2.02 | <0.01 |
| Lysine | 65.13 a | 42.56 b | 66.14 a | 68.75 a | 72.45 a | 69.46 a | 41.45 b | 65.98 a | 65.16 a | 73.11 a | 63.02 | 2.14 | <0.01 |
| Methionine | 68.50 b | 58.17 bc | 50.56 c | 63.35 bc | 54.25 c | 55.89 bc | 88.28 a | 83.89 a | 86.86 a | 85.35 a | 69.51 | 2.37 | <0.01 |
| Phenylalanine | 82.48 bc | 81.12 bc | 82.99 bc | 76.56 c | 83.4 bc | 84.35 b | 93.44 a | 98.38 a | 99.03 a | 93.04 a | 87.48 | 1.17 | <0.01 |
| Threonine | 77.48 ab | 75.38 b | 77.77 ab | 74.42 b | 76.23 ab | 83.11 ab | 79.75 ab | 80.25 ab | 87.66 a | 85.42 ab | 79.75 | 1.19 | 0.17 |
| Tryptophan | 96.07 | 94.77 | 91.41 | 93.7 | 96.26 | 97.76 | 93.97 | 96.05 | 92.48 | 93.84 | 94.63 | 0.60 | 0.43 |
| Valine | 86.98 a | 84.24 a | 84.28 a | 87.48 a | 89.87 a | 86.50 a | 77.49 ab | 88.21 a | 88.97 a | 67.88 c | 84.19 | 1.42 | <0.01 |
| Non-essential amino acids, % | |||||||||||||
| Alanine | 84.45 ab | 82.60 ab | 84.78 ab | 83.38 ab | 88.14 ab | 87.94 ab | 79.83 b | 83.77 ab | 90.09 a | 86.10 ab | 85.11 | 0.94 | 0.40 |
| Aspartate | 79.25 | 74.56 | 78.49 | 76.27 | 80.00 | 80.57 | 74.43 | 75.40 | 81.17 | 75.46 | 77.56 | 0.85 | 0.47 |
| Cystine | 76.69 bc | 76.51 bc | 80.32 abc | 74.20 c | 79.87 abc | 66.76 c | 90.02 ab | 92.42 a | 91.76 a | 79.52 abc | 80.80 | 1.58 | <0.01 |
| Glutamine | 89.23 ab | 84.38 bc | 86.56 abc | 85.03 abc | 87.48 abc | 88.36 abc | 81.35 c | 86.24 abc | 93.52 a | 88.26 abc | 87.04 | 0.78 | <0.05 |
| Glycine | 84.86 a | 77.54 cd | 80.22 a | 76.36 a | 74.57 a | 76.12 a | 47.70 b | 49.84 b | 70.30 a | 74.11 a | 71.16 | 2.09 | <0.01 |
| Proline | 118.68 ab | 127.87 a | 130.46 a | 101.85 abc | 95.63 bc | 97.43 bc | 88.76 b | 103.74 abc | 128.22 a | 123.64 ab | 111.63 | 3.38 | <0.01 |
| Serine | 85.58 ab | 79.05 b | 84.68 ab | 81.01 ab | 84.5 ab | 78.68 b | 76.25 b | 83.83 ab | 90.07 a | 91.06 a | 83.47 | 1.08 | 0.021 |
| Tyrosine | 86.76 | 84.49 | 86.09 | 86.26 | 89.38 | 90.12 | 87.13 | 81.96 | 87.51 | 87.92 | 86.76 | 0.84 | 0.70 |
CP, crude protein; Values for SID were calculated by correcting the AID values with the basal endogenous losses (IAAend). IAAend (g/kg DM intake) averaged as CP, 21.24; Arg, 1.96; His, 0.23; Ile, 0.56; Leu, 0.43; Lys, 0.08; Met, 0.15; Phe, 0.48; Thr, 0.80; Trp, 0.19; Val, 0.6; Ala, 1.01; Asp, 0.77; Cys, 0.04; Glu,1.03; Gly, 1.02; Pro, 6.39; Ser, 0.64; Tyr, 0.63. a,b,c,d,e Means that values in the same row with no letter or the same letter are not different at p < 0.05.
3.3. Correlation Analysis and Prediction Equations for SID of CP and AA
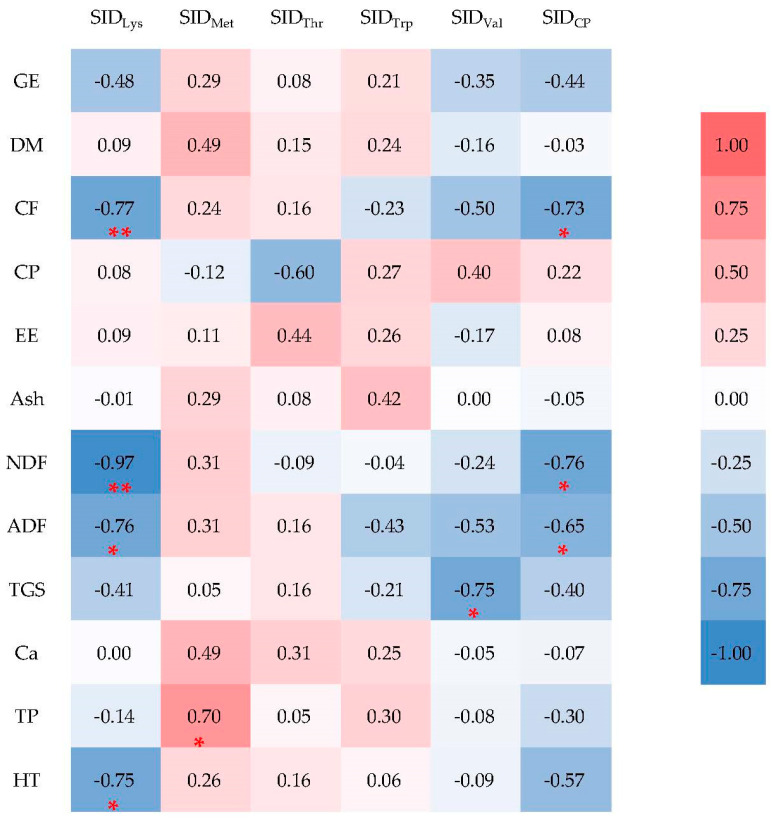
Significant negative correlation was found between the SID of Lys and heating temperature (p < 0.05, Figure 1), as well as with the levels of CF and NDF (p < 0.01, Figure 1). In Table 8, the optimal fitting equations for SIDCP, SIDLys, and SIDVal are as follows: SIDCP = 90.124 − 0.540NDF (R2 = 0.58, RSD = 4.39, p < 0.05), SIDLys = 100.107 − 1.229NDF (R2 = 0.94, RSD = 2.88, p < 0.01), and SIDVal = 151.012 − 2.990TGS (R2 = 0.57, RSD = 4.69, p < 0.05).
Figure 1.
Correlation coefficients (r) among physical characteristics, chemical constituents, and the SID of the first four limiting amino acids of the 10 rapeseed cake samples. * means significant difference (p < 0.05); ** means extremely significant difference (p < 0.01). SIDCP, SIDLys, SIDMet, SIDThr, SIDTrp, and SIDVal indicate the SID of CP, Lys, Met, Thr, Trp, and Val, respectively. DM, dry matter; GE, gross energy; CP, crude protein; EE, ether extract; Ash, crude ash; CF, crude fiber; NDF, neutral detergent fiber; ADF, acid detergent fiber; Ca, calcium; TP, total phosphorus; TGS, total glucosinolate; HT, heating temperature.
Table 8.
Stepwise regression equations for SID of CP, Lys, Met, Arg, and Val based upon the chemical characteristics of the 10 rapeseed cake samples (air-dry basis, %).
| Items | Prediction Equations | RSD | R2 | p-Value |
|---|---|---|---|---|
| SIDCP | SIDCP = 90.124 − 0.540NDF | 4.39 | 0.58 | <0.05 |
| SIDLys | SIDLys = 100.107 − 1.229NDF | 2.88 | 0.94 | <0.01 |
| SIDLys | SIDLys = 90.662 − 0.234HT | 8.04 | 0.51 | <0.05 |
| SIDVal | SIDVal = 151.012 − 2.990TGS | 4.69 | 0.57 | <0.05 |
SID, standardized ileal digestibility; CP, crude protein; NDF, neutral detergent fiber; HT, heating temperature; TGS, total glucosinolate; Lys, Lysine; Val, valine; R2, R-square; RSD, relative standard deviation. p < 0.05 means significant difference; p < 0.01 means extremely significant difference.
4. Discussion
4.1. Chemical Composition and AA Profile of Rapeseed Cake
The information of the rest of our samples is shown in Table 1. The majority of the analyzed chemical compositions of the 10 RSC samples in our study were within the range of the tabulated values [4,6,22], indicating the reliability of our obtained data. In this experiment, there was high variability (CV > 10%) in EE, CF, NDF, ADF, and TGS among the 10 RSC samples, indicating significant differences among the RSC samples. The average CP content was slightly higher than the reported values [4,6,22], but lower than some observed values [5,8]. These differences may be due to variations in rapeseed meal varieties and processing techniques. Research indicates that the traits and genetic selection of rapeseed can result in differences in its crude protein content [23]. Additionally, differences in processing conditions directly affect the EE content and other nutrients in RSC [2,24,25,26,27,28,29]. The average GE was similar to the reported values in the Nutrient Requirements of Swine in China (2020) [4] and by Li et al. (2015) [22], but lower than those in the NRC (2012) [6]. The mean CF, ADF, and NDF contents were within the range of tabulated values [4,6,22]. Among them, the NDF of the RSC7 sample was as high as 48.61%, which might be due to the excessive temperature during its thermal processing, resulting in a vigorous Maillard reaction. In the study by Mosenthin et al. [30], the NDF content of rapeseed meal (based on DM) varied between 40.7% and 47.6%, and it was pointed out that the occurrence of the Maillard reaction in rapeseed meal under heating and prolonged heating time would lead to an increase in NDF content. The TGS content of all 10 RSC samples was below 30 μmol/g, aligning with the range in previous studies [31,32]. The Ca and TP contents were also close to the values reported in previous literature [4,6].
Lys, Met, Thr, and Trp are crucial limiting amino acids for pig growth [33,34,35,36,37], which play an indispensable role in pig growth and development [33,34,35,38,39]. The CV values for the Lys and Met content in our RSC samples exceeded 20%, and those for Thr and Trp content were more than 10%, indicating significant differences in the amino acid compositions of our RSC samples. These changes in amino acids may result from different natural conditions and processing methods [30,40,41]. Through multivariate analysis of variance, we found that there was no significant difference (p > 0.05) in the overall amino acid composition of the ten samples compared with the NRC (2012) [6] and the Nutrient Requirements of Swine in China (2020) [4]. And they were closely consistent with some reports [4,6,7,22].
4.2. SID of AA in Rapeseed Cake
SID was derived based on AID by correcting with basic endogenous losses. One advantage of doing this was that it made the SID values additive [42]. Generally, nitrogen-free diets were used to obtain basic endogenous losses [3]. In Table 6, we compared the basic endogenous losses obtained by previous researchers in growing pigs. The results showed that our basic endogenous losses were basically within or close to the range of the table values. Mathai [18], Espinosa et al. [19], Son et al. [20], and Li et al. [9] all used the nitrogen-free diet method to determine the basic endogenous losses, and the cellulose proportions of their nitrogen-free diets were 3%, 4%, 4%, and 4%, respectively, but the method adopted by Zhang et al. [21] was not described in their report. Studies showed that fiber could seriously affect endogenous losses [43], and the fiber content of the nitrogen-free diet method, under the suggestions of previous researchers, had basically remained within the range of 3% to 4% [10]. The cellulose proportion of our nitrogen-free diet was 4%, which was consistent with the cellulose usage range of previous researchers.
The mean AID and SID values for Lys and Met were lower than those reported in the NRC (2012) [6] (Lys, 70% and 71%; Met, 82% and 83%) and the Nutrient Requirements of Swine in China (2020) [4] (Lys, 66% and 69%; Met, 88% and 89%), which may be related to the fiber components and the processing of the RSC. Several studies have shown that fiber components, such as NDF, can hinder the absorption of other nutrients and increase the endogenous losses of amino acids [10,24,29,44,45]. Elkund et al. [46] found that in the case where the NDF content of the sample (47.6%) was similar to that of our RSC7 sample with the highest NDF content, the SIDLys of the low glucosinolate rapeseed meal obtained was 55%, which was lower than that of the NRC (2012) [6] and the Nutrient Requirements of Swine in China (2020) [4].
According to the additional amount of RSC in our diet, the content of NDF contributed by RSC in the diet is approximately between 8.8% and 19.4%. Previous studies [47] have reported that adding wheat bran and soybean hulls to the diet would reduce the AID of dietary AA. Adding 2.5% of wheat bran compared to 0% (dietary NDF level, 14.2% vs. 13.5%) would reduce the AID of AA by 1.4% to 5.2%. Adding 2.5% of soybean hulls compared to 0% (dietary NDF level, 14.8% vs. 13.5%) would reduce the apparent ileal digestibility of amino acids by 4.7% to 10.8%. However, further adding wheat bran or soybean meal at a ratio of 2.5% to 5% or 7.5% had little effect on the AID of AA, and it was pointed out that different types of NDF have different effects on reducing the AID of AA. The inhibitory effect of fibers on the apparent digestibility of amino acids might mainly be due to the cause of higher endogenous amino acid losses, and it was speculated that this endogenous loss has a plateau after increasing [47]. But there were also studies indicating that as the NDF increases, the AID of AA decreases linearly [47,48]. In our study, the predictive equation shows that the SID of lysine has a linear relationship with NDF; the reason for this phenomenon might be that the Maillard reaction caused lysine to combine with the fibrous components, forming an indigestible part. Fiber components could undergo Maillard reactions with AA during the heating process, especially Lys, which was highly susceptible to Maillard reactions with reducing sugars due to its exposed ε-amino group [33]. This reaction leads to the binding of AA to fiber components, making them unavailable for absorption [33]. The lowest SID values or AID of lysine in our two RSC samples were similar to or higher than those of Seneviratne et al. (cold-pressed rapeseed cake: AIDLys, 40.8%; SIDLys, 41.4%) [45], Almeida et al. (rapeseed meal autoclaved at 130 °C for 45 min: AIDLys, 12.9%; SIDLys, 20.8%) [49], and Li et al. (2002) (rapeseed cake heated at >130 °C for 25 min, AIDLys, 40.6%) [50]. Considering the sample information in our Table 1, it could be seen that in the case of the highest heating temperature and similar time for the RSC2 and RSC7 samples in our study, the digestibility of lysine was also extremely similar. Such a phenomenon indicated that the low digestibility of lysine was the multiple result of endogenous losses, Maillard reactions, and other effects. The lowest AID and SID values of methionine in our study were lower than those of Almeida et al. (rapeseed meal autoclaved at 130 °C for 45 min: AIDMet, 64%; SIDMet, 68.5%) [49], Li et al. (2002) (rapeseed cake heated at >130 °C for 25 min: AIDMet, 83.8%) [50], and Elkund et al. (rapeseed cake with NDF content of 47.6%: SIDMet, 81%) [46]. The digestibility of most methionine in our samples was higher than or close to the results of Almeida et al. [49]. Considering the sample information of ours, the relationship between the digestibility of methionine in our samples and the processing temperature and time seemed difficult to capture, and the factors causing the low digestibility of methionine might be more complex.
The average AID values of Thr and Trp were similar to those in previous studies [4,6,22,24], but the SID values were slightly higher than the reported values [4,6,22,24,51]. This may be ascribed to a higher level of endogenous losses of Thr and Trp measured in this experiment compared to previous studies [6,32,33,52]. Additionally, the range of SID of proline in this study falls within reported values in the NRC (2012) [6] and the Nutrient Requirements of Swine in China (2020) [4].
4.3. Correlation Analysis and Prediction Equations for SID of AA in Rapeseed Cake
Our study found that the SID Lys had a negative correlation with CF, NDF, ADF, and heating temperature, and the NDF was the best predictor for the prediction equation of SIDLys and SIDCP. This suggested that fiber components, especially NDF, are critical for the digestion and absorption of AA. Fiber components could undergo Maillard reactions with AA during the heating process, especially Lys, which was highly susceptible to Maillard reactions with reducing sugars due to its exposed ε-amino group [33]. This reaction led to a portion of the protein becoming a component of the NDF matrix and no longer being free during digestion in the small intestine [53]. This also explained why there was a significant negative correlation between heating temperature and SIDLys in our study and why the heating temperature becomes its predictor. In addition, NDF and its lignin fraction had a high-water retention capacity and thereby increased the viscosity of the digesta, which slowed mass transfer and enzymatic reactions, and hence the digestion and absorption of nutrients [10,24,29,44,45,53]. Moreover, the SID of AA and CP was achieved via apparent ileal digestibility coefficients correcting for the ileal basal endogenous losses, and dietary composition, especially fiber components, was responsible for ileal basal endogenous CP and AA losses [9]. While there are no direct findings in previous studies to support our findings, Messad et al. [53] reported that NDF was negatively correlated with SIDLys, which was the best predictor for SIDLys and the essential amino acid SID of rapeseed meal in growing pigs. Toghyani et al. [54] found that NDF was negatively correlated with SIDLys and SIDCP in canola meal for broiler chickens. NDF was also the single predictor for SIDCP from the study on barley fed to pigs [9]. These studies indicated that the nutritional level of rapeseed cake meal could be improved via controlling the fiber contents.
In this study, the single predictor for SIDVal was TGS. Pigs are sensitive to TGS [55]; Wang et al. [56] reported that serum triiodothyronine and tetraiodothyronine concentrations were linearly decreased with the increased contents of double-low rapeseed cake. Spiegel et al. [57] reported TGS induced hypothyroidism, leading to delayed gastric emptying, and reduced intestinal motility and bacterial overgrowth, which affected amino acid absorption [58,59]. However, factors such as fiber fractions might have masked the effect of TGS on SIDAA [9,10,27,32,50,51,59], making the influence of TGS difficult to observe in studies. This led to fewer researchers having results similar to us, but a previous study reported that TGS was negatively correlated with SIDVal in RSC for laying hens [60]. Unfortunately, there is no literature specifically explained the effect of TGS on amino acid digestion and absorption and its mechanism of influence. More efforts and works are needed to do so in the future.
4.4. Validate the Prediction Equations for SIDCP and SIDAA of Rapeseed Cake Based on Database
The predicted SIDCP and SIDLys values for RSC using the best prediction equations were 77.66% and 70.90%, respectively, which were similar to the reported values in the NRC (2012) [6] (75% and 71%). Since the NRC (2012) [6] lacks the TGS value, we used the parameters from Seneviratne [61] and Li et al. [22]. The predicted values of SIDVal were 78.44% and 74.83%, respectively, which were higher than or similar to the reported values [22,61] of 70.5% and 76.93%.
5. Conclusions
The source and processing factors significantly affected the chemical composition and AA profile of RSC in the Yangtze River Basin and its adjacent areas, resulting in great variations in the digestibility of AA for pigs. NDF, TGS, and heating temperature might be the predictors for the SID of AA.
Acknowledgments
We thank the staff of the Key Laboratory of Subtropical Agroecological Processes, the National Engineering Laboratory of Poultry Breeding Pollution Control and Resource Technology, the Key Laboratory of Animal Nutritional Physiology and Metabolic Processes, institute of Subtropical Agriculture, Chinese Academy of Sciences, and the Center for Innovative Research on Animal Nutritional Genome and Germplasm, College of Animal Science and Technology, Hunan Agricultural University for support.
Author Contributions
H.T., R.L., X.Z. and Y.Y. conceived the study. H.T., J.Z., G.F., Q.O., X.L., X.J. and M.D. performed the experiments. H.T., G.F., Z.X. and F.C. analyzed the data. H.T. and G.F. visualized the statistical results. H.T. wrote the draft. H.T. and R.L. wrote and edited the final manuscript. R.L. improved the language and grammar of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The experimental design and procedures in this study were reviewed and approved by the Animal Ethics Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (IACUC#201302). The animal experiment was carried out in the metabolism laboratory of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (Changsha, China).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Z.X. was employed by the company Twins Group, Nanchang, China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This research was jointly funded by the “National Natural Science Foundation of China (32472957), National Key Research and Development Program (2021YFD1300201, 2021YFD1301004), Major Science and Technology Special Program of Yunnan Province (202202AE090032), Natural Science Foundation of Hunan Province (2022JJ40532), Open Fund of Key Laboratory of Agro-ecological Processes in Subtropical Regions, Chinese Academy of Sciences (ISA2021103, ISA2023201), and the Cooperation Project between institutions and enterprises (E241020501).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rakita S., Kokic B., Manoni M., Mazzoleni S., Lin P., Luciano A., Ottoboni M., Cheli F., Pinotti L. Cold-Pressed Oilseed Cakes as Alternative and Sustainable Feed Ingredients: A Review. Foods. 2023;12:432. doi: 10.3390/foods12030432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng H., Liu X., Xiao Q.R., Zhang F., Liu N., Tang L.Z., Wang J., Ma X.K., Tan B., Chen J.S., et al. Rapeseed Meal and Its Application in Pig Diet: A Review. Agriculture. 2022;12:849. doi: 10.3390/agriculture12060849. [DOI] [Google Scholar]
- 3.Kong C., Adeola O. Evaluation of Amino Acid and Energy Utilization in Feedstuff for Swine and Poultry Diets. Asian Australas J. Anim. Sci. 2014;27:917–925. doi: 10.5713/ajas.2014.r.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D.F. Nutrient Requirements of Swine in China. China Agriculture Press; Beijing, China: 2020. [Google Scholar]
- 5.Liu Y.H., Oliveira M.S.F., Stein H.H. Canola meal produced from high-protein or conventional varieties of canola seeds may substitute soybean meal in diets for gestating and lactating sows without compromising sow or litter productivity. J. Anim. Sci. 2018;96:5179–5187. doi: 10.1093/jas/sky356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NRC . Nutrient Requirements of Swine. 11th ed. Natl. Acad. Press; Washington, DC, USA: 2012. [Google Scholar]
- 7.Stein H.H., Lagos L.V., Casas G.A. Nutritional value of feed ingredients of plant origin fed to pigs. Anim. Feed. Sci. Technol. 2016;218:33–69. doi: 10.1016/j.anifeedsci.2016.05.003. [DOI] [Google Scholar]
- 8.Parr C.K., Liu Y., Parsons C.M., Stein H.H. Effects of high-protein or conventional canola meal on growth performance, organ weights, bone ash, and blood characteristics of weanling pigs. J. Anim. Sci. 2015;93:2165–2173. doi: 10.2527/jas.2014-8439. [DOI] [PubMed] [Google Scholar]
- 9.Li R., Tian M., Feng G., Hou G., Jiang X., Yang G., Xiang Q., Liu X., Long C., Huang R., et al. Determination and prediction of digestible energy, metabolizable energy, and standardized ileal digestibility of amino acids in barley for growing pig. Anim. Feed. Sci. Technol. 2023;298:115607. doi: 10.1016/j.anifeedsci.2023.115607. [DOI] [Google Scholar]
- 10.Stein H.H., Sève B., Fuller M.F., Moughan P.J., de Lange C.F.M. Invited review: Amino acid bioavailability and digestibility in pig feed ingredients: Terminology and application. J. Anim. Sci. 2007;85:172–180. doi: 10.2527/jas.2005-742. [DOI] [PubMed] [Google Scholar]
- 11.Tian Z., Ji Y., Sun L., Xu X., Fan D., Zhong H., Liang Z., Gunther F. Changes in production potentials of rapeseed in the Yangtze River Basin of China under climate change: A multi-model ensemble approach. J. Geogr. Sci. 2018;28:1700–1714. doi: 10.1007/s11442-018-1538-1. [DOI] [Google Scholar]
- 12.Feng G., Li R., Jiang X., Yang G., Tian M., Xiang Q., Liu X., Ouyang Q., Long C., Huang R., et al. Prediction of available energy and amino acid digestibility of Chinese sorghum fed to growing–finishing pigs. J. Anim. Sci. 2023;101:skad262. doi: 10.1093/jas/skad262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein H.H., Shipley C.F., Easter R.A. Technical note: A technique for inserting a T-cannula into the distal ileum of pregnant sows. J. Anim. Sci. 1998;76:1433–1436. doi: 10.2527/1998.7651433x. [DOI] [PubMed] [Google Scholar]
- 14.AOAC . Official Methods of Analysis. 18th ed. Association of the Official Analytical Chemists; Arlington, VA, USA: 2006. [Google Scholar]
- 15.Vansoest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 16.Determination of Tryptophan in Feeds. Standards Press of China, National Standards Press of China Press; Beijing, China: 2018. [Google Scholar]
- 17.Determination of Titanium Dioxide in Food. Standards Press of China, National Standards Press of China Press; Beijing, China: 2016. [Google Scholar]
- 18.Mathai J.K. Digestible Indispensable Amino Acid Scores for Food Proteins. University of Illinois at Urbana-Champaign; UIUC Press; Urbana, IL, USA: 2018. [Google Scholar]
- 19.Espinosa C., Fanelli N., Stein H. Digestibility of amino acids and concentration of metabolizable energy are greater in high-oil corn than in conventional corn when fed to growing pigs. Anim. Feed. Sci. Technol. 2021;280:115040. doi: 10.1016/j.anifeedsci.2021.115040. [DOI] [Google Scholar]
- 20.Son A.R., Hyun Y., Htoo J.K., Kim B.G. Amino acid digestibility in copra expellers and palm kernel expellers by growing pigs. Anim. Feed. Sci. Technol. 2014;187:91–97. doi: 10.1016/j.anifeedsci.2013.09.015. [DOI] [Google Scholar]
- 21.Zhang S., Zhong R., Gao L., Liu Z., Chen L., Zhang H. Effects of optimal carbohydrase mixtures on nutrient digestibility and digestible energy of corn-and wheat-based diets in growing pigs. Animals. 2020;10:1846. doi: 10.3390/ani10101846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li P.L., Wu F., Chen Y.F., Wang J.R., Guo P.P., Li Z.C., Liu L., Lai C.H. Determination of the energy content and amino acid digestibility of double-low rapeseed cakes fed to growing pigs. Anim. Feed. Sci. Technol. 2015;210:243–253. doi: 10.1016/j.anifeedsci.2015.10.012. [DOI] [Google Scholar]
- 23.Azam S.M., Farhatullah F., Adnan Nasim A.N., Sikandar Shah S.S., Sidra Iqbal S.I. Correlation studies for some agronomic and quality traits in Brassica napus L. Sarhad J. Agric. 2013;29:547–550. [Google Scholar]
- 24.Liu Y., Song M., Maison T., Stein H.H. Effects of protein concentration and heat treatment on concentration of digestible and metabolizable energy and on amino acid digestibility in four sources of canola meal fed to growing pigs. J. Anim. Sci. 2014;92:4466–4477. doi: 10.2527/jas.2013-7433. [DOI] [PubMed] [Google Scholar]
- 25.Khajali F., Slominski B.A. Factors that affect the nutritive value of canola meal for poultry. Poult. Sci. 2012;91:2564–2575. doi: 10.3382/ps.2012-02332. [DOI] [PubMed] [Google Scholar]
- 26.Kiarie E., Romero L.F., Nyachoti C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 2013;26:71–88. doi: 10.1017/S0954422413000048. [DOI] [PubMed] [Google Scholar]
- 27.Le M.H.A., Buchet A.D.G., Beltranena E., Gerrits W.J.J., Zijlstra R.T. Digestibility energy and amino acids of canola meal from two species (Brassica juncea and Brassica napus) fed to distal ileum cannulated grower pigs. J. Anim. Sci. 2012;90:218–220. doi: 10.2527/jas.53952. [DOI] [PubMed] [Google Scholar]
- 28.Maison T., Liu Y., Stein H.H. Digestibility of energy and detergent fiber and digestible and metabolizable energy values in canola meal, 00-rapeseed meal, and 00-rapeseed expellers fed to growing pigs. J. Anim. Sci. 2015;93:652–660. doi: 10.2527/jas.2014-7792. [DOI] [PubMed] [Google Scholar]
- 29.Mejicanos G., Sanjayan N., Kim I.H., Nyachoti C.M. Recent advances in canola meal utilization in swine nutrition. J. Anim. Sci. Technol. 2016;58:7. doi: 10.1186/s40781-016-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosenthin R., Messerschmidt U., Sauer N., Carré P., Quinsac A., Schöne F. Effect of the desolventizing/toasting process on chemical composition and protein quality of rapeseed meal. J. Anim. Sci. Biotechnol. 2016;7:36. doi: 10.1186/s40104-016-0095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P.L., Chen Y.F., Lyu Z.Q., Yu S.B., Wu F., Huang B.B., Liu L., Lai C.H. Concentration of metabolizable energy and digestibility of amino acids in Chinese produced dehulled double-low rapeseed expellers and non-dehulled double-low rapeseed co-products fed to growing-finishing pigs. Anim. Feed. Sci. Technol. 2017;234:10–19. doi: 10.1016/j.anifeedsci.2017.09.001. [DOI] [Google Scholar]
- 32.Li P., Lyu Z., Wang L., Huang B., Lai C. Nutritive values of double-low rapeseed expellers and rapeseed meal with or without supplementation of multi-enzyme in pigs. Can. J. Anim. Sci. 2020;100:729–738. doi: 10.1139/cjas-2019-0097. [DOI] [Google Scholar]
- 33.Brusov V., Stocklan W.L., Pick R.I., Meade R.J. Limiting amino acids in a low-protein diet forgrowing pigs. J. Anim. Sci. 1966;25:1241–1246. [Google Scholar]
- 34.Friesen K.G., Tokach M.D., Nelssen J.L., Goodband R.D. A review of current amino acid estimates for swine 2. Compend. Contin. Educ. Pract. Vet. 1996;18:1368–1372. [Google Scholar]
- 35.Zhang H., Hu B., Pang W. Research Progress on Physiological Effects of Threonine in Pigs. Chin. J. Anim. Nutr. 2023;35:708–717. [Google Scholar]
- 36.Lee C.Y., Song A.A.-L., Loh T.C., Rahim R.A. Effects of lysine and methionine in a low crude protein diet on the growth performance and gene expression of immunity genes in broilers. Poult. Sci. 2020;99:2916–2925. doi: 10.1016/j.psj.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maynard C.W., Kidd M.T., Chrystal P.V., McQuade L.R., McInerney B.V., Selle P.H., Liu S.Y. Assessment of limiting dietary amino acids in broiler chickens offered reduced crude protein diets. Anim. Nutr. 2022;10:1–11. doi: 10.1016/j.aninu.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langer S., Fuller M.F. Interactions among the branched-chain amino acids and their effects on methionine utilization in growing pigs: Effects on nitrogen retention and amino acid utilization. Br. J. Nutr. 2000;83:43–48. doi: 10.1017/S0007114500000076. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z., Liu M., Zhang H., He J. Advance on Effects of Dietary Lysine Restriction in Pigs. Chin. J. Anim. Nutr. 2019;31:4909–4916. [Google Scholar]
- 40.Hosseini P., Mohsenifar K., Rajaie M., Babaeinejad T. Plant growth regulators affecting canola (Brasica napus L.) biochemistry including oil yield under drought stress. Physiol. Mol. Biol. Plants. 2023;29:1663–1674. doi: 10.1007/s12298-023-01399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu J., Hou D., Li Y., Chao H., Zhang K., Wang H., Xiang J., Raboanatahiry N., Wang B., Li M. Integration of proteomic and genomic approaches to dissect seed germination vigor in Brassica napus seeds differing in oil content. BMC Plant Biol. 2019;19:21. doi: 10.1186/s12870-018-1624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein H.H., Fuller M., Moughan P., Sève B., Mosenthin R., Jansman A., Fernández J., De Lange C. Definition of apparent, true, and standardized ileal digestibility of amino acids in pigs. Livest. Sci. 2007;109:282–285. doi: 10.1016/j.livsci.2007.01.019. [DOI] [Google Scholar]
- 43.Adeola O., Xue P., Cowieson A., Ajuwon K. Basal endogenous losses of amino acids in protein nutrition research for swine and poultry. Anim. Feed. Sci. Technol. 2016;221:274–283. doi: 10.1016/j.anifeedsci.2016.06.004. [DOI] [Google Scholar]
- 44.Nursten H., Nursten H.E. Maillard Reaction. The Royal Society of Chemistry Press; London, UK: 2005. Nutritional Aspects. [Google Scholar]
- 45.Seneviratne R.W., Beltranena E., Newkirk R.W., Goonewardene L.A., Zijlstra R.T. Processing conditions affect nutrient digestibility of cold-pressed canola cake for grower pigs. J. Anim. Sci. 2011;89:2452–2461. doi: 10.2527/jas.2010-3569. [DOI] [PubMed] [Google Scholar]
- 46.Eklund M., Sauer N., Schöne F., Messerschmidt U., Rosenfelder P., Htoo J., Mosenthin R. Effect of processing of rapeseed under defined conditions in a pilot plant on chemical composition and standardized ileal amino acid digestibility in rapeseed meal for pigs. J. Anim. Sci. 2015;93:2813–2825. doi: 10.2527/jas.2014-8210. [DOI] [PubMed] [Google Scholar]
- 47.Dégen L., Halas V., Tossenberger J., Babinszky L. Dietary impact of NDF from different sources on the apparent ileal digestibility of amino acids. Acta Agrar. Kaposváriensis. 2011;15:1–11. [Google Scholar]
- 48.Dégen L., Halas V., Babinszky L. Effect of dietary fibre on protein and fat digestibility and its consequences on diet formulation for growing and fattening pigs: A review. Acta Agric. Scand Sect. A. 2007;57:1–9. doi: 10.1080/09064700701372038. [DOI] [Google Scholar]
- 49.Almeida F.N., Htoo J.K., Thomson J., Stein H.H. Effects of heat treatment on the apparent and standardized ileal digestibility of amino acids in canola meal fed to growing pigs. Anim. Feed. Sci. Technol. 2014;187:44–52. doi: 10.1016/j.anifeedsci.2013.09.009. [DOI] [Google Scholar]
- 50.Li D., Pengbin X., Liming G., Shijun F., Canghai H. Determination of apparent ileal amino acid digestibility in rapeseed meal and cake processed at different temperatures using the direct and difference method with growing pigs. Arch. Anim. Nutr. 2002;56:339–349. doi: 10.1080/00039420215629. [DOI] [PubMed] [Google Scholar]
- 51.Heyer C.M.E., Wang L., Beltranena E., Zijlstra R.T. Nutrient digestibility of extruded canola meal in ileal-cannulated growing pigs and effects of its feeding on diet nutrient digestibility and growth performance in weaned pigs. J. Anim. Sci. 2021;99:skab135. doi: 10.1093/jas/skab135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lahaye L., Ganier P., Thibault J.N., Sève B. Technological processes of feed manufacturing affect protein endogenous losses and amino acid availability for body protein deposition in pigs. Anim. Feed. Sci. Technol. 2004;113:141–156. doi: 10.1016/j.anifeedsci.2003.07.005. [DOI] [Google Scholar]
- 53.Messad F., Létourneau-Montminy M.P., Charbonneau E., Sauvant D., Guay F. Meta-analysis of the amino acid digestibility of oilseed meal in growing pigs. Animal. 2016;10:1635–1644. doi: 10.1017/S1751731116000732. [DOI] [PubMed] [Google Scholar]
- 54.Toghyani M., Rodgers N., Iji P.A., Swick R.A. Standardized ileal amino acid digestibility of expeller-extracted canola meal subjected to different processing conditions for starter and grower broiler chickens. Poult. Sci. 2015;94:992–1002. doi: 10.3382/ps/pev047. [DOI] [PubMed] [Google Scholar]
- 55.Hossen I., Hua W., Ting L., Mehmood A., Jingyi S., Duoxia X., Yanping C., Hongqing W., Zhipeng G., Kaiqi Z., et al. Phytochemicals and inflammatory bowel disease: A review. Crit. Rev. Food Sci. Nutr. 2020;60:1321–1345. doi: 10.1080/10408398.2019.1570913. [DOI] [PubMed] [Google Scholar]
- 56.Wang L., Hu Q., Li P., Lai C., Li D., Zang J., Ni S. Development and Validation of Equations for Predicting the Metabolizable Energy Value of Double-Low Rapeseed Cake for Growing Pigs. Animals. 2021;11:1168. doi: 10.3390/ani11041168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spiegel C., Bestetti G.E., Rossi G.L., Blum J.W. Normal circulating triiodothyronine concentrations are maintained despite severe hypothyroidism in growing pigs fed rapeseed presscake meal. J. Nutr. 1993;123:1554–1561. doi: 10.1093/jn/123.9.1554. [DOI] [PubMed] [Google Scholar]
- 58.Ebert E.C. The thyroid and the gut. J. Clin. Gastroenterol. 2010;44:402–406. doi: 10.1097/MCG.0b013e3181d6bc3e. [DOI] [PubMed] [Google Scholar]
- 59.Daher R., Yazbeck T., Jaoude J.B., Abboud B. Consequences of dysthyroidism on the digestive tract and viscera. World J. Gastroenterol. 2009;15:2834–2838. doi: 10.3748/wjg.15.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rezvani M., Kluth H., Bulang M., Rodehutscord M. Variation in amino acid digestibility of rapeseed meal studied in caecectomised laying hens and relationship with chemical constituents. Br. Poult. Sci. 2012;53:665–674. doi: 10.1080/00071668.2012.729130. [DOI] [PubMed] [Google Scholar]
- 61.Seneviratne R.W., Young M.G., Beltranena E., Goonewardene L.A., Newkirk R.W., Zijlstra R.T. The nutritional value of expeller-pressed canola meal for grower-finisher pigs. J. Anim. Sci. 2010;88:2073–2083. doi: 10.2527/jas.2009-2437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.