Abstract
Japanese encephalitis virus (JEV) is the leading cause of human encephalitis in Asia. JEV is a vector-borne disease, mainly transmitted by Culex mosquitoes, with Ardeidae birds as maintenance hosts and pigs as amplifying hosts. Other vertebrate animal hosts have been suggested to play a role in the epidemiology of JEV. This scoping review followed PRISMA guidelines to identify species in which evidence of naturally occurring JEV infection was detected in vertebrates other than ardeid birds, pigs and people. Following systematic searches, 4372 records were screened, and data were extracted from 62 eligible studies. Direct evidence (virus, viral antigen or viral RNA) of JEV infection was identified in a variety of mammals and birds (not always identified to the species level), including bats, passerine birds (family Turdidae), livestock (cattle [Bos taurus] and a goat [Capra hircus]), carnivores (two meerkats [Suricata suricatta]), and one horse (Equus caballus). Bat families included Pteropodidae, Vespertilionidae, Rhinolophidae, Miniopteridae, Hipposideridae. Indirect evidence (antibodies) was identified in several mammalian and avian orders, as well as reported in two reptile species. However, a major limitation of the evidence of JEV infection identified in this review was diagnostic test accuracy, particularly for serological testing. Studies generally did not report diagnostic sensitivity or specificity which is critical given the potential for cross-reactivity in orthoflavivirus detection. We hypothesise that bats and passerine birds could play an underappreciated role in JEV epidemiology; however, development of diagnostic tests to differentiate JEV from other orthoflaviviruses will be essential for effective surveillance in these, as well as the companion and livestock species that could be used to evaluate JEV control measures in currently endemic regions.
Author summary
Japanese encephalitis virus (JEV) is the leading cause of encephalitis in Asia and the Western Pacific, with estimated 100,000 cases in people (mainly children) annually. Up to one third of cases result in death, and of those who survive, half can suffer ongoing neurological problems. Japanese encephalitis virus circulation is complex. It is transmitted via mosquitoes between hosts. Although well-known key hosts include pigs and water birds, our review showed that there are others that should be considered in surveillance programs such as bats and perching birds. We also found that detection is challenging because diagnostic tests cross-react with other viruses. Both improved understanding of hosts and better diagnostic tests are needed to develop surveillance systems to reduce JEV incidence in people.
1. Introduction
Japanese encephalitis (JE) is caused by the zoonotic, mosquito-borne Japanese encephalitis virus (JEV), a single stranded RNA virus of the genus Orthoflavivirus [1–3]. Japanese encephalitis was first described in Japan in 1924 and is now endemic in >20 countries in South-East Asia and the Western Pacific region [4]. Although JE incidence is uncertain due to limited surveillance in many countries in which JEV circulates, JEV is considered to be the leading cause of viral encephalitis in Asia, with an estimated 67,900–100,000 JE cases annually, of which 75% occur in children <14 years old [4,5]. Manifestations of JEV infection vary from asymptomatic (approximately 98% of infections) or nonspecific febrile illness to severe meningoencephalitis, acute flaccid paralysis, and death [1]. Among those with reported symptomatic infection, JE is estimated to have a case fatality rate of 20–30%, with up to 50% of survivors reporting long term neurological sequelae including ‘locked-in syndrome’ in which a JE survivor is cognitively aware but paralysed [6,7].
Japanese encephalitis virus is transmitted primarily by mosquitoes of genus Culex [8] and waterbirds of the family Ardeidae are the major maintenance hosts in endemic transmission cycles [9]. In pigs, JEV infection also causes a viraemia sufficient to infect mosquitoes for ongoing transmission. Pigs are considered amplifying hosts and major contributors to the local transmission risk of JEV to people in endemic regions with high densities of domestic pigs [10,11]. Infection in pigs can be asymptomatic or manifest as reproductive failure characterised by abortions, stillbirths, and mummified foetuses [10,11]; for example, stillbirths and abortions affected up to 50% of unvaccinated pregnant sows during a JEV outbreak in Japan [12]. People are dead-end hosts because their associated viremia is not sufficient for transmission to mosquitoes [9].
Japanese encephalitis virus and other mosquito-borne orthoflaviviruses, such as Zika virus, dengue haemorrhagic fever virus, and St Louis (SLEV), West Nile (WNV) and Murray Valley encephalitis viruses, are ongoing global health threats to human and animal health due to their mutability and propensity for emergence and re-emergence [12]. There is growing concern about the possibility of emergence of JEV globally [13–15]. The mechanisms of emergence are not well understood. Climate change and associated changes in temperature and rainfall, and increasingly frequent extreme weather events are all likely to influence the abundance and distribution of mosquito vectors [16,17]. Changes in the distribution of JEV circulation can also be influenced by factors such as bird migration, movement of infected domesticated hosts, and land use changes [18]. For example, an increase in urban pig farming has resulted in increased JEV circulation in urban and peri-urban settings [19], and expansion of rice farming has resulted in more standing water, thus increasing vector presence [20].
Emergence and re-emergence of JEV might also be influenced by the availability of alternative vectors and hosts. For example, whilst Culex tritaeniorhynchus is the predominant vector mosquito across Southeast Asia, other Culex species are regionally predominant or suggested as secondary vectors, including Culex vishnui, Culex annulirostris, Culex palpalis, Culex quinquefasciatus and Culex sitiens, and JEV has also been isolated from other mosquito genera such as Aedes, Anopheles, and Mansonia [21]. In the recent geographic expansion of JEV across eastern and southern Australia, where Cx. tritaeniorhynchus has not been detected (unlike in northern Australia [22]), the major JEV vector was considered to be Culex annulirostris [23].
The occurrence of JE in people in regions without high densities of ardeid birds or pigs also suggests the presence of alternative maintenance and amplifying hosts (competent hosts) [4,24,25]. For example, epidemiological investigations in India indicate an important role of domestic chickens [19]. The role of domestic poultry (orders Galliformes and Anseriformes) as amplifying hosts has been suggested elsewhere too but is poorly understood due to varying levels of reported seroprevalence and uncertainty about levels of viraemia [26]. Although most studies of JEV epidemiology focus on competent hosts that contribute to virus transmission [27], there could also be an epidemiologically important role of non-competent hosts, i.e. dead-end hosts that become infected but develop insufficient viraemia for ongoing transmission. These species might contribute to a dilution effect by reducing viral circulation among hosts within maintenance communities and their mosquito vectors, as well as have a role in JEV surveillance. Examples include domestic animals such as dogs, horses and cattle, as well as wild bird or mammal species [28–30].
The objective of the current study was to systematically review and collate evidence of naturally occurring JEV infection in vertebrate species other than humans, ardeid birds and pigs. Whilst some alternative hosts are well-documented, others might be less well-documented and their role in the epidemiology of JEV worth further consideration and investigation. Therefore, the aim of this review was to inform surveillance as well as further research to understand the potential contribution of alternative host species in JEV epidemiology and JE mitigation strategies.
2. Materials and methods
2.1. Study overview
A PRISMA-guided scoping review [31] was conducted to identify evidence of naturally occurring JEV infection in vertebrate animals other than humans, ardeid birds (herons, egrets and bitterns) and pigs, either by direct detection (virus, viral antigen, or viral RNA) suggesting current infection, or indirect detection (antibody) indicating previous exposure.
2.2. Search strategy
Search terms included: “Japanese encephalitis virus” OR JEV OR JE, AND detection OR detected (details in Table A in S1 Text). Searches for eligible records were conducted in April 2023 and January 2024 in Web of Science, Scopus, and ProQuest Central databases. The first 100 search results from Google Scholar were also screened with a date range of 1935–1980 to identify records published without inclusion in the searched databases. To supplement the database search strategy, records were also identified by snowballing, starting with the bibliographies of two recent reviews on the pathogenesis of JEV infection [32,33].
2.3. Eligibility criteria
Records were eligible if they were peer-reviewed and described primary research in which evidence of naturally occurring JEV infection was found in any vertebrate animal other than humans, ardeid birds or pigs using a laboratory test. Animals could be sampled for any reason, including population-level surveillance and outbreak investigation, or diagnosis of disease in individual animals. Investigations of vaccinated animals (for example, horses) were excluded unless studies were investigating natural infection. Laboratory tests included methods to detect virus, viral antigen or viral RNA, or JEV antibody. All observational study designs were eligible, including case reports, but experimental studies in which animals were inoculated with JEV were excluded because routes and doses might not reflect natural infection, or the species might not be involved in JEV epidemiology. Records published in English, in any year, and from any location, were eligible.
2.4. Screening and data charting
Following removal of duplicates, two reviewers (ZL and VB) screened records for eligibility based on title and abstract (Level 1) then full manuscript text (Level 2).
Following screening, data were extracted from eligible records (Level 3) including: month and year of sampling, country and region of study, species investigated, number of animals tested (including number positive), the animals’ circumstances (owned, captive, farmed, wild, and whether sampling had been targeted to identify infection in animals with clinical signs of disease), and the method of detection of JEV infection. Data relating to pigs and ardeid birds were also charted if they had been included in eligible studies to provide the full range and context of the animals investigated in the studies.
2.5. Analysis
Data were summarised narratively, and descriptive statistical analyses were conducted in R [34] with packages plyr [32], ggplot2 [33], meta [35], and metafor [36]. Forest plots were used to visualise point estimates of JEV prevalence (direct detection) and JEV seroprevalence (antibody detection) with 95% confidence intervals (Clopper-Pearson method) in animals grouped by the lowest taxonomic rank that was feasible given the aggregation of species reported in the studies. Estimates were not combined across studies due to the methodological heterogeneity of studies (for example, different sampling strategies, and different methods used for detection of infection). A probability density plot was used to visualise detection method usage throughout the period during which studies were conducted.
3. Results
3.1. Search outcomes
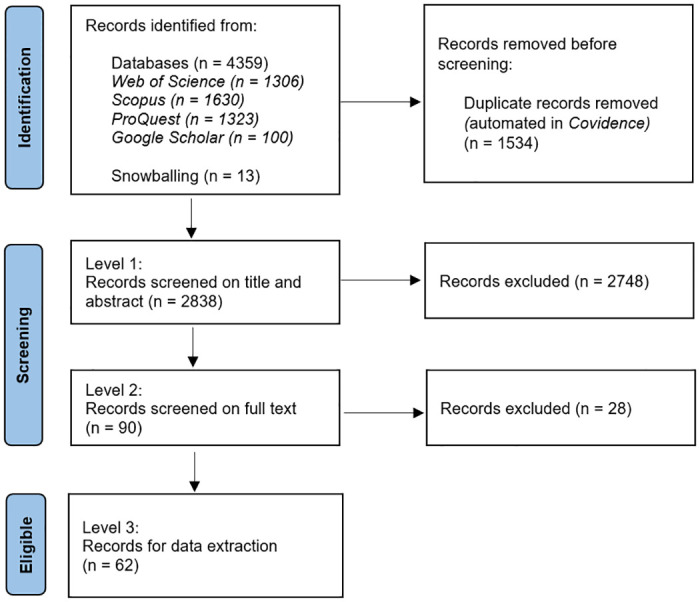
The search strategy identified 4,359 records (Fig 1). Following removal of 1,534 duplicates, 2,748 and 28 records were removed during Level 1 and 2 screening, respectively. Most excluded records were not relevant to the review (did not investigate evidence for naturally occurring JEV infection in vertebrate animals other than people, ardeid birds and pigs). From the records identified by snowballing (n = 13), six were excluded at Level 1, and seven were eligible for data extraction. A total of 62 records were eligible for data extraction (extracted data are available in S1 Table).
Fig 1. PRISMA diagram of records at each level in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs.
3.2. Study distribution
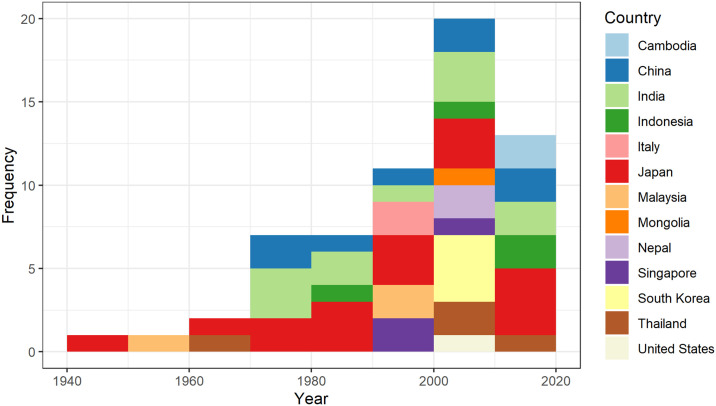
Studies were published from 1947 [37] to 2023 [38] and were mainly conducted in Asia (n = 55; Fig A in S1 Text), with the greatest number in Japan (n = 17), followed by India (n = 11), and China (n = 8). Three studies were conducted in locations outside the current known distribution of JEV circulation: North America (n = 1; United States [39]) and Europe (n = 2; Italy [40,41]). Data were collected over periods of 1–6 years from 1946–2020 inclusive; the distribution of the earliest year of data collection in each study (for studies in which this was recorded [n = 57]) or the date of publication by country is shown in Fig 2. Naturally occurring JEV infection in vertebrate animals other than humans, ardeid birds and pigs was first investigated in Japan in the 1940s, followed by Malaysia in the 1950s. Since then, the number of studies increased each decade, peaking in 2000–2010, then declining in 2011–2020.
Fig 2. Histogram of the starting year of data collection (or date of publication for 5 studies) by country in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs.
3.3. Laboratory tests
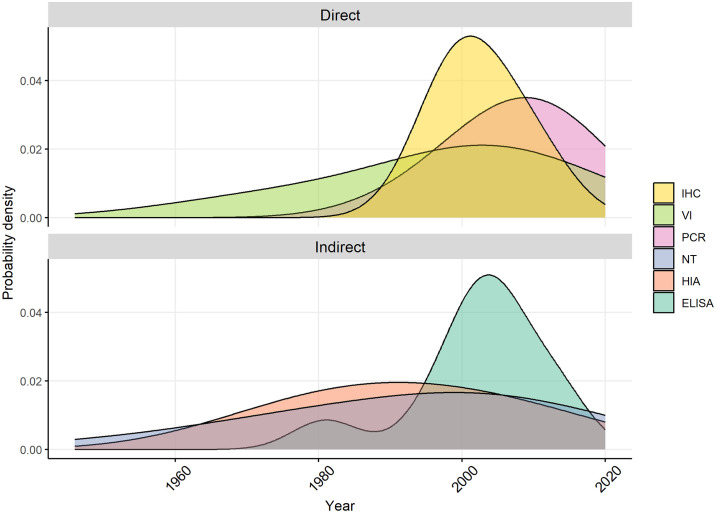
Tests for direct and indirect detection of JEV infection were conducted in 29% (n = 18) and 85% of studies (n = 51), respectively. The methods used varied according to the years in which the study was conducted (Fig 3).
Fig 3. Density plots of methods used in studies identified in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs.
IHC = immunohistochemistry, VI = virus isolation, PCR = polymerase chain reaction, NT = neutralisation test, HIA = haemagglutinin inhibition test, ELISA = enzyme-linked immunosorbent assay.
Direct methods of detection of JEV infection included virus isolation which was conducted in five studies (using inoculation of live mice or Vero, MK2 and Ae. albopictus C6/36 cell cultures) with subsequent phylogenetic analyses or transmission confirmation studies [42–45]. Immunohistochemistry (IHC) was conducted in three studies to detect the presence of JEV antigen in tissues from animals that had exhibited neurological signs [42,43,46]. Initial detection by IHC was followed by visualisation with electron microscopy in two deceased meerkats (Suricata suricatta) in Thailand [46]. Immunohistochemistry was also used to detect virus in archived samples from passerine birds from Tuscany, Italy, following its detection in mosquitoes in the same region in 1997–2000 [40,41]. Reverse transcriptase-PCR (RT-PCR) tests were conducted from the 1990s onwards in 17 studies (Table B in S1 Text). Reported primers targeted the non-structural (NS) protein1, NS3, NS5 and E (envelope) gene regions of JEV.
Indirect tests included haemagglutination-inhibition assays (HIA; n = 26 studies), virus neutralisation tests (NT; n = 38 studies), enzyme-linked immunosorbent assays (ELISA; n = 10 studies), immunofluorescence assay (IF, n = 1), and immunochemical staining (n = 1). Studies in which HIA and NT tests were conducted used a range of JEV strains including, most commonly, the Nakayama strain (n = 15), followed by P20778 (n = 5) and JaGAr01 (n = 3). ELISAs were designed to detect antibody rather than virus and used envelope protein antigen (when reported) from a range of JEV strains (including Beijing 01 and SA14) as well as vaccine strains.
The diagnostic performance of tests or test strategies was reported in only two studies. In a study from Japan, authors reported the diagnostic sensitivity and specificity of an ELISA as 82% and 96%, respectively, when compared to a virus neutralisation test as a gold standard [47]. In another study from Japan, a test strategy (diagnostic algorithm using ELISA, IF, and NT) was used in which tests were conducted sequentially and dependent on the previous test result in an algorithm, and authors reported 100% accuracy in differentiating detection of WNV exposure from JEV exposure [48]. Throughout most of the studies, however, authors recognised the limitations of potentially poor test performance due to cross-reactivity with other flaviviruses, especially when using antibody tests. Serial testing was often conducted to increase specificity (for example, authors sometimes stated that more specific NT tests were used to confirm HIA positive samples [NT was use in series in 62% of studies using HIA]). Due to the overall lack of reported diagnostic sensitivity and specificity in studies in this review, we make no assumptions about test performance and report apparent prevalence (direct testing) and apparent seroprevalence (indirect testing).
3.4. Evidence of JEV infection
Reported direct and indirect evidence is described below according to the finest-resolved animal taxa available in the studies (Figs 4–9 and Figs B-C in S1 Text), and is shown by country in the Supplementary Material (Figs D-G in S1 Text).
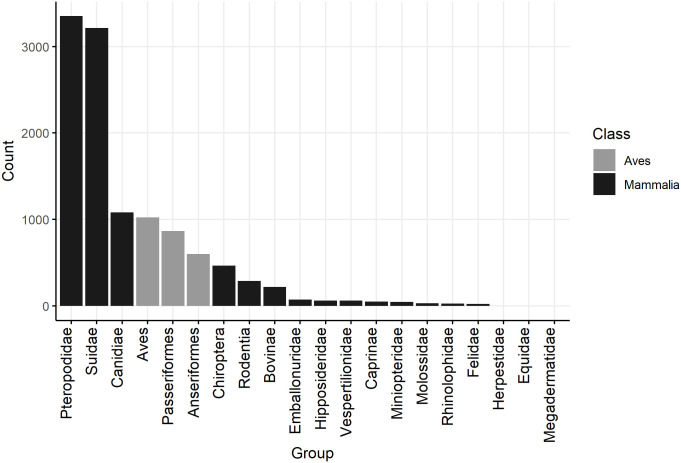
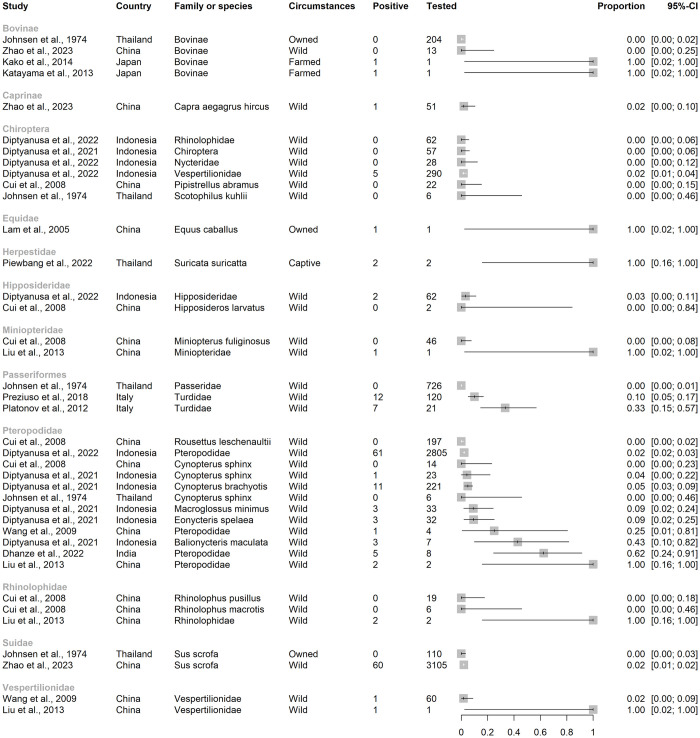
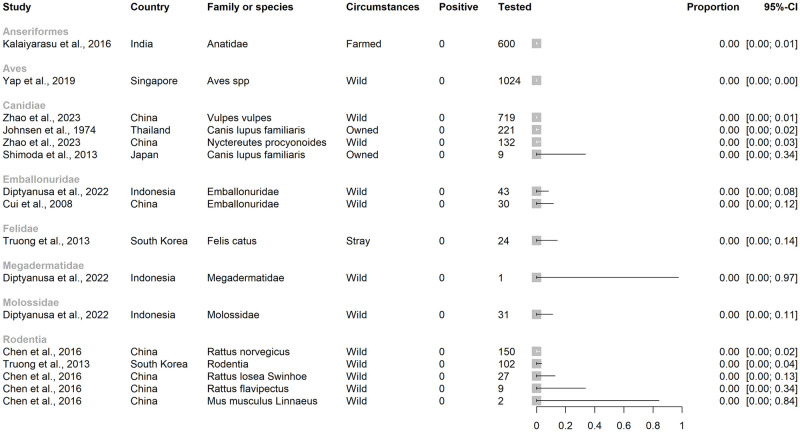
Fig 4. Barplots of the number of animals tested for direct evidence of naturally occurring JEV infection, to lowest taxonomic rank given the aggregation of species reported in studies of vertebrate animals other than humans, ardeid birds and pigs.
Suidae are included from studies in which vertebrate animals other than humans, ardeid birds or pigs were also tested.
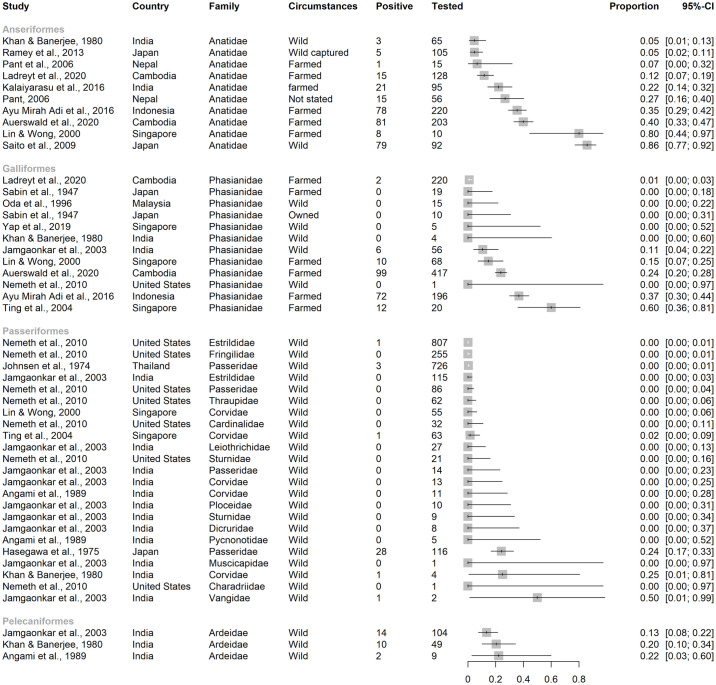
Fig 9. Reported seroprevalence of JEV antibodies of Anseriformes, Galliformes, Passeriformes, and Pelecaniformes in a scoping review of direct and indirect evidence of Japanese encephalitis virus infection in vertebrate animals other than humans, pigs and ardeid birds.
Ardeidae (Pelecaniformes) are included from studies in which vertebrate animals other than ardeids, pigs and humans were also tested. Horizontal lines = 95% confidence intervals.
3.4.1. Direct evidence of JEV
In the studies identified, 9004 mammalian (including 3215 Suidae) and 2491 avian (no Ardeidae specified) individual animals were tested for direct evidence of JEV infection (virus, viral antigen or viral RNA). Reptiles, fish and amphibia were not tested.
The largest family of mammals tested was Pteropodidae (pteropodid bats, also known as Megachiroptera or megabats, fruit bats, old world bats, and flying foxes; n = 3346; Figs 4 and 5) and direct evidence of JEV infection was detected in pteropodid bats in Indonesia and India but not in Thailand or China. In a survey from 11 provinces in Indonesia, JEV viral RNA was detected by RT-PCR targeting the NS3 gene in blood samples from 61 of 2805 live, wild-caught pteropodid bats (2.3%) including the genera Cynopterus, Dobsonia, Eonycteris, Macroglossus, Pteropus, Rousettus, and Thoopterus [49]. Viral RNA was also detected in pteropodid bats in an earlier study in Indonesia, also by RT-PCR targeting the NS3 gene (up to 43% of Balionycterus maculata; other species were up to 9% prevalence) [50]. The first detection of JEV infection in bats in India was in 2020, associated with a mortality event in which 52 pteropodid bats were found dead in Gorakhpur district, Uttar Pradesh, a province which has a high incidence of JE in people and JEV circulating in domestic pigs [51–53]. Brain tissue samples from 8 bats were tested by conventional RT-PCR and TaqMan real-time RT-PCR using primers targeting the JEV envelope and NS1 genes. Five bats were positive by TaqMan real-time RT-PCR (62%, with 8–18 copies/reaction). Other bat families in which evidence of JEV infection was detected included Vespertilionidae, Rhinolophidae, Miniopteridae, and Hipposideridae in Indonesia and China [49,54]. Complete genome sequencing was conducted on eight different isolates from bats (Rhinolophidae, Miniopteridae, and Vespertilionidae, as well as Pteropodidae) which were sampled in China in Yunnan, Hainan, Guangdong, and Hunan provinces from 1986–2007 [54,55]. The sequence data were consistent with JEV Genotype III, showed 99.4–99.9% genetic homogeneity in the full-length nucleotide sequences, and were phylogenetically clustered into the same subgroup despite wide spatial and temporal sampling. JEV was not detected in bat families Emballonuridae (Indonesia and China), Megadermatidae (Indonesia), and Molossidae (Indonesia; Fig 6) [49,56]. In all the studies of bats identified above except Dhanze et al. (2022) in India [53], sampled bats had appeared healthy.
Fig 5. Studies by family or subfamily with reported direct evidence of naturally occurring Japanese encephalitis virus infection in studies of vertebrate animals other than humans, ardeid birds and pigs.
Suidae are included from studies in which vertebrate animals other than solely humans, ardeid birds and pigs were also tested. Horizontal lines = 95% confidence intervals.
Fig 6. Studies by order or family in which evidence of JEV infection was not detected in a scoping review of Japanese encephalitis virus infection in vertebrate animals other than humans, pigs and ardeid birds.
Horizontal lines = 95% confidence intervals.
Japanese encephalitis virus was also detected in other mammals including farmed Caprinae and Suidae in China (each with prevalence 2% in a survey which also included fox [Vulpes vulpes], racoon dog [Nyctereutes procyonoides] and yak [Bos grunniens] in which no direct evidence of JEV infection was detected [38]), a single Thoroughbred racehorse in Hong Kong (Equus caballus [42]), cattle in Japan [43,45], and two pet meerkats in Thailand (S. suricatta [46]). The racehorse had shown signs of severe neurological disease and viral RNA consistent with both Genotype I and II JEV strains was detected in spinal cord tissue by RT-PCR targeting the NS5 and E genes. Along with a rising JEV antibody titre, it was considered that the clinical signs had been consistent with JEV infection. The cattle in Japan were two Japanese black cattle (B. taurus; 141-day-old calf in Miyazaki Prefecture in 2009 and a 114-month-old beef cow in Aichi Prefecture in 2010) that had both shown neurological signs; sequenced cerebral virus isolates were consistent with JEV Group 1. The meerkats had died after developing signs of neurological disease. Brain samples were positive for JEV by immunohistochemistry and yielded JEV partial sequences that aligned with JEV strain Beijing-015, and lung samples from one meerkat also had inclusion bodies consistent with JEV infection.
Lastly, JEV was detected in samples from birds of the order Passeriformes and family Turdidae (thrushes) that were either found dead in mortality events in Tuscany, Italy 1997–2000, or were healthy birds hunted during the same period [40,41]. In birds found dead and which were positive for JEV by IHC, JEV sequences were detected that were consistent with genotype III (RT-PCR targeting NS5 and E genes) [41]. JEV was detected in bone marrow by IHC in 10% of healthy birds (none found in kidney, spleen, liver, lung, brain, intestine), and of these, four yielded JEV sequences also most consistent with genotype III (RT-PCR targeting NS5 and E genes) [41].
3.4.2. Indirect evidence of JEV
In the studies identified, 33,505 mammalian, 8738 avian, and 158 reptilian individuals were tested for indirect evidence (antibodies) of JEV infection (Fig 7). Fish and amphibia were not tested.
Fig 7. Barplots of the number of mammalian and avian individuals tested for JEV antibody in studies identified in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs.
Suidae are ardeid birds (in Pelecaniformes) are included from studies in which vertebrate animals other than solely humans, ardeid birds and pigs were also tested.
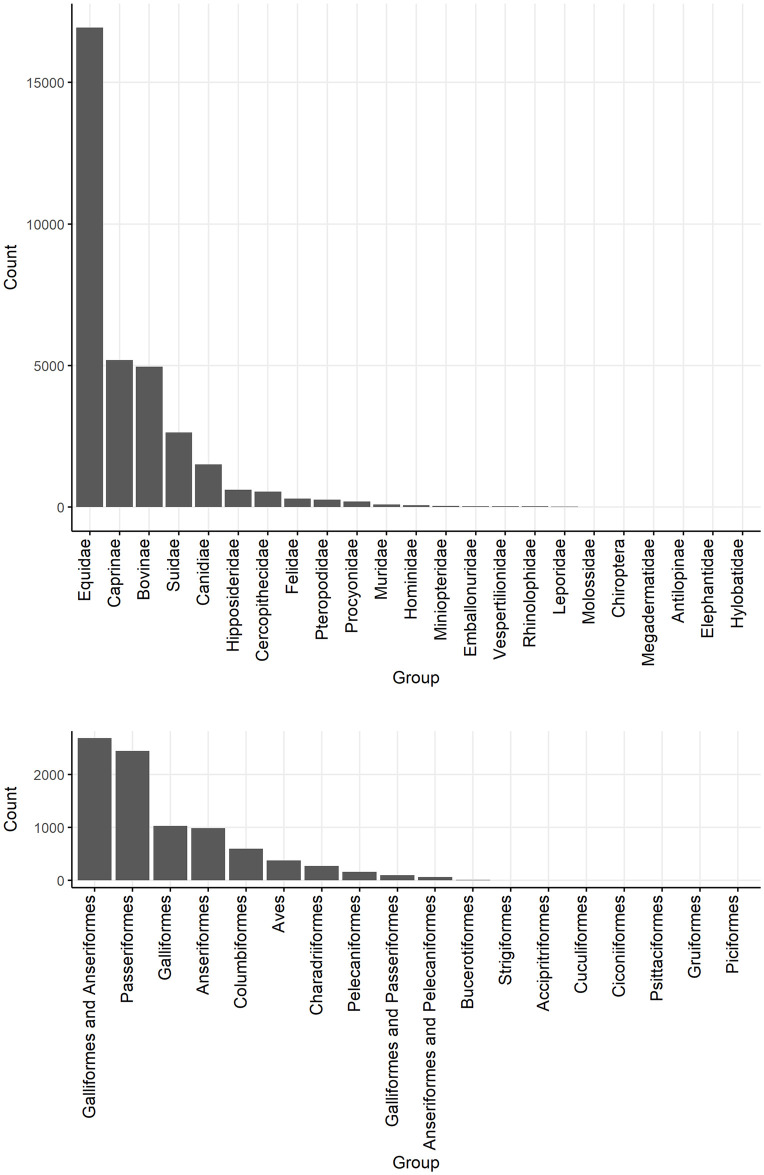
3.4.1.1 Mammalia. Of the mammalian individuals tested (Fig 7), 16,931 were horses (E. caballus), 5197 were Caprinae (2819 sheep [Ovis aries], 1 mouflon [O. gmelina], 2377 goats [Capra aegagrus hircus]), 2996 were Bovinae (at least 703 buffalo [Bubalis bubalis], and other cattle [B. taurus], or unspecified), 2633 were Suidae (mainly Sus scrofa, as well as 13 bearded pigs [S. barbatus], and four babirusa [Babyrousa quadricornua]), and 1516 were Canidae (>100 racoon dogs [N. procyonoides], one African wild dog [Lycaon pictus], 1496 domestic dogs [Canis lupus familiaris]). Fewer than 1000 individuals were tested in each of the other families, although together, 1021 bats (order Chiroptera) were tested from the following families: Emballonuridae, Hipposideridae, Megadermatidae, Miniopteridae, Molossidae, Pteropodidae, Rhinolophidae, and Vespertilionidae.
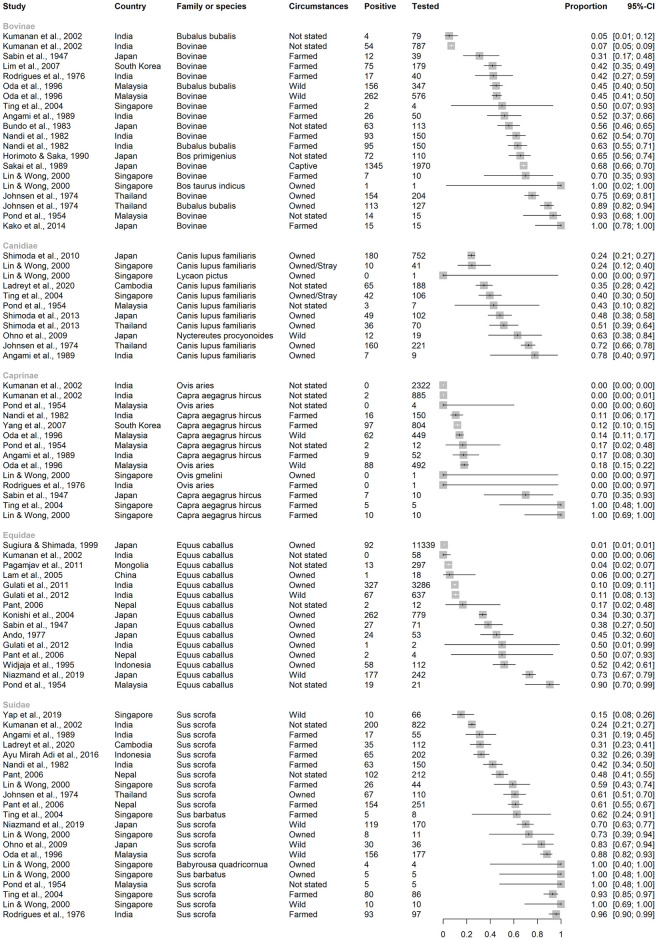
Seroprevalence point estimates in studies ranged from 0–100% (inclusive) in subfamilies Bovinae and Caprinae and family Suidae, 0–90% in Equidae, and 0–78% in Canidae, (Fig 8).
Fig 8. Reported seroprevalence of JEV antibodies in Bovinae, Canidae, Caprinae, Equidae, and Suidae in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs.
Suidae are included only from studies in which vertebrate animals other than humans, ardeid birds or pigs were also tested. Horizontal lines = 95% confidence intervals.
Seroprevalence point estimates in studies ranged less widely in other groups of mammals (Fig B in S1 Text). In studies of bats, seroprevalence ranged up to 10% in Emballonuridae, 15% in Hipposideridae (Hipoposideros bicolor), 11% in Miniopteridae (Miniopterus fuliginosus), and 18% in Pteropodidae (Rousettus leschenaultia) [56,57]. Antibodies to JEV were not detected in studies of bat families Hylobatidae, Megadermatidae, Molossidae, Rhinolophidae, and Vespertilionidae [56–59]. In studies of primates, seroprevalence ranged up to 30% in Cercopithecidae (Japanese macaque, Macaca fuscata), and 50% in Hominidae (Bornean orangutan, Pongo pygmaeus) [58,60]. In Felidae, Leporidae, Procyonidae, and Rodentia, studies detected seroprevalence up to 1%, 33%, 53% and 52%, respectively [37,47,61,62]. In Singapore, antibodies to JEV were not detected in a Springbok (Antidorcas marsupialis; family Antilopinae), but archived serum from an Elephas maximus (family Elephantidae) in Singapore was positive by HIA [58].
3.4.1.2 Aves. Birds were often grouped in studies so that separation of avian families by species was not possible (Fig 7). Over 1000 individuals were tested in orders Galliformes (land fowl), Passeriformes (perching birds, including songbirds), and Anseriformes (waterfowl). At least 150 ardeid birds were tested for JEV antibody alongside other species in eligible records (Pelecaniformes, including little egret [Egretta garzetta] and cattle egret [Bubulcus ibis]).
The widest range of JEV seroprevalence was in studies of Anseriformes (0–86%), followed by Galliformes (0–60%), then Passeriformes (0–50%) (Fig 9). Of the Passeriformes, families in which JEV antibodies were detected were Estrilididae in Hawai’i, USA (<0.01% [63]), Passeridae in Thailand and Japan (<0.01% and 24%, respectively [59,64]), Corvidae in Singapore and India (0.02% and 25%, respectively [65,66]), and Vangidae in India [67]. Seroprevalence in Pelecaniformes ranged from 13–22% in Ardeidae in India [66,67]. In the study in Hawai’i, which is outside the known geographic distribution of JEV circulation, neutralising antibody consistent with JEV seropositivity was also detected in two Columbiformes (pigeons and doves; from a total of 1835 Passeriformes and Columbiformes).
Low seroprevalence was detected in Charadriiformes (shorebirds; up to 8–10% in families Chionidae, Charadriidae, and Scolopacidae in India and Singapore [66,68]), and Columbiformes in Hawaii, USA and India (up to 12% [39,69]) (Fig C in S1 Text). Antibody to JEV was not detected in Accipitriformes, Bucerotiformes, Ciconiiformes, Cuculiformes, Gruiformes, Piciformes, Psittaciformes, and Strigiformes in India [67].
3.4.1.3 Reptilia. Eighty-two Squamata (snakes) were tested for JEV antibody. Antibodies were not detected in two reticulated pythons (Malayopython reticulatus) in Singapore [58]. In Kwantung Province, China, antibodies were detected in 68 and 63 Indian cobras (Naja naja, N = 80; seroprevalence up to 85%) by HIA and NT, respectively (80% agreement, Cohen’s kappa 0.35 [“fair” agreement]), with seven cobras negative by both tests [70]. The cobras were adult females, obtained monthly in batches of 5–10 from a dealer in Hong Kong from October 1971 to September 1972. All were sourced within the previous 2 days from unknown locations in Kwantung Province. Seroprevalence and mean HIA and NT titres in cobras collected in the spring and summer months were significantly greater than those collected in the autumn and winter months.
Seventy-six Testudines (turtles) were tested for JEV antibody. Antibodies were not detected in one red-eared terrapin (Trachemys scripta elegans) in Singapore [58]. Seventy-five wild-caught soft-shelled turtles (Trionyx sinensis) from Wuchow, Kwangsi Province, China were tested for JEV antibody and 45 and 58 were positive by HIA and NT tests, respectively [71] (58% agreement, Cohen’s kappa 0.07 [“none to slight” agreement]). Unlike the cobras from the same region, seroprevalence and titres were similar between seasons.
4. Discussion
In this scoping review of naturally occurring JEV in vertebrates other than humans, ardeid birds and pigs, direct evidence of JEV infection through detection of virus, viral antigen or viral RNA, was found in Chiroptera (Pteropodidae, Vespertilionidae, Rhinolophidae, Miniopteridae, Hipposideridae), Passeriformes (thrushes; family Turdidae), Bovidae (cattle [B. taurus] and a goat [C. hircus]), one horse (E. caballus), and two meerkats (S. suricatta). Indirect evidence of JEV infection (antibodies) was reported for several mammalian and avian orders with high apparent seroprevalence in some studies, as well as two species of reptiles (Indian cobra [N. naja] and soft-shelled turtles [T. sinensis]).
A major limitation of the evidence of naturally occurring JEV infection identified in this review was lack of information on diagnostic test accuracy, especially for detection of JEV-specific antibodies because tests can cross-react with similar orthoflaviviruses [12,72,73]. Such viruses include those that already occur in recognised geographic regions of JEV circulation including West Nile virus (for example, in India and Australia [Kunjin virus]) and Murray Valley encephalitis virus (Australia), as well as potential emergent and re-emergent viruses including Zika virus and yellow fever virus [74]. The diagnostic sensitivity and specificity of JEV tests are therefore region specific and often unknown [74]. Examples of tests with known sensitivity and specificity include those used for JEV sero-surveillance in pigs in Uttar Pradesh, India (the diagnostic sensitivity and specificity of an IgG ELISA test is 91% and 97%, respectively, whilst the diagnostic sensitivity and specificity of an IgM ELISA is 95.34% and 98.6%, respectively [75,76]). Due to usually unknown diagnostic test characteristics, as well as additional sources of heterogeneity associated with study design (for example, sampling strategies) and JEV epidemiology (for example, seasonality), study estimates were not combined. Therefore, we focus on the broad patterns of the review findings, identifying plausible alternative hosts and surveillance opportunities, rather than focusing on species’ point estimates of prevalence or seroprevalence.
Bats are known reservoirs of many viruses—for example, Hendra virus and lyssavirus—but their role in the epidemiology of orthoflaviruses, including JEV, is unknown despite research interest [77,78]. This interest was reflected in the current review in which bats comprised >40% of all individuals sampled for direct evidence of JEV infection over an extensive spatiotemporal window (China, India, Indonesia, and Thailand from 1974 to 2022) [49,50,53–56,59]. Whilst ecological studies have not found evidence that bats are involved in the epidemiology of JEV, experimental investigation indicates that bats can be competent JEV hosts [79,80]. In the context of arboviruses, competent hosts are species in which viraemia develops to a sufficiently high titre for ongoing transmission to other individuals via the vector [81,82]. Well-known competent hosts for JEV include ardeid birds and pigs, in which a short viraemia (duration 3–5 days) starting 1–5 days post-challenge has been shown in experimental studies [10,83–85]. In an experimental study based in Australia, seroprevalence in ten flying foxes (P. alecto) was 60% following exposure to JEV-infected mosquitoes (Cx. annulirostris), and although viraemia was not detected in these flying foxes, ongoing JEV transmission was demonstrated to JEV-naïve mosquitoes that fed on two of the flying foxes [79]. Given this and the direct evidence of JEV infection in bats identified in the current review, it is plausible that JEV circulation could circulate in bat populations with occasional spillover to other species. Viruses that are maintained in bat populations at low titres and prevalence might have increased replication and shedding during periods of allostatic overload on the host (cumulative and increased energy needs with external stressors such as habitat degradation and fragmentation, as well as climate anomalies), as recently described for Hendra virus [86]. If JEV infection in bat populations follows a similar mechanism, spillover of JEV from bat populations to local ardeid birds—and potentially other populations such as people, pigs, other domestic or wildlife species—during periods of allostatic overload could occur [87]. Therefore, whilst not an identified driver of detected JEV outbreaks, the role of bats in the circulation of JEV in different ecological scenarios should be considered.
Another mechanism by which bats might be involved in JEV persistence or apparent re-emergence is overwintering of the virus in regions in which vector mosquitoes are seasonally inactive. To conserve energy, bats within the families Vespertilionidae and Rhinolophidae exhibit daily torpor and seasonal hibernation during cold nights and winter months, coinciding with decreased vector activity [77]. Altered immune capacity during these periods is thought to allow viruses to remain blood-borne and infectious, so that re-infection of vectors can occur post-torpor or hibernation [88]. In an experimental infection of Vespertilionidae (big brown bats [Eptesicus fuscus] and little brown bats [Myotis lucifigus]) with JEV, individuals maintained viraemia for 95–108 days after being subjected to temperatures mimicking hibernation (8–24°C) [89]. Overwintering of JEV in birds or pigs is not considered possible because their viraemia is short [90]. Prolonged viraemia could also occur in reptiles; in an experimental study in Japan, JEV viraemia was detected in lizards for several weeks following infection via Culex mosquitoes [91]. The apparent prevalence of antibodies suggestive of naturally occurring JEV infection in reptiles was also identified in the current review [70,71]. Although this might have been cross-reactivity with another orthoflavivirus, in a recent review of experimental studies of reptile infections, six studies were identified in which several species of Squamata were infected following JEV inoculation [92]. Therefore, overwintering of JEV in reptiles, as well as bats is another possible feature worth consideration in the epidemiology of JEV.
The potential role of Passeriformes in JEV epidemiology is also of interest because Passeriform birds are known reservoir and amplificatory hosts for other arboviruses, including Sindbis virus, Tahyna orthobunyavirus and Batai orthobunyavirus in Europe and WNV in north America [93,94]. In the current review, direct evidence of JEV infection was found in thrushes (Turdidae) collected from mortality events or hunting in Tuscany, Italy in the late 1990s [40,41]. Seropositivity was reported in other Passeriform families including Scolopacidae, Estrilidae, Corvidae, and Vangidae [39,59,64,65,67]. Serosurveillance of Passeriformes could be valuable, especially as a component of JEV preparedness activities in non-endemic regions. However, accurate diagnostic tests are needed to differentiate orthoflavivirus antibodies in the known geographic range of JEV such as WNV, as well as those in non-endemic regions, such as SLEV in North America, and this has been the subject of recent reviews [95,96]. For example, in a study in Hawai’i in which neutralising antibody consistent with JEV seropositivity was detected in two Columbiformes, previous JEV infection was considered plausible due to the presence of competent hosts, vectors, and the proximity of air travel from Asia. However, cross-reactivity with WNV and SLEV antibodies or another, unknown flavivirus, could not be ruled out. Nevertheless, these findings are consistent with experimental studies in which a variety of avian species from eight taxonomic orders (including Columbiformes and Passeriformes) were inoculated and demonstrated infection with JEV genotypes I and III [97].
In addition, we identified numerous species that although unlikely to play a role as competent hosts, could be used as sentinels for JEV surveillance. In the current review, high seroprevalence (if JEV specific) of companion and livestock animals (cattle, dogs, goats, horses, ducks and land fowl) suggested that they could be useful for surveillance, especially given that these animals are kept in proximity to people. We found no direct evidence of naturally occurring JEV infection in waterfowl or land fowl (Anseriformes and Galliformes), despite epidemiological indication that they could be important in India and experimentally induced viraemia in ducklings [19,98]. Therefore, whilst they are useful in serosurveillance programs, their inclusion in JEV spread modelling as competent hosts is not well supported.
Overall, studies identified in the current review focused on evidence of JEV infection in known and ‘known unknown’ alternative hosts (for example, horses and bats, respectively). However, there were some unexpected findings. In species which are considered non-competent (dead-end) hosts, only horses (rarely) and people (estimated 0-5-1% of those infected) are generally considered to show clinical signs. However, disease attributable to JE was also reported in cattle (known non-competent JEV hosts) and meerkats (order Carnivora, suborder Feliformia) [43,45,46]. Whilst it could be expected that occasional clinical disease could occur in cattle (order Artiodactyla, like pigs), detection of disease in meerkats is unexpected and highlights the importance of comprehensive clinical reasoning in neurological cases.
In considering the limitations of the current review, in addition to the challenges of accurate diagnostic tests and false positive results, the adage that absence of evidence is not evidence of absence is highly applicable [99]. Studies were only included if they were in English and in peer-reviewed publications. Therefore, we would have excluded publications in other languages from JE-affected countries, and findings that are only available in local government reports or media; for example, there is a local government webpage report of a confirmed JE case in an alpaca in South Australia in 2022 [100]. In addition, studies in which animals were tested but findings were negative are as important as positive findings but are less likely to be published at all. Finally, the geographic distribution of JE-cases will affect the research effort. For example, JE has only recently emerged as a significant human and animal health concern in Australia, which has a unique marsupial fauna. Australian marsupials are known reservoirs of arboviruses including potentially flaviviruses, and macropods and possums have been experimentally infected with JEV [101,102]; however, naturally occurring JEV in marsupials has yet to be reported.
5. Conclusion
We conclude that several species other than humans, ardeid birds and pigs may contribute to the epidemiology of JEV. Potential competent hosts included passerine birds and bats and given intensifying anthropogenic ecological stressors and the propensity of orthoflaviruses for emergence, this could be of increasing importance. This review also demonstrates a range of species for serosurveillance. These included known companion and livestock non-competent hosts, as well as species which might be less frequently considered in serosurveillance programs (for example, passerine birds). However, we also demonstrated a critical need for more accurate diagnostic tests to differentiate co-circulating cross-reactive flaviviruses. Nearly all studies highlighted this as a limitation of antibody testing, and this is currently a major limitation not only in diagnosis of JEV infection, but also for JEV serosurveillance.
Supporting information
Table A Search terms in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs.
(DOCX)
(separate Excel file) Bibliography and extracted data of eligible studies in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Fig A Bar plot of the frequency of study location by country, in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Fig B Reported seroprevalence (indirect detection of JEV infection [antibody]) in studies of mammals other than Bovinae, Canidae, Caprinae, Equidae, Suidae, humans and ardeid birds. Horizontal lines = 95% confidence intervals. Fig C Reported seroprevalence (indirect detection of JEV infection [antibody]) in bird orders in which maximum seroprevalence was <10% in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Ardeidae (Pelecaniformes) are included from studies in which vertebrate animals other than pigs and humans were also tested. Horizontal lines = 95% confidence intervals. Fig D Reported prevalence (direct detection of JEV infection [virus, viral antigen, or viral RNA]) stratified by country in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Suidae are included from studies in which vertebrate animals other than ardeid birds and humans were also tested. Horizontal lines = 95% confidence intervals. Fig E Reported seroprevalence (indirect detection of JEV infection [antibody]) of mammals in China, India, Japan, and Thailand, in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Suidae are included from studies in which vertebrate animals other than ardeid birds and humans were also tested. Horizontal lines = 95% confidence intervals. Fig F Reported seroprevalence (indirect detection of JEV infection [antibody]) of mammals in Cambodia, Indonesia, Mongolia, Nepal, Singapore and South Korea in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Suidae are included from studies in which vertebrate animals other than ardeid birds and humans were also tested. Horizontal lines = 95% confidence intervals. Fig G Reported seroprevalence (indirect detection of JEV infection [antibody]) of birds by country in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Suidae are included from studies in which vertebrate animals other than ardeid birds and humans were also tested. Horizontal lines = 95% confidence intervals.
(XLSX)
Acknowledgments
We thank the librarians at the University of Sydney for their assistance with this study. This study was completed in partial fulfilment of the requirements of the Doctor of Veterinary Medicine degree, The University of Sydney (ZAL).
Data Availability
All underlying data are available in the Supplementary Material.
Funding Statement
This study was part funded by the Sydney School of Veterinary Science, The University of Sydney to ZAL. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ghosh D, Basu A. Japanese encephalitis—A pathological and clinical perspective. PLoS Neglected Tropical Diseases. 2009;3(9). doi: 10.1371/journal.pntd.0000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postler TS, Beer M, Blitvich BJ, Bukh J, de Lamballerie X, Drexler JF, et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch Virol. 2023;168(9):224. Epub 20230810. doi: 10.1007/s00705-023-05835-1 . [DOI] [PubMed] [Google Scholar]
- 3.Cardona GA, Carmena D. A review of the global prevalence, molecular epidemiology and economics of cystic echinococcosis in production animals. Veterinary parasitology. 2013;192(1–3):10–32. doi: 10.1016/j.vetpar.2012.09.027 [DOI] [PubMed] [Google Scholar]
- 4.Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bulletin of the World Health Organization. 2011;89(10):766–74. doi: 10.2471/BLT.10.085233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quan TM, Thao TTN, Duy NM, Nhat TM, Clapham H. Estimates of the global burden of Japanese encephalitis and the impact of vaccination from 2000–2015. eLife. 2020;9:e51027. doi: 10.7554/eLife.51027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turtle L, Easton A, Defres S, Ellul M, Bovill B, Hoyle J, et al. ’More than devastating’-patient experiences and neurological sequelae of Japanese encephalitis. Journal of Travel Medicine. 2019;26(7). doi: 10.1093/jtm/taz064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin Z, Wang H, Yang J, Luo H, Li Y, Hadler SC, et al. Japanese encephalitis disease burden and clinical features of Japanese encephalitis in four cities in the People’s Republic of China. American Journal of Tropical Medicine and Hygiene. 2010;83(4):766–73. doi: 10.4269/ajtmh.2010.09-0748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franklinos LHV, Redding DE, Lucas TCDS, Gibb RS, Abubakar IS, Jones KS. Joint spatiotemporal modelling reveals seasonally dynamic patterns of Japanese encephalitis vector abundance across India. Plos Neglected Tropical Diseases. 2022;16(2). doi: 10.1371/journal.pntd.0010218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulvey P, Duong V, Boyer S, Burgess G, Williams DT, Dussart P, et al. The Ecology and Evolution of Japanese Encephalitis Virus. Pathogens. 2021;10(12):1534. doi: 10.3390/pathogens10121534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladreyt H, Durand B, Dussart P, Chevalier V. How central is the domestic pig in the epidemiological cycle of Japanese encephalitis virus? A review of scientific evidence and implications for disease control. Viruses. 2019;11(10):949. doi: 10.3390/v11100949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cappelle J, Duong V, Pring L, Kong L, Yakovleff M, Prasetyo DB, et al. Intensive circulation of Japanese encephalitis virus in peri-urban sentinel pigs near Phnom Penh, Cambodia. PLoS neglected tropical diseases. 2016;10(12):e0005149. doi: 10.1371/journal.pntd.0005149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierson TC, Diamond MS. The continued threat of emerging flaviviruses. Nature Microbiology. 2020;5(6):796–812. doi: 10.1038/s41564-020-0714-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson MF, Brooks C, Kakazu A, Promma P, Sornjai W, Smith DR, et al. Mosquito surveillance on U.S military installations as part of a Japanese encephalitis virus detection program: 2016 to 2021. PLoS neglected tropical diseases. 2023;17(10):e0011422–e. MEDLINE:37856569. doi: 10.1371/journal.pntd.0011422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monath TP. Japanese Encephalitis: Risk of Emergence in the United States and the Resulting Impact. Viruses. 2023;16(1):54. doi: 10.3390/v16010054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tolsá-García MJ, Wehmeyer ML, Lühken R, Roiz D. Worldwide transmission and infection risk of mosquito vectors of West Nile, St. Louis encephalitis, Usutu and Japanese encephalitis viruses: a systematic review. Scientific Reports. 2023;13(1). doi: 10.1038/s41598-022-27236-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartlow AW, Manore C, Xu C, Kaufeld KA, Del Valle S, Ziemann A, et al. Forecasting Zoonotic Infectious Disease Response to Climate Change: Mosquito Vectors and a Changing Environment. Veterinary Sciences. 2019;6(2):40. doi: 10.3390/vetsci6020040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramasamy R, Surendran SN. Global climate change and its potential impact on disease transmission by salinity-tolerant mosquito vectors in coastal zones. Frontiers in physiology. 2012;3:198. doi: 10.3389/fphys.2012.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai T. Factors in the changing epidemiology of Japanese encephalitis and West Nile fever. Factors in the emergence of arbovirus diseases. 1997:179–89. [Google Scholar]
- 19.Walsh MG, Pattanaik A, Vyas N, Saxena D, Webb C, Sawleshwarkar S, et al. High-risk landscapes of Japanese encephalitis virus outbreaks in India converge on wetlands, rain-fed agriculture, wild Ardeidae, and domestic pigs and chickens. International Journal of Epidemiology. 2022. doi: 10.1093/ije/dyac050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raju KHK, Sabesan S, Rajavel AR, Subramanian S, Natarajan R, Thenmozhi V, et al. A Preliminary Study to Forecast Japanese Encephalitis Vector Abundance in Paddy Growing Area, with the Aid of Radar Satellite Images. Vector-Borne and Zoonotic Diseases. 2016;16(2):117–23. doi: 10.1089/vbz.2014.1757 [DOI] [PubMed] [Google Scholar]
- 21.Pearce JC, Learoyd TP, Langendorf BJ, Logan JG. Japanese encephalitis: The vectors, ecology and potential for expansion. Journal of Travel Medicine. 2018;25:S16–S26. doi: 10.1093/jtm/tay009 [DOI] [PubMed] [Google Scholar]
- 22.Lessard BD, Kurucz N, Rodriguez J, Carter J, Hardy CM. Detection of the Japanese encephalitis vector mosquito Culex tritaeniorhynchus in Australia using molecular diagnostics and morphology. Parasites and Vectors. 2021;14(1). doi: 10.1186/s13071-021-04911-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Hurk AF, Skinner E, Ritchie SA, Mackenzie JS. The Emergence of Japanese Encephalitis Virus in Australia in 2022: Existing Knowledge of Mosquito Vectors. Viruses. 2022;14(6):1208. doi: 10.3390/v14061208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim MJ, Loh ZY, Yeo HL, Yenamandra SP, Kong M, Pang HY, et al. Isolation and Genetic Characterization of Japanese Encephalitis Virus Two Decades after Its Elimination in Singapore. Viruses. 2022;14(12). doi: 10.3390/v14122662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lord JS, Gurley ES, Pulliam JRC. Rethinking Japanese Encephalitis Virus Transmission: A Framework for Implicating Host and Vector Species. PLoS Neglected Tropical Diseases. 2015;9(12). doi: 10.1371/journal.pntd.0004074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhattacharya S, Basu P. Japanese Encephalitis Virus (JEV) infection in different vertebrates and its epidemiological significance: a Review. International Journal of Fauna and Biological Studies. 2014;1(6):32–7. [Google Scholar]
- 27.Oliveira ARS, Strathe E, Etcheverry L, Cohnstaedt LW, McVey DS, Piaggio J, et al. Assessment of data on vector and host competence for Japanese encephalitis virus: A systematic review of the literature. Preventive Veterinary Medicine. 2018;154:71–89. doi: 10.1016/j.prevetmed.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 28.Ladreyt H, Auerswald H, Tum S, Ken S, Heng L, In S, et al. Comparison of Japanese encephalitis force of infection in pigs, poultry and dogs in Cambodian villages. Pathogens. 2020;9(9):1–13. doi: 10.3390/pathogens9090719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalil H, Ecke F, Evander M, Magnusson M, Hörnfeldt B. Declining ecosystem health and the dilution effect. Scientific Reports. 2016;6(1):31314. doi: 10.1038/srep31314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konishi E, Kitai Y, Nishimura K, Harada S. Follow-up survey of Japanese encephalitis virus infection in Kumamoto Prefecture, South-West Japan: Status during 2009–2011. Japanese Journal of Infectious Diseases. 2012;65(5):448–50. doi: 10.7883/yoken.65.448 [DOI] [PubMed] [Google Scholar]
- 31.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Annals of Internal Medicine. 2018;169(7):467–73. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 32.Wickham H. The split-apply-combine strategy for data analysis. Journal of Statistical Software. 2011;40(1):1–29. [Google Scholar]
- 33.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York; 2016. [Google Scholar]
- 34.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2022.
- 35.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. BMJ Ment Health. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of statistical software. 2010;36:1–48. [Google Scholar]
- 37.Sabin AB, Ginder DR, Matumoto M. Difference in dissemination of the virus of Japanese-B-encephalitis among domestic animals and human beings in Japan. American Journal of Hygiene. 1947;46(3):341–55. doi: 10.1093/oxfordjournals.aje.a119173 [DOI] [PubMed] [Google Scholar]
- 38.Zhao G, Gao Y, Shi N, Zhang S, Xiao P, Zhang J, et al. Molecular Detection and Genetic Characterization of Japanese Encephalitis Virus in Animals from 11 Provinces in China. Viruses. 2023;15(3):625. 2791714301. doi: 10.3390/v15030625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nemeth NM, Bosco-Lauth AM, Sciulli RH, Gose RB, Nagata MT, Bowen RA. Serosurveillance for Japanese encephalitis and West Nile viruses in resident birds in Hawai’i. Journal of Wildlife Diseases. 2010;46(2):659–64. doi: 10.7589/0090-3558-46.2.659 [DOI] [PubMed] [Google Scholar]
- 40.Preziuso S, Mari S, Mariotti F, Rossi G. Detection of Japanese Encephalitis Virus in bone marrow of healthy young wild birds collected in 1997–2000 in Central Italy. Zoonoses and Public Health. 2018;65(7):798–804. doi: 10.1111/zph.12501 [DOI] [PubMed] [Google Scholar]
- 41.Platonov A, Rossi G, Karan L, Mironov K, Busani L, Rezza G. Does the Japanese encephalitis virus (JEV) represent a threat for human health in Europe? Detection of JEV RNA sequences in birds collected in Italy. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2012;17(32). MEDLINE:22913940. doi: 10.2807/ese.17.32.20241-en [DOI] [PubMed] [Google Scholar]
- 42.Lam KHK, Ellis IM, Williams DT, Lunt RA, Daniels PW, Watkins KL, et al. Japanese encephalitis in a racing thoroughbred gelding in Hong Kong. Veterinary Record. 2005;157(6):168-+. doi: 10.1136/vr.157.6.168 [DOI] [PubMed] [Google Scholar]
- 43.Kako N, Suzuki S, Sugie N, Kato T, Yanase T, Yamakawa M, et al. Japanese encephalitis in a 114-month-old cow: pathological investigation of the affected cow and genetic characterization of Japanese encephalitis virus isolate. BMC Veterinary Research. 2014;10(1). doi: 10.1186/1746-6148-10-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gulati BR, Singha H, Singh BK, Virmani N, Kumar S, Singh RK. Isolation and genetic characterization of Japanese encephalitis virus from equines in India. Journal of Veterinary Science. 2012;13(2):111–8. doi: 10.4142/jvs.2012.13.2.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katayama T, Saito S, Horiuchi S, Maruta T, Kato T, Yanase T, et al. Nonsuppurative encephalomyelitis in a calf in Japan and isolation of Japanese encephalitis virus genotype 1 from the affected calf. Journal of Clinical Microbiology. 2013;51(10):3448–53. doi: 10.1128/JCM.00737-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piewbang C, Wardhani SW, Chaiyasak S, Yostawonkul J, Kasantikul T, Techangamsuwan S. Japanese encephalitis virus infection in meerkats (Suricata suricatta). Zoonoses and Public Health. 2022;69(1):55–60. doi: 10.1111/zph.12882 [DOI] [PubMed] [Google Scholar]
- 47.Shimoda H, Ohno Y, Mochizuki M, Iwata H, Okuda M, Maeda K. Dogs as sentinels for human infection with japanese encephalitis Virus. Emerging Infectious Diseases. 2010;16(7):1136–9. doi: 10.3201/eid1607.091757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeh J, Park J, Ostlund EN. Serologic evidence of West Nile Virus in wild ducks captured in major inland resting sites for migratory waterfowl in South Korea. Veterinary Microbiology. 2012;154(1/2):96–103. CABI:20123005360. [DOI] [PubMed] [Google Scholar]
- 49.Diptyanusa A, Herini ES, Indarjulianto S, Satoto TBT. Estimation of Japanese encephalitis virus infection prevalence in mosquitoes and bats through nationwide sentinel surveillance in Indonesia. PLoS One. 2022;17(10):e0275647–e. ZOOREC:ZOOR15905028972. doi: 10.1371/journal.pone.0275647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diptyanusa A, Herini ES, Indarjulianto S, Satoto TBT. The detection of Japanese encephalitis virus in Megachiropteran bats in West Kalimantan, Indonesia: A potential enzootic transmission pattern in the absence of pig holdings. International Journal for Parasitology: Parasites and Wildlife. 2021;14:280–6. doi: 10.1016/j.ijppaw.2021.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jain P, Singh AK, Khan DN, Pandey M, Kumar R, Garg R, et al. Trend of Japanese encephalitis in Uttar Pradesh, India from 2011 to 2013. Epidemiology and Infection. 2016;144(2):363–70. doi: 10.1017/S0950268815000928 [DOI] [PubMed] [Google Scholar]
- 52.Dhanze H, Singh BB, Walsh M, Kumar MS, Kumar A, Bhilegaonkar KN, et al. Spatio-temporal epidemiology of Japanese encephalitis virus infection in pig populations of eastern Uttar Pradesh, India, 2013–2022. Zoonoses and Public Health. 2024;0(0):1–13. doi: 10.1111/zph.13123 [DOI] [PubMed] [Google Scholar]
- 53.Dhanze H, Karikalan M, Mehta D, Gupta M, Mote A, Kumar M, et al. First report on the detection of Japanese encephalitis virus in fruit bats from India. Journal of Vector Borne Diseases. 2022;59(2):190–2. doi: 10.4103/0972-9062.335769 [DOI] [PubMed] [Google Scholar]
- 54.Wang JL, Pan XL, Zhang HL, Fu SH, Wang HY, Tang Q, et al. Japanese encephalitis viruses from bats in Yunnan, China. Emerging Infectious Diseases. 2009;15(6):939–42. doi: 10.3201/eid1506.081525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S, Li X, Chen Z, Chen Y, Zhang Q, Liao Y, et al. Comparison of genomic and amino acid sequences of eight Japanese encephalitis virus isolates from bats. Archives of Virology. 2013;158(12):2543–52. doi: 10.1007/s00705-013-1777-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui J, Counor D, Shen D, Sun G, He H, Deubel V, et al. Detection of Japanese encephalitis virus antibodies in bats in Southern China. American Journal of Tropical Medicine and Hygiene. 2008;78(6):1007–11. doi: 10.4269/ajtmh.2008.78.1007 [DOI] [PubMed] [Google Scholar]
- 57.Banerjee K, Bhat HR, Geevarghese G, Jacob PG, Malunjkar AS. Antibodies against Japanese encephalitis virus in insectivorous bats from Karnataka. Indian Journal of Medical Research. 1988;87:527–30. [PubMed] [Google Scholar]
- 58.Lin YN, Wong WK. Sero-prevalence of Japanese encephalitis virus in various species of animals in Singapore—a preliminary study. Singapore Journal of Primary Industries. 2000;28:57–61. CABI:20013104959. [Google Scholar]
- 59.Johnsen DO, Edelman R, Grossman RA, Muangman D, Pomsdhit J, Gould DJ. Study of Japanese encephalitis-virus in Chiangmai Valley, Thailand. 5. Animal infections. American Journal of Epidemiology. 1974;100(1):57–68. doi: 10.1093/oxfordjournals.aje.a112009 [DOI] [PubMed] [Google Scholar]
- 60.Yuwono J, Suharyono W, Koiman I, Tsuchiya Y, Tagaya I. Seroepidemiological survey on dengue and Japanese encephalitis virus infections in Asian monkeys. Southeast Asian Journal of Tropical Medicine and Public Health. 1984;15(2):194–200. BIOSIS:PREV198579050697. [PubMed] [Google Scholar]
- 61.Ohno Y, Sato H, Suzuki K, Yokoyama M, Uni S, Shibasaki T, et al. Detection of antibodies against Japanese encephalitis virus in raccoons, raccoon dogs and wild boars in Japan. Journal of Veterinary Medical Science. 2009;71(8):1035–9. doi: 10.1292/jvms.71.1035 [DOI] [PubMed] [Google Scholar]
- 62.Chen S-w Jiang L-n, Zhong X-s Zheng X-y, Ma S-j Xiong Y-q, et al. Serological Prevalence Against Japanese Encephalitis Virus-Serocomplex Flaviviruses in Commensal and Field Rodents in South China. Vector-Borne and Zoonotic Diseases. 2016;16(12):777–80. doi: 10.1089/vbz.2015.1934 [DOI] [PubMed] [Google Scholar]
- 63.Nemeth N, Bosco-Lauth A, Bowen R. North American birds as potential amplifying hosts of Japanese encephalitis virus. American Journal of Tropical Medicine and Hygiene. 2009;81(5):324-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hasegawa T, Takehara Y, Takahashi K. Natural and experimental infections of Japanese tree sparrows with Japanese encephalitis-virus. Archives of Virology. 1975;49(4):373–6. doi: 10.1007/BF01318247 [DOI] [PubMed] [Google Scholar]
- 65.Ting SHL, Tan HC, Wong WK, Ng ML, Chan SH, Ooi EE. Seroepidemiology of neutralizing antibodies to Japanese encephalitis virus in Singapore: Continued transmission despite abolishment of pig farming? Acta Tropica. 2004;92(3):187–91. doi: 10.1016/j.actatropica.2004.04.010 [DOI] [PubMed] [Google Scholar]
- 66.Khan FU, Banerjee K. Mosquito collection in heronries and antibodies to Japanese encephalitis-virus in birds in Asansol-Dhanbad region. Indian Journal of Medical Research. 1980;71(JAN):1–5. [PubMed] [Google Scholar]
- 67.Jamgaonkar AV, Yergolkar PN, Geevarghese G, Joshi GD, Joshi MV, Mishra AC. Serological evidence for Japanese encephalitis virus and West Nile virus infections in water frequenting and terrestrial wild birds in Kolar District, Karnataka State, India. A retrospective study. Acta Virologica. 2003;47(3):185–8. [PubMed] [Google Scholar]
- 68.Yap G, Lim XF, Chan S, How CB, Humaidi M, Yeo G, et al. Serological evidence of continued Japanese encephalitis virus transmission in Singapore nearly three decades after end of pig farming. Parasites and Vectors. 2019;12(1). doi: 10.1186/s13071-019-3501-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Angami K, Chakravarty SK, Das MS, Chakraborty MS, Mukherjee KK. Seroepidemiological study of Japanese encephalitis in Dimapur, Nagaland. Journal of Communicable Diseases. 1989;21(2):87–95. [PubMed] [Google Scholar]
- 70.Shortridge KF, Ng MH, Oya A, Kobayashi M, Munro R, Wong F, et al. Arbovirus infections in reptiles: Immunological evidence for a high incidence of Japanese encephalitis virus in the cobra Naja naja. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1974;68(6):454–60. doi: 10.1016/0035-9203(74)90068-6 [DOI] [PubMed] [Google Scholar]
- 71.Shortridge KF, Oya A, Kobayashi M, Yip DY. Arbovirus infections in reptiles: studies on the presence of Japanese encephalitis virus antibody in the plasma of the turtle, Trionyx sinensis. Southeast Asian Journal of Tropical Medicine and Public Health. 1975;6(2):161–9. [PubMed] [Google Scholar]
- 72.Mansfield KL, Hernández-Triana LM, Banyard AC, Fooks AR, Johnson N. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Veterinary Microbiology. 2017;201:85–92. doi: 10.1016/j.vetmic.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 73.Mansfield KL, Horton DL, Johnson N, Li L, Barrett AD, Smith DJ, et al. Flavivirus-induced antibody cross-reactivity. The Journal of general virology. 2011;92(Pt 12):2821. doi: 10.1099/vir.0.031641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pham D, Howard-Jones AR, Hueston L, Jeoffreys N, Doggett S, Rockett RJ, et al. Emergence of Japanese encephalitis in Australia: a diagnostic perspective. Pathology. 2022;54(6):669–77. doi: 10.1016/j.pathol.2022.07.001 [DOI] [PubMed] [Google Scholar]
- 75.Dhanze H, Bhilegaonkar K, Rawat S, Kumar HC, Kumar A, Gulati B, et al. Development of recombinant nonstructural 1 protein based indirect enzyme linked immunosorbent assay for sero-surveillance of Japanese encephalitis in swine. Journal of virological methods. 2019;272:113705. doi: 10.1016/j.jviromet.2019.113705 [DOI] [PubMed] [Google Scholar]
- 76.Dhanze H, Kumar MS, Singh V, Gupta M, Bhilegaonkar KN, Kumar A, et al. Detection of recent infection of Japanese encephalitis virus in swine population using IgM ELISA: a suitable sentinel to predict infection in humans. Journal of immunological methods. 2020;486:112848. doi: 10.1016/j.jim.2020.112848 [DOI] [PubMed] [Google Scholar]
- 77.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: important reservoir hosts of emerging viruses. Clinical microbiology reviews. 2006;19(3):531–45. doi: 10.1128/CMR.00017-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fagre AC, Kading RC. Can Bats Serve as Reservoirs for Arboviruses? Viruses-Basel. 2019;11(3). doi: 10.3390/v11030215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Den Hurk AF, Smith CS, Field HE, Smith IL, Northill JA, Taylor CT, et al. Transmission of Japanese encephalitis virus from the black flying fox, Pteropus alecto, to Culex annulirostris mosquitoes, despite the absence of detectable viremia. American Journal of Tropical Medicine and Hygiene. 2009;81(3):457–62. doi: 10.4269/ajtmh.2009.81.457 [DOI] [PubMed] [Google Scholar]
- 80.La Motte LC. Japanese B encephalitis in bats during simulated hibernation. 1958. [DOI] [PubMed]
- 81.Martin LB, Burgan S, Adelman JS, Gervasi SS. Host competence: an organismal trait to integrate immunology and epidemiology. Integrative and Comparative Biology. 2016;56(6):1225–37. doi: 10.1093/icb/icw064 [DOI] [PubMed] [Google Scholar]
- 82.Kramer LD, Li J, Shi P-Y. West nile virus. The Lancet Neurology. 2007;6(2):171–81. doi: 10.1016/S1474-4422(07)70030-3 [DOI] [PubMed] [Google Scholar]
- 83.Boyle DB, Dickerman RW, Marshall ID. Primary viraemia and responses of herons to experimental infection with Murray Valley encepahlitis, Kunjin and Japanese encephalitis viruses. Australian Journal of Experimental Biology and Medical Science. 1983;61(DEC):655–64. doi: 10.1038/icb.1983.62 [DOI] [PubMed] [Google Scholar]
- 84.Williams DT, Daniels PW, Lunt RA, Wang LF, Newberry KM, Mackenzie JS. Experimental infections of pigs with Japanese encephalitis virus and closely related Australian flaviviruses. American Journal of Tropical Medicine and Hygiene. 2001;65(4):379–87. doi: 10.4269/ajtmh.2001.65.379 [DOI] [PubMed] [Google Scholar]
- 85.Hameed M, Wahaab A, Nawaz M, Khan S, Nazir J, Liu K, et al. Potential role of birds in japanese encephalitis virus zoonotic transmission and genotype shift. Viruses. 2021;13(3). doi: 10.3390/v13030357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Plowright RK, Ahmed AN, Coulson T, Crowther TW, Ejotre I, Faust CL, et al. Ecological countermeasures to prevent pathogen spillover and subsequent pandemics. Nature Communications. 2024;15(1):2577. doi: 10.1038/s41467-024-46151-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, et al. Pathways to zoonotic spillover. Nature Reviews Microbiology. 2017;15(8):502–10. doi: 10.1038/nrmicro.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuno G. Persistence of arboviruses and antiviral antibodies in vertebrate hosts: its occurrence and impacts. Reviews in Medical Virology. 2001;11(3):165–90. doi: 10.1002/rmv.314 [DOI] [PubMed] [Google Scholar]
- 89.Sulkin SE, Allen R. Virus infections in bats1974. viii+103pp.-viii+pp. p.
- 90.van den Hurk AF A R S., Mackenzie J. S. Ecology and Geographical Expansion of Japanese Encephalitis Virus. Annual Review of Entomology. 2009;54:17–35. CCC:000262482300003. doi: 10.1146/annurev.ento.54.110807.090510 [DOI] [PubMed] [Google Scholar]
- 91.Doi R, Oya A, Shirasaka A, Yabe S, Sasa M. Studies on Japanese encephalitis virus infection of reptiles. II. Role of lizards on hibernation of Japanese encephalitis virus. Japanese Journal of Experimental Medicine. 1983;53(2):125–34. [PubMed] [Google Scholar]
- 92.Wesson JP, apos, Dea MA, Hyndman TH. A review of reptile virus experimental infection studies. Journal of General Virology. 2023;104(4). doi: 10.1099/jgv.0.001832 [DOI] [PubMed] [Google Scholar]
- 93.Juřicová Z, Hubálek Z, Halouzka J, Šikutová S. Serological examination of songbirds (Passeriformes) for mosquito-borne viruses Sindbis, Ťahyňa, and Batai in a South Moravian wetland (Czech Republic). Vector-Borne and Zoonotic Diseases. 2009;9(3):295–9. [DOI] [PubMed] [Google Scholar]
- 94.Beasley DWC, Barrett ADT, Tesh RB. Resurgence of West Nile neurologic disease in the United States in 2012: What happened? What needs to be done? Antiviral Research. 2013;99(1):1–5. doi: 10.1016/j.antiviral.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 95.Endale A, Medhin G, Darfiro K, Kebede N, Legesse M. Magnitude of antibody cross-reactivity in medically important mosquito-borne flaviviruses: a systematic review. Infection and drug resistance. 2021:4291–9. doi: 10.2147/IDR.S336351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chan KR, Ismail AA, Thergarajan G, Raju CS, Yam HC, Rishya M, et al. Serological cross-reactivity among common flaviviruses. Frontiers in Cellular and Infection Microbiology. 2022;12. doi: 10.3389/fcimb.2022.975398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nemeth N, Bosco-Lauth A, Oesterle P, Kohler D, Bowen R. North American birds as potential amplifying hosts of Japanese encephalitis virus. American Journal of Tropical Medicine and Hygiene. 2012;87(4):760–7. doi: 10.4269/ajtmh.2012.12-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dhanda V, Banerjee K, Deshmukh PK, Ilkal MA. Experimental viraemia and transmission of Japanese encephalitis virus by mosquitoes in domestic ducks. Indian J Med Res. 1977;66(6):881–8. . [PubMed] [Google Scholar]
- 99.Marsh O. Life cycle of a star: Carl Sagan and the circulation of reputation. The British Journal for the History of Science. 2019;52(3):467–86. doi: 10.1017/S0007087419000049 [DOI] [PubMed] [Google Scholar]
- 100.Agriculture Victoria. Japanese encephalitis—Frequently asked questions: Department of Energy, Environment and Climate Action; 2024 [01/04/2024]. https://agriculture.vic.gov.au/biosecurity/animal-diseases/important-animal-diseases/japanese-encephalitis/japanese-encephalitis-frequently-asked-questions.
- 101.Ong OTW, Skinner EB, Johnson BJ, Old JM. Mosquito-borne viruses and non-human vertebrates in australia: A review. Viruses. 2021;13(2). doi: 10.3390/v13020265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van den Hurk AF, Pyke AT, Mackenzie JS, Hall-Mendelin S, Ritchie SA. Japanese Encephalitis Virus in Australia: From Known Known to Known Unknown. Trop Med Infect Dis. 2019;4(1). Epub 20190220. doi: 10.3390/tropicalmed4010038 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A Search terms in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs.
(DOCX)
(separate Excel file) Bibliography and extracted data of eligible studies in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Fig A Bar plot of the frequency of study location by country, in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Fig B Reported seroprevalence (indirect detection of JEV infection [antibody]) in studies of mammals other than Bovinae, Canidae, Caprinae, Equidae, Suidae, humans and ardeid birds. Horizontal lines = 95% confidence intervals. Fig C Reported seroprevalence (indirect detection of JEV infection [antibody]) in bird orders in which maximum seroprevalence was <10% in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Ardeidae (Pelecaniformes) are included from studies in which vertebrate animals other than pigs and humans were also tested. Horizontal lines = 95% confidence intervals. Fig D Reported prevalence (direct detection of JEV infection [virus, viral antigen, or viral RNA]) stratified by country in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Suidae are included from studies in which vertebrate animals other than ardeid birds and humans were also tested. Horizontal lines = 95% confidence intervals. Fig E Reported seroprevalence (indirect detection of JEV infection [antibody]) of mammals in China, India, Japan, and Thailand, in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Suidae are included from studies in which vertebrate animals other than ardeid birds and humans were also tested. Horizontal lines = 95% confidence intervals. Fig F Reported seroprevalence (indirect detection of JEV infection [antibody]) of mammals in Cambodia, Indonesia, Mongolia, Nepal, Singapore and South Korea in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Suidae are included from studies in which vertebrate animals other than ardeid birds and humans were also tested. Horizontal lines = 95% confidence intervals. Fig G Reported seroprevalence (indirect detection of JEV infection [antibody]) of birds by country in a scoping review of direct and indirect evidence of naturally occurring Japanese encephalitis virus infection in vertebrate animals other than humans, ardeid birds and pigs. Suidae are included from studies in which vertebrate animals other than ardeid birds and humans were also tested. Horizontal lines = 95% confidence intervals.
(XLSX)
Data Availability Statement
All underlying data are available in the Supplementary Material.