Abstract
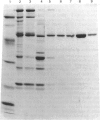
Adenylosuccinate lyase from rat skeletal muscle was purified to apparent homogeneity by a combination of ion-exchange chromatography and affinity chromatography on agarose containing covalently bound adenylophosphonopropionate. The purified enzyme is stable when stored in 20% glycerol at -70 degrees C, and can be thawed and re-frozen with minimal loss of activity. Adenylosuccinate lyase has a specific activity of 11 mumol/min per mg of protein at 25 degrees C. Its subunit Mr is 52,000, by SDS/polyacrylamide-gel electrophoresis, and its apparent native Mr is approx. 200,000, by gel filtration. The purified enzyme has Km values for adenylosuccinate and 4-(N-succino)-5-aminoimidazole-4-carboxamide ribonucleotide (SAICAR) of 1.5 microM and approximately 1 microM respectively, in Hepes/KOH buffer, pH 7.4. Several monoanions and dianions activate the enzyme at low concentration; several of these inhibit the enzyme at high concentrations. Fluoro analogues of adenylosuccinate and SAICAR were synthesized by using highly purified adenylosuccinate synthase and SAICAR synthase respectively, and erythro-beta-fluoroaspartate in place of aspartate. Both analogues are competitive inhibitors of adenylosuccinate lyase in both of the reactions catalysed by the enzyme, with Ki values well below the Km values for the two substrates.
Full text
PDF

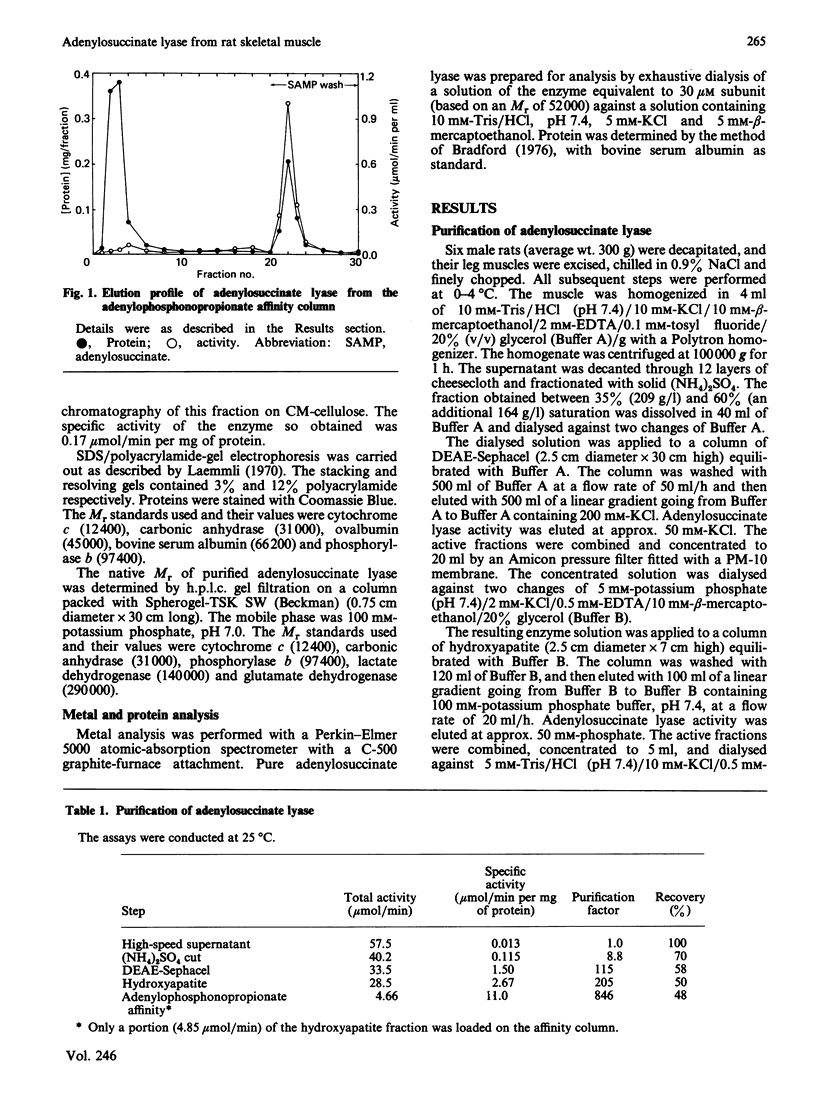
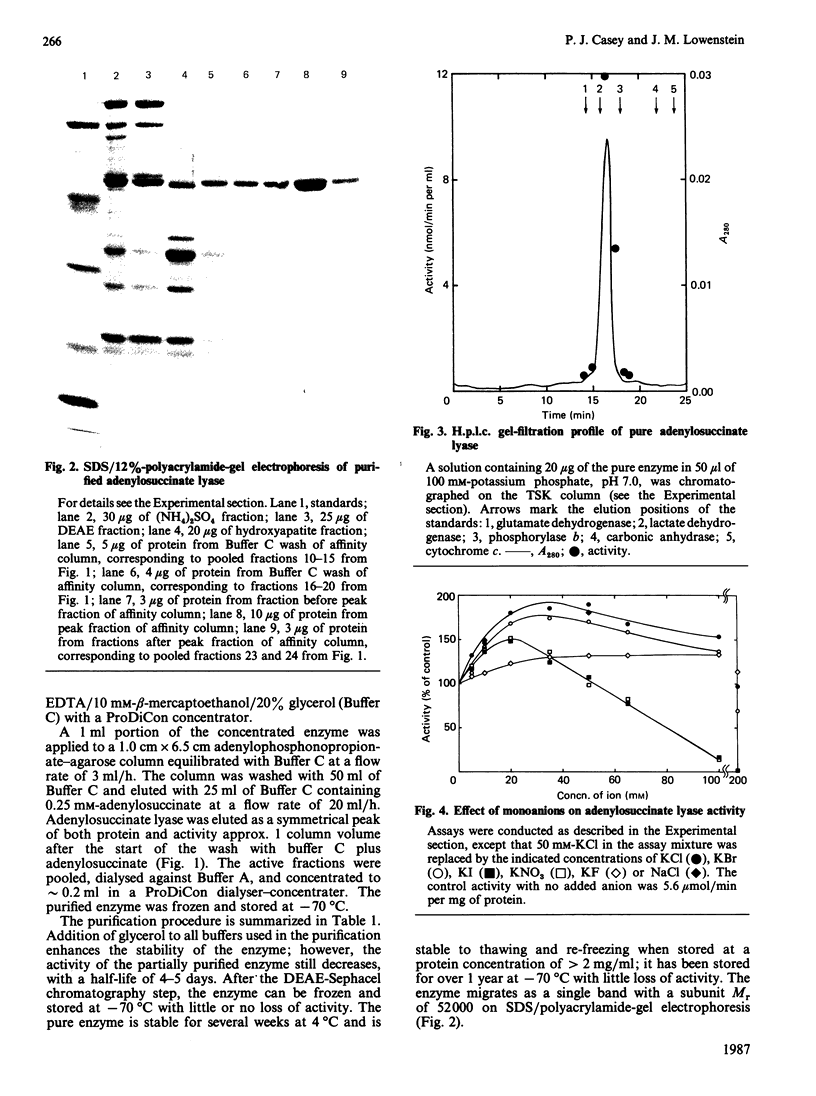
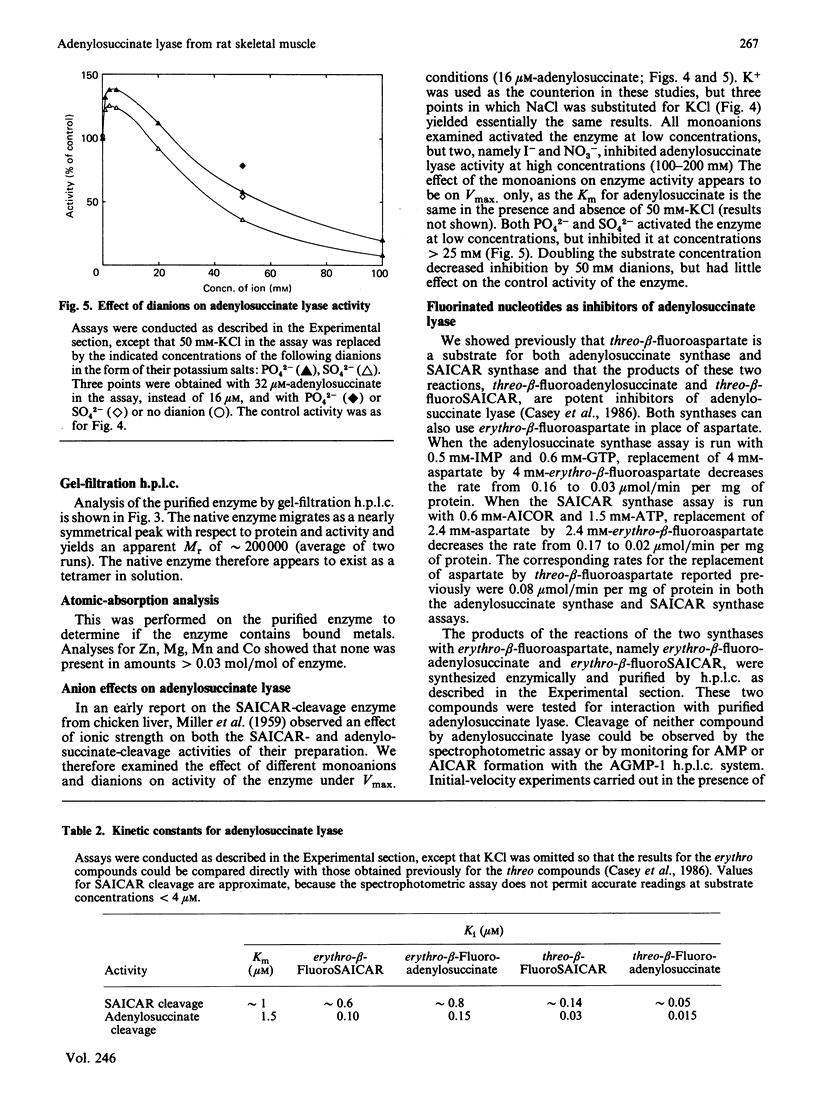


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brand L. M., Lowenstein J. M. Effect of diet on adenylosuccinase activity in various organs of rat and chicken. J Biol Chem. 1978 Oct 10;253(19):6872–6878. [PubMed] [Google Scholar]
- Brand L. M., Lowenstein J. M. Inhibition of adenylosuccinase by adenylophosphonopropionate and related compounds. Biochemistry. 1978 Apr 18;17(8):1365–1370. doi: 10.1021/bi00601a002. [DOI] [PubMed] [Google Scholar]
- Bright H. J. Divalent metal activation of beta-methylaspartase. The importance of ionic radius. Biochemistry. 1967 Apr;6(4):1191–1203. doi: 10.1021/bi00856a032. [DOI] [PubMed] [Google Scholar]
- Brox L. W. The cleavage of adenylosuccinate and 5-amino-4-imidazole-N-succino-carboxamide ribonucleotide by an adenylosuccinate lyase from Ehrlich ascites tumor cells. Can J Biochem. 1973 Jul;51(7):1072–1076. doi: 10.1139/o73-140. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Abeles R. H., Lowenstein J. M. Metabolism of threo-beta-fluoroaspartate by H4 cells. Inhibition of adenylosuccinate lyase by fluoro analogs of its substrates. J Biol Chem. 1986 Oct 15;261(29):13637–13642. [PubMed] [Google Scholar]
- Cleland W. W. The use of pH studies to determine chemical mechanisms of enzyme-catalyzed reactions. Methods Enzymol. 1982;87:390–405. doi: 10.1016/s0076-6879(82)87024-9. [DOI] [PubMed] [Google Scholar]
- ENGLARD S. Studies on the mechanism of the enzymatic amination and hydration of fumarate. J Biol Chem. 1958 Oct;233(4):1003–1009. [PubMed] [Google Scholar]
- Fields G. A., Bright H. J. Magnetic resonance and kinetic studie of the activation of beta-methylaspartase by manganese. Biochemistry. 1970 Sep 15;9(19):3801–3809. doi: 10.1021/bi00821a020. [DOI] [PubMed] [Google Scholar]
- Garrard L. J., Bui Q. T., Nygaard R., Raushel F. M. Acid-base catalysis in the argininosuccinate lyase reaction. J Biol Chem. 1985 May 10;260(9):5548–5553. [PubMed] [Google Scholar]
- Givot I. L., Abeles R. H. Mammalian histidine ammonia lyase. In vivo inactivation and presence of an electrophilic center at the active site. J Biol Chem. 1970 Jun;245(12):3271–3273. [PubMed] [Google Scholar]
- Hanson K. R., Havir E. A. L-phenylalanine ammonia-lyase. IV. Evidence that the prosthetic group contains a dehydroalanyl residue and mechanism of action. Arch Biochem Biophys. 1970 Nov;141(1):1–17. doi: 10.1016/0003-9861(70)90100-1. [DOI] [PubMed] [Google Scholar]
- Havir E. A., Hanson K. R. L-phenylalanine ammonia-lyase. II. Mechanism and kinetic properties of the enzyme from potato tubers. Biochemistry. 1968 May;7(5):1904–1914. doi: 10.1021/bi00845a039. [DOI] [PubMed] [Google Scholar]
- Jackson R. C., Goulding F. J., Weber G. Enzymes of purine metabolism in human and rat renal cortex and renal cell carcinoma. J Natl Cancer Inst. 1979 Apr;62(4):749–754. [PubMed] [Google Scholar]
- Jackson R. C., Morris H. P., Weber G. Increased adenylosuccinase activity in hepatomas and kidney tumors. Life Sci. 1976 May 15;18(10):1043–1048. doi: 10.1016/0024-3205(76)90136-3. [DOI] [PubMed] [Google Scholar]
- Jaeken J., Van den Berghe G. An infantile autistic syndrome characterised by the presence of succinylpurines in body fluids. Lancet. 1984 Nov 10;2(8411):1058–1061. [PubMed] [Google Scholar]
- Kamlay M. T., Halvorson H. R., Shore J. D. Anion effects on the liver alcohol dehydrogenase reaction. Arch Biochem Biophys. 1985 Aug 15;241(1):58–66. doi: 10.1016/0003-9861(85)90361-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MILLER R. W., BUCHANAN J. M. Biosynthesis of the purines. 28. Mechanism of action of adenylosuccinase. J Biol Chem. 1962 Feb;237:491–496. [PubMed] [Google Scholar]
- MILLER R. W., LUKENS L. N., BUCHANAN J. M. Biosynthesis of the purines. XXV. The enzymatic cleavage of N-(5-amino-1-ribosyl-4-imidazolylcarbonyl)-L-aspartic acid 5'-phosphate. J Biol Chem. 1959 Jul;234(7):1806–1811. [PubMed] [Google Scholar]
- Patey C. A., Shaw G. Purification and properties of an enzyme duet, phosphoribosylaminoimidazole carboxylase and phosphoribosylaminoimidazolesuccinocarboxamide synthetase, involved in the biosynthesis of purine nucleotides de novo. Biochem J. 1973 Nov;135(3):543–545. doi: 10.1042/bj1350543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto R. M., Faraldo A., Fernández A., Canales J., Sillero A., Sillero M. A. Adenylosuccinate lyase from Artemia embryos. Purification and properties. J Biol Chem. 1983 Oct 25;258(20):12513–12519. [PubMed] [Google Scholar]
- Porter D. J., Rudie N. G., Bright H. J. Nitro analogs of substrates for adenylosuccinate synthetase and adenylosuccinate lyase. Arch Biochem Biophys. 1983 Aug;225(1):157–163. doi: 10.1016/0003-9861(83)90019-x. [DOI] [PubMed] [Google Scholar]
- Smith L. D., Emerson R. L., Morrical S. W. A comparison of hepatic adenylosuccinate lyase from rats fed either a chow diet or a semisynthetic basal diet low in riboflavin. Int J Biochem. 1982;14(10):875–878. doi: 10.1016/0020-711x(82)90067-2. [DOI] [PubMed] [Google Scholar]
- Spector T., Berens R. L., Marr J. J. Adenylosuccinate synthetase and adenylosuccinate lyase from Trypanosoma cruzi, Specificity studies with potential chemotherapeutic agents. Biochem Pharmacol. 1982 Jan 15;31(2):225–229. doi: 10.1016/0006-2952(82)90215-5. [DOI] [PubMed] [Google Scholar]
- Spector T., Jones T. E., Elion G. B. Specificity of adenylosuccinate synthetase and adenylosuccinate lyase from Leishmania donovani. Selective amination of an antiprotozoal agent. J Biol Chem. 1979 Sep 10;254(17):8422–8426. [PubMed] [Google Scholar]
- Stern A. M., Foxman B. M., Tashjian A. H., Jr, Abeles R. H. DL-threo-beta-Fluoroaspartate and DL-threo-beta-fluoroasparagine: selective cytotoxic agents for mammalian cells in culture. J Med Chem. 1982 May;25(5):544–550. doi: 10.1021/jm00347a013. [DOI] [PubMed] [Google Scholar]
- Swain J. L., Hines J. J., Sabina R. L., Harbury O. L., Holmes E. W. Disruption of the purine nucleotide cycle by inhibition of adenylosuccinate lyase produces skeletal muscle dysfunction. J Clin Invest. 1984 Oct;74(4):1422–1427. doi: 10.1172/JCI111553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornheim K., Lowenstein J. M. The purine nucleotide cycle. The production of ammonia from aspartate by extracts of rat skeletal muscle. J Biol Chem. 1972 Jan 10;247(1):162–169. [PubMed] [Google Scholar]
- Tyagi A. K., Cooney D. A., Bledsoe M., Jayaram H. N. Two complementary radiometric methods for the measurement of 5-amino-4-imidazole-N-succinocarboxamide ribonucleotide synthetase (SAICAR synthetase). J Biochem Biophys Methods. 1980 Mar;2(3):123–132. doi: 10.1016/0165-022x(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Woodward D. O., Braymer H. D. Purification and properties of Neurospora adenylosuccinase. J Biol Chem. 1966 Feb 10;241(3):580–587. [PubMed] [Google Scholar]