Abstract
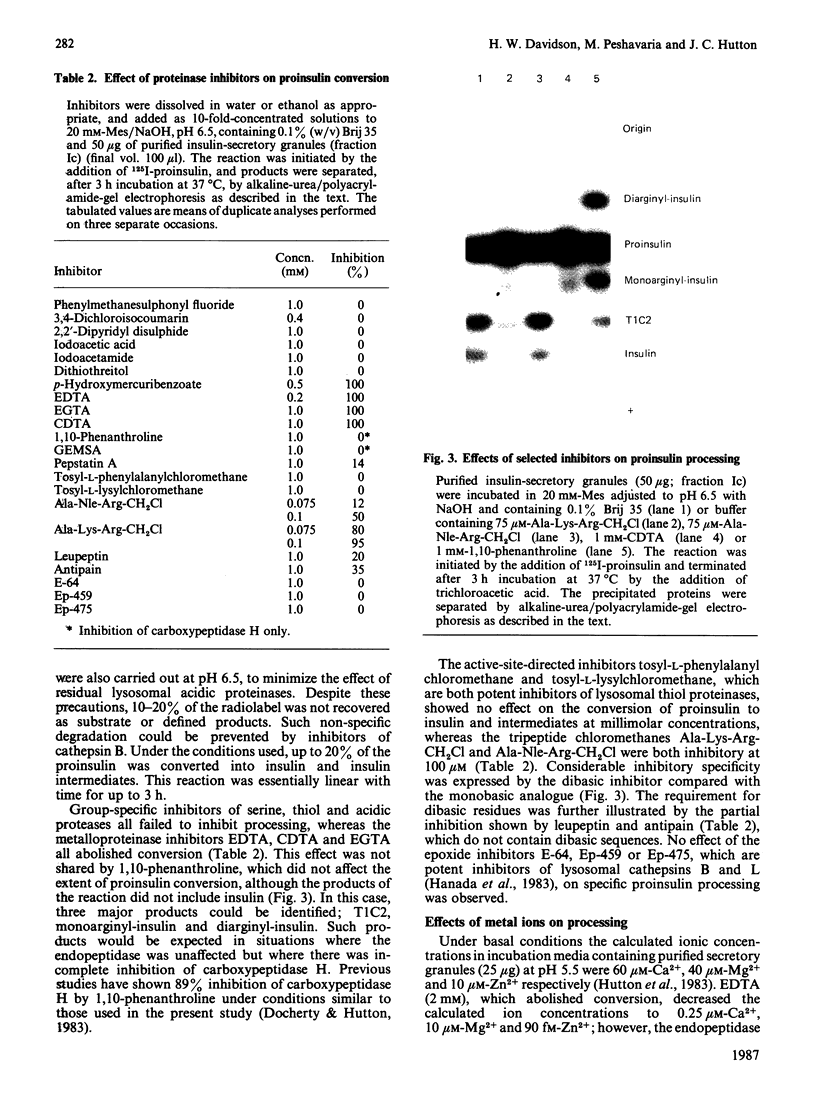
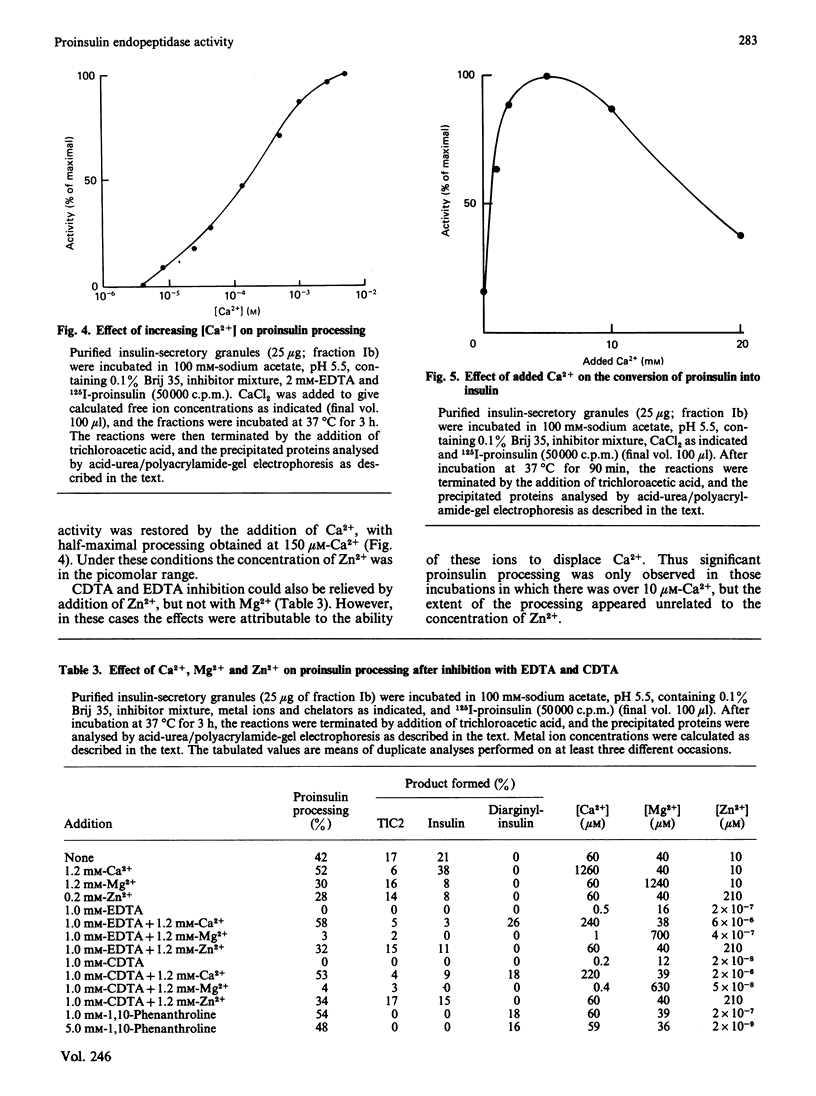
The nature of the endoproteolytic activity involved in the post-translational processing of proinsulin has been investigated in rat insulinoma tissue. 125I-proinsulin was converted by lysed insulin-secretory granules into insulin via an intermediate form identified as des-dibasic-proinsulin. This activity co-localized with immunoreactive (endogenous) insulin and carboxypeptidase H upon subcellular fractionation of the tissue, indicating a secretory-granular location. Under optimized conditions, conversion was quantitative. Inhibitor studies demonstrated that processing occurred by a reaction sequence involving cleavage on the C-terminal side of the pairs of basic amino acids, with subsequent removal of the newly exposed basic residues by carboxypeptidase H. Endoproteolytic activity was abolished by EDTA and CDTA (1,2-cyclohexanediaminetetra-acetic acid), but not by 1,10-phenanthroline or by group-specific inhibitors of serine, thiol or acidic proteinases. Inhibition by EDTA and CDTA could be reversed by both Ca2+ and Zn2+, although the former appeared to be the ion of physiological importance. Addition of Ca2+ in the absence of chelators stimulated endoproteinase activity, with a maximal effect at 5 mM, a concentration consistent with the intragranular environment. Similarly the pH optimum of 5.5 coincides with the prevailing intragranular pH. Together these properties suggest that the Ca2+-dependent endopeptidase described here is involved in vivo in the proteolytic processing of proinsulin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstetter T., Wolf D. H. Hormone processing and membrane-bound proteinases in yeast. EMBO J. 1985 Jan;4(1):173–177. doi: 10.1002/j.1460-2075.1985.tb02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amory A., Foury F., Goffeau A. The purified plasma membrane ATPase of the yeast Schizosaccharomyces pombe forms a phosphorylated intermediate. J Biol Chem. 1980 Oct 10;255(19):9353–9357. [PubMed] [Google Scholar]
- Andersson T., Berggren P. O., Gylfe E., Hellman B. Amounts and distribution of intracellular magnesium and calcium in pancreatic beta-cells. Acta Physiol Scand. 1982 Feb;114(2):235–241. doi: 10.1111/j.1748-1716.1982.tb06977.x. [DOI] [PubMed] [Google Scholar]
- Ansorge S., Kirschke H., Friedrich K. Conversion of proinsulin into insulin by cathepsins B and L from rat liver lysosomes. Acta Biol Med Ger. 1977;36(11-12):1723–1727. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chick W. L., Warren S., Chute R. N., Like A. A., Lauris V., Kitchen K. C. A transplantable insulinoma in the rat. Proc Natl Acad Sci U S A. 1977 Feb;74(2):628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson H. W., Hutton J. C. The insulin-secretory-granule carboxypeptidase H. Purification and demonstration of involvement in proinsulin processing. Biochem J. 1987 Jul 15;245(2):575–582. doi: 10.1042/bj2450575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Carroll R. J., Steiner D. F. Conversion of proinsulin to insulin: involvement of a 31,500 molecular weight thiol protease. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4613–4617. doi: 10.1073/pnas.79.15.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Carroll R., Steiner D. F. Identification of a 31,500 molecular weight islet cell protease as cathepsin B. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3245–3249. doi: 10.1073/pnas.80.11.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Hutton J. C. Carboxypeptidase activity in the insulin secretory granule. FEBS Lett. 1983 Oct 3;162(1):137–141. doi: 10.1016/0014-5793(83)81065-5. [DOI] [PubMed] [Google Scholar]
- Docherty K., Hutton J. C., Steiner D. F. Cathepsin B-related proteases in the insulin secretory granule. J Biol Chem. 1984 May 25;259(10):6041–6044. [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Fletcher D. J., Quigley J. P., Bauer G. E., Noe B. D. Characterization of proinsulin- and proglucagon-converting activities in isolated islet secretory granules. J Cell Biol. 1981 Aug;90(2):312–322. doi: 10.1083/jcb.90.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L. D., Snyder S. H. Purification and characterization of enkephalin convertase, an enkephalin-synthesizing carboxypeptidase. J Biol Chem. 1983 Sep 25;258(18):10950–10955. [PubMed] [Google Scholar]
- Given B. D., Cohen R. M., Shoelson S. E., Frank B. H., Rubenstein A. H., Tager H. S. Biochemical and clinical implications of proinsulin conversion intermediates. J Clin Invest. 1985 Oct;76(4):1398–1405. doi: 10.1172/JCI112116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold G., Gishizky M. L., Chick W. L., Grodsky G. M. Contrasting patterns of insulin biosynthesis, compartmental storage, and secretion. Rat tumor versus islet cells. Diabetes. 1984 Jun;33(6):556–561. doi: 10.2337/diab.33.6.556. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Chang H. T., Potts J. T., Jr Enzymic processing of proparathyroid hormone by cell-free extracts of parathyroid glands. Biochemistry. 1977 Aug 23;16(17):3910–3917. doi: 10.1021/bi00636a029. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Davidson H. W., Grimaldi K. A., Peshavaria M. Biosynthesis of betagranin in pancreatic beta-cells. Identification of a chromogranin A-like precursor and its parallel processing with proinsulin. Biochem J. 1987 Jun 1;244(2):449–456. doi: 10.1042/bj2440449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Davidson H. W., Peshavaria M. Proteolytic processing of chromogranin A in purified insulin granules. Formation of a 20 kDa N-terminal fragment (betagranin) by the concerted action of a Ca2+-dependent endopeptidase and carboxypeptidase H (EC 3.4.17.10). Biochem J. 1987 Jun 1;244(2):457–464. doi: 10.1042/bj2440457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Jackson P., Hales C. N. Isolation and characterization of calmodulin from an insulin-secreting tumour. Biochem J. 1981 Mar 1;193(3):875–885. doi: 10.1042/bj1930875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Peshavaria M. Isolation and characterisation of insulin secretory granules from a rat islet cell tumour. Diabetologia. 1982 Oct;23(4):365–373. doi: 10.1007/BF00253746. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983 Feb 15;210(2):297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Peshavaria M. Proton-translocating Mg2+-dependent ATPase activity in insulin-secretory granules. Biochem J. 1982 Apr 15;204(1):161–170. doi: 10.1042/bj2040161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C. The internal pH and membrane potential of the insulin-secretory granule. Biochem J. 1982 Apr 15;204(1):171–178. doi: 10.1042/bj2040171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Kemmler W., Peterson J. D., Steiner D. F. Studies on the conversion of proinsulin to insulin. I. Conversion in vitro with trypsin and carboxypeptidase B. J Biol Chem. 1971 Nov 25;246(22):6786–6791. [PubMed] [Google Scholar]
- Kemmler W., Steiner D. F., Borg J. Studies on the conversion of proinsulin to insulin. 3. Studies in vitro with a crude secretion granule fraction isolated from rat islets of Langerhans. J Biol Chem. 1973 Jul 10;248(13):4544–4551. [PubMed] [Google Scholar]
- Kemmler W., Steiner D. F. Conversion of proinsulin to insulin in a subcellular fraction from rat islets. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1223–1230. doi: 10.1016/0006-291x(70)90217-2. [DOI] [PubMed] [Google Scholar]
- Kitahara A., Ohtsuki H., Kirihata Y., Yamagata Y., Takano E., Kannagi R., Murachi T. Selective localization of calpain I (the low-Ca2+-requiring form of Ca2+-dependent cysteine proteinase) in B-cells of human pancreatic islets. FEBS Lett. 1985 May 6;184(1):120–124. doi: 10.1016/0014-5793(85)80666-9. [DOI] [PubMed] [Google Scholar]
- Loh Y. P., Brownstein M. J., Gainer H. Proteolysis in neuropeptide processing and other neural functions. Annu Rev Neurosci. 1984;7:189–222. doi: 10.1146/annurev.ne.07.030184.001201. [DOI] [PubMed] [Google Scholar]
- Loh Y. P., Parish D. C., Tuteja R. Purification and characterization of a paired basic residue-specific pro-opiomelanocortin converting enzyme from bovine pituitary intermediate lobe secretory vesicles. J Biol Chem. 1985 Jun 25;260(12):7194–7205. [PubMed] [Google Scholar]
- Nolan C., Margoliash E., Peterson J. D., Steiner D. F. The structure of bovine proinsulin. J Biol Chem. 1971 May 10;246(9):2780–2795. [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Madsen O., Perrelet A., Vassalli J. D., Anderson R. G. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J Cell Biol. 1986 Dec;103(6 Pt 1):2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Madsen O., Vassalli J. D., Perrelet A. Direct identification of prohormone conversion site in insulin-secreting cells. Cell. 1985 Sep;42(2):671–681. doi: 10.1016/0092-8674(85)90124-2. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Parish D. C., Tuteja R., Altstein M., Gainer H., Loh Y. P. Purification and characterization of a paired basic residue-specific prohormone-converting enzyme from bovine pituitary neural lobe secretory vesicles. J Biol Chem. 1986 Nov 5;261(31):14392–14397. [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D. C., Blix P. M., Rubenstein A. H., Kanazawa Y., Kosaka K., Tager H. S. A human proinsulin variant at arginine 65. Nature. 1981 Jun 25;291(5817):679–681. doi: 10.1038/291679a0. [DOI] [PubMed] [Google Scholar]
- Sorenson R. L., Shank R. D., Lindall A. W. Effect of pH on conversion of proinsulin to insulin by a subcellular fraction of rat islets. Proc Soc Exp Biol Med. 1972 Feb;139(2):652–655. doi: 10.3181/00379727-139-36207. [DOI] [PubMed] [Google Scholar]
- Sorenson R. L., Steffes M. W., Lindall A. W. Subcellular localization of proinsulin to insulin conversion in isolated rat islets. Endocrinology. 1970 Jan;86(1):88–96. doi: 10.1210/endo-86-1-88. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M. A., Vassalli J. D., Estensen R. D., Reich E. Plasminogen activator of islets of Langerhans: modulation by glucose and correlation with insulin production. Proc Natl Acad Sci U S A. 1980 Feb;77(2):875–879. doi: 10.1073/pnas.77.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji K., Tada K., Trakatellis A. C. On the biosynthesis of insulin in anglerfish islets. J Biol Chem. 1972 Jun 25;247(12):4080–4088. [PubMed] [Google Scholar]
- Yip C. C. A bovine pancreatic enzyme catalyzing the conversion of proinsulin to insulin. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1312–1315. doi: 10.1073/pnas.68.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoi O. O., Seldin D. C., Spragg J., Pinkus G. S., Austen K. F. Sequential cleavage of proinsulin by human pancreatic kallikrein and a human pancreatic kininase. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3612–3616. doi: 10.1073/pnas.76.8.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]




