Abstract
Recombinant mouse hepatitis viruses (MHV) differing only in the spike gene, containing A59, MHV-4, and MHV-2 spike genes in the background of the A59 genome, were compared for their ability to replicate in the liver and induce hepatitis in weanling C57BL/6 mice infected with 500 PFU of each virus by intrahepatic injection. Penn98-1, expressing the MHV-2 spike gene, replicated to high titer in the liver, similar to MHV-2, and induced severe hepatitis with extensive hepatocellular necrosis. SA59R13, expressing the A59 spike gene, replicated to a somewhat lower titer and induced moderate to severe hepatitis with zonal necrosis, similar to MHV-A59. S4R21, expressing the MHV-4 spike gene, replicated to a minimal extent and induced few if any pathological changes, similar to MHV-4. Thus, the extent of replication and the degree of hepatitis in the liver induced by these recombinant viruses were determined largely by the spike protein.
Various strains of mouse hepatitis virus (MHV) induce different patterns of pathogenesis, including enteritis, hepatitis, encephalitis, and demyelination in the mouse (20, 21). We are considering three strains here, MHV-A59, MHV-2, and MHV-4 (an isolate of MHV-JHM). The MHV-A59 strain is dualtropic, producing moderate to severe hepatitis as well as mild to moderate acute meningoencephalitis and chronic demyelination in C57BL/6 weanling mice (29, 30). The MHV-4 strain causes severe acute encephalitis, chronic demyelination, and only minimal levels of hepatitis (6, 23). The MHV-2 strain causes severe hepatitis and meningitis but is unable to cause encephalitis (7, 20, 42). There are previous studies demonstrating a relationship between attenuation of neurovirulence (6, 10, 13, 42) or hepatitis (14, 28) and the presence of mutations and variations in the spike (S) gene. The S protein, found on the virion envelope and on the plasma membrane of infected cells, is responsible for attachment to viral receptor and virus-cell fusion during viral entry and for cell-to-cell fusion later during infection. S is a 180-kDa glycoprotein, which (in the case of most MHV spike proteins) is cleaved into two noncovalently associated 90-kDa subunits, the amino-terminal S1 and carboxy-terminal S2 subunits (14, 33). It is speculated that the S1 subunit forms the globular head of the spike and the S2 subunit forms the membrane-bound stalk (8). Recently, a receptor-binding activity has been demonstrated using a recombinant protein containing the amino-terminal 330 residues of the S1 subunit of MHV-JHM (25, 41). S2 is believed to contain the domain that mediates fusion of viral and cell membranes (5, 8). The MHV-2 spike, while highly homologous in sequence to the spike proteins of other MHV strains, remains uncleaved and does not mediate fusion (44, 45).
Using targeted recombination technology (11, 12, 35), we have directly demonstrated that the spike protein is a major determinant of the neuropathogenic properties of MHV (39). When the S gene of MHV-4 was introduced into the background of MHV-A59, the resulting recombinant viruses (S4R21 and S4R22) were highly neurotropic, displaying similar pathogenic properties to parental MHV-4 after intracerebral inoculation into mice. These experiments did not address the question of whether the hepatitis phenotype of the recombinant viruses is also determined by the spike protein.
In order to more completely explore the role of the spike protein in controlling the ability to induce hepatitis, we compared three isogenic recombinant viruses that differ only in the spike gene, expressing the spike protein of A59 (SA59R13), MHV-2 (Penn98-1), or MHV-4 (S4R21) in the background of the A59 genome. This study shows that the level of viral replication in the liver is determined by the spike gene and that the amount of antigen staining and necrosis in the liver correlates with the level of viral replication. Thus, the ability to induce hepatitis is largely determined by the spike gene.
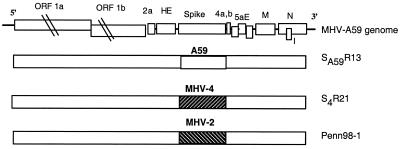
We have previously described the targeted recombination technology used to select recombinant viruses differing only in the spike gene, including the wild-type A59 recombinants and the MHV-4 spike-containing recombinant viruses that we have compared for neuropathogenesis (32, 39). In this study, we are using, in addition, a third type of recombinant virus only containing the MHV-2 spike gene, as described by Das Sarma et al. (7). Briefly, recombinant viruses were selected using the Alb4 mutant of MHV-A59 and synthetic capped RNA transcribed from pMH54, a plasmid containing a portion of the A59 HE pseudogene through the 3′ end of the genome (26) with the MHV-4 or MHV-2 spike gene substituted for the MHV-A59 spike gene (7, 39). In the experiments described below, we used SA59R13, a wild-type recombinant expressing the MHV-A59 spike protein; S4R21, expressing the MHV-4 spike protein; and Penn 98-1, expressing the MHV-2 spike protein. The spike genes of Penn98-1, S4R21, and SA59R13 were sequenced and shown to be identical to those from the parental viruses. In every case, a second independent recombinant had the identical spike gene sequence and displayed the same properties. Figure 1 shows a schematic diagram of these recombinant viruses.
FIG. 1.
Schematic diagram of MHV-A59 genome and generated recombinant viruses. The MHV genome is 31 kb. The sizes of the genes are drawn approximately to scale except for gene 1. Viral genes encoding nonstructural proteins (1a, 1b, 2a, 4a and b, and 5a) and structural proteins (HE, spike, E, M, N, and the internal gene I) are indicated. SA59R13, used as a control recombinant virus, has the A59 spike reintroduced in the A59 background genome. S4R21 and Penn98-1 recombinant viruses present the MHV-4 and the MHV-2 spike genes in the MHV-A59 background, respectively.
We initially compared the replication in the liver of the parental MHV-A59, MHV-2, and MHV-4 (Fig. 2) by inoculating 500 PFU of each virus directly into the liver of weanling C57BL/6 mice (The Jackson Laboratory, Bar Harbor, Maine). We used direct intrahepatic rather than intracerebral inoculation because this eliminates the central nervous system (CNS) disease induced by MHV-A59 and MHV-4 and isolates the hepatitis. We have previously shown that this method delivers virus into the liver (17). Mice were sacrificed at 1, 3, 5, and 7 days postinfection (p.i.). After perfusion of animals with phosphate-buffered saline, livers were homogenized, and virus was titrated on L-2 cells, as previously described (17). While the nonhepatotropic MHV-4 replicated only slightly above the level of detection, both A59 and MHV-2 replicated efficiently, higher in the case of MHV-2, peaking at day 5, as previously observed for MHV-A59 (18, 31) (Fig. 2). This is consistent with previous results reported for these strains of virus (20).
FIG. 2.
Titers of MHV in livers of animals infected with parental and recombinant viruses. C57BL/6 mice were infected intrahepatically with 500 PFU of MHV-A59, MHV-2, MHV-4, and the recombinants SA59R13, Penn98-1, and S4R21, and sacrificed at days 1, 3, 5, and 7 p.i. Virus titers were determined by plaque assay. Virus titers are presented as logarithmic means of PFU per gram of liver. Error bars represent logarithmic standard deviation. The limit of detection was 300 PFU/g of liver. Numbers of mice examined were as follows: MHV-A59, 18; MHV-2, 15; MHV-4, 8; SA59R13, 23; Penn98-1, 19; and S4R21, 24. At day 5 p.i., replication of Penn98-1 was significantly higher than that of SA59R13 and S4R21 (Wilcoxon's rank sum test: P < 0.03 and P < 0.004, respectively).
Recombinant viruses SA59R13, Penn98-1, and S4R21 were inoculated intrahepatically into weanling mice, which were sacrificed at selected times postinfection, and viruses from liver homogenates were titrated as described above (Fig. 2). All of the recombinant viruses replicated at levels similar to those of the parental strains. At day 5 p.i., Penn98-1 virus replicated to one log higher than SA59R13 and 3 logs over S4R21 (Penn98-1 versus SA59R13, P < 0.03; Penn98-1 versus S4R21, P < 0.004; Wilcoxon's rank sum test). The MHV-4 spike-containing recombinant virus (S4R21), like MHV-4 itself, replicated to a minimal extent above the level of detection (Fig. 2). Thus, since all the recombinants had the same MHV-A59 background, the level of replication was largely determined by the spike.
In order to further assess hepatitis induced by these viruses, histopathology studies on liver sections were performed. For histological diagnosis, formalin-fixed liver tissue was embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Hepatitis was scored as mild, moderate, or severe with the following criteria: (i) mild hepatitis (level 1): very few, small foci of inflammation and hepatocellular necrosis; (ii) moderate hepatitis (level 2): multiple foci of hepatocellular necrosis separated by normal liver; and (iii) severe hepatitis (level 3): either diffuse confluent (bridging) lesions or multiple foci. Replicate sections were stained immunohistochemically. All slides were read in a blinded manner. Briefly, sections were incubated with a monoclonal antibody (MAb) against the nucleocapsid protein (N) of MHV-JHM (MAb clone 1-16-1, kindly provided by J. L. Leibowitz, Texas A&M University), and immunohistochemistry was performed by the avidin-biotin-immunoperoxidase technique (Vector Laboratories, Burlingame, Calif.) using diaminobenzadine tetrahydrochloride as the substrate and counterstained with methyl green (Dako, Carpinteria, Calif.). As controls, replicate sections of each sample were incubated with an unrelated antibody (mouse immunoglobulin G, 10 μg/ml) and with secondary antibody alone. Furthermore, liver sections from mock-infected mice were incubated with the anti-N (MHV) MAb. These data are shown in Figs. 3, 4, and 5. Only the data obtained with the recombinant viruses are shown, as representative of data also observed in their parental viruses.
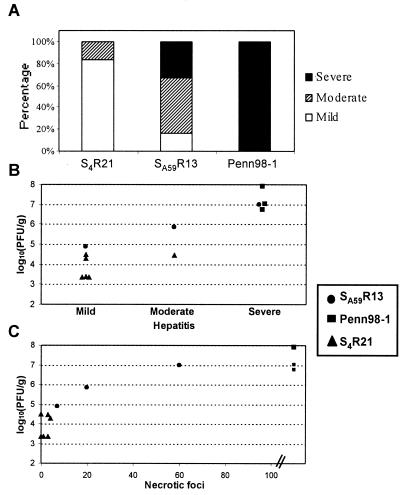
FIG. 3.
Spike protein determines degree of hepatitis and level of replication in the liver. The extent of hepatitis (scored as described in the text) and virus titers of recombinant viruses in livers of C57BL/6 mice after intrahepatic inoculation with 500 PFU of SA59R13, Penn98-1, and S4R21 viruses were studied. (A) Hepatitis induced by SA59R13 was milder than the severe hepatitis induced by Penn98-1 (P < 0.05, Wilcoxon's rank sum test), whereas S4R21 induced a low extent of hepatitis (SA59R13 versus S4R21, P < 0.05; Penn98-1 versus S4R21, P < 0.01; Wilcoxon's rank sum test). The results are shown as the percentage of mice with mild, moderate, and severe hepatitis, with six animals included per recombinant virus. Viral replication correlated both with the degree of hepatitis induced by each virus (B) and with the number of nonconfluent necrotic foci (C) (Spearman's correlation coefficients: r = 0.85, P < 0.001, and r = 0.88, P < 0.001, respectively). Each point represents viral replication and type of hepatitis or number of nonconfluent necrotic foci at day 5 p.i. of individual mice (three with SA59R13, 3 with Penn98-1, and six with S4R21).
FIG. 4.
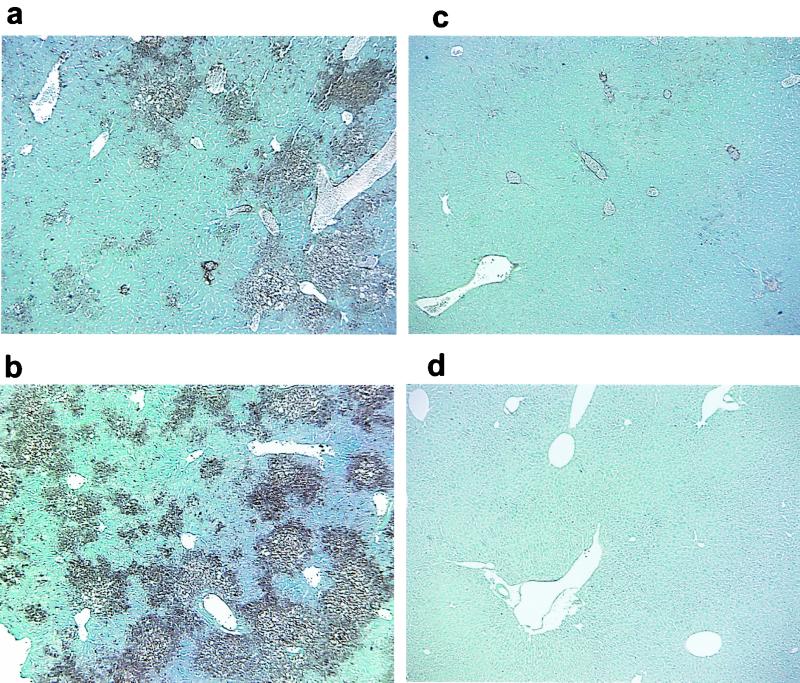
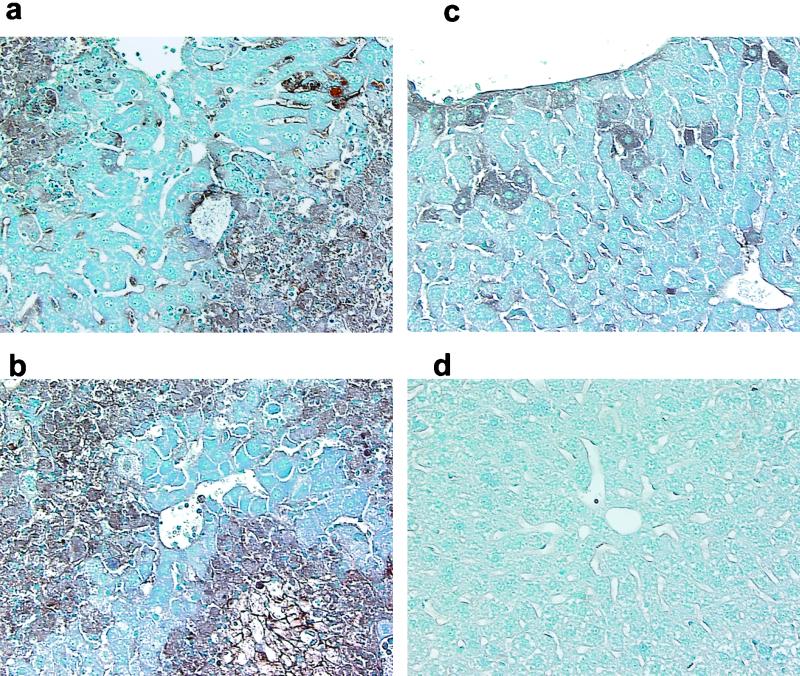
Immunohistochemistry of liver sections of C57BL/6 mice infected with the recombinant viruses SA59R13 (a), Penn98-1 (b), and S4R21 (c) and a mock-infected mouse (d). Mice were infected as described for Fig. 2 and then sacrificed at day 5 p.i. Livers were removed, fixed and sectioned. MHV was detected by immunolabeling with an MAb against the nucleocapsid (N) protein of MHV, using the avidin-biotin-immunoperoxidase technique (Vector) as described in the text. Viral antigen was associated with areas of hepatocellular necrosis in SA59R13 (a) and Penn98-1 (b). S4R21-infected mice (c) showed low levels of viral antigen staining. No signs of pathology or viral antigen were found in a mock-infected control (d). Magnification, ×40.
FIG. 5.
Immunohistochemistry of liver sections of C57BL/6 mice infected with the recombinant viruses SA59R13 (a), Penn98-1 (b), and S4R21 (c) and a mock-infected mouse (d) at day 5 p.i. Immunolabeling was performed as described for Fig. 4. Viral antigen staining was found in sinusoidal lining cells (consistent with endothelial and/or Kupfer cells) of livers infected with SA59R13 (a), Penn98-1 (b), and S4R21 (c). Viral antigen colocalized with focal necrosis areas in SA59R13 (a), confluent necrosis areas in Penn98-1 (b), and small clusters of hepatocytes in S4R21 (c). No signs of pathology or viral antigen were found in a mock-infected control (d). Magnification, ×200.
First, we found that the spike protein determines the degree of hepatitis (Fig. 3A). The degree of hepatitis induced by SA59R13 was less severe than the extreme hepatitis induced by Penn 98-1 (P < 0.05, Wilcoxon's rank sum test), whereas S4R21 induced mild hepatitis (SA59R13 versus S4R21, P < 0.05; Penn98-1 versus S4R21, P < 0.01; Wilcoxon's rank sum test). Second, we found a correlation between the viral replication titers and the degree of hepatitis induced by each virus (Spearman's correlation coefficient: r = 0.85, P < 0.001, Fig. 3B). Third, the extent of hepatocellular injury was also monitored by counting the number of nonconfluent necrotic foci per liver section, and this analysis also demonstrated a positive correlation between virus titers and the severity of hepatitis (Spearman's correlation coefficient: r = 0.88, P < 0.001) (Fig. 3C).
Figures 4 and 5 show immunohistochemistry staining of liver sections from SA59R13-, Penn98-1-, and S4R21-infected mice by day 5 p.i. At low magnification (Fig. 4), Penn98-1 showed evidence of extensive hepatocellular necrosis, whereas SA59R13 showed several areas of focal necrosis. The antigen staining always colocalized with the necrotic areas. In contrast, in S4R21-infected livers, the hepatic parenchyma appeared almost normal, with very low levels of antigen staining. Higher magnification (Fig. 5) revealed inflammatory cell infiltration associated with necrotic areas in the livers infected by SA59R13 and Penn98-1. Livers from animals infected with either SA59R13 or Penn98-1 also exhibited staining of sinusoidal lining cells (consistent with Kupfer or endothelial cells or both). In the case of Penn98-1-infected animals, hepatocellular degeneration and necrosis were so extreme that it was not possible to find isolated hepatocytes immunoreactive for viral antigen, while in the livers of SA59R13-infected animals, staining was observed both in necrotic foci and in isolated hepatocytes. Finally, livers from animals infected with S4R21 showed viral antigen staining in sinusoidal lining cells and small clusters of hepatocytes as well as in isolated hepatocytes. Little if any tissue destruction or inflammation was observed. This is consistent with the minimal level of virus replication observed with S4R21.
Thus, our results indicate that substitution of the spike gene of either MHV-2 or MHV-4 for the MHV-A59 spike gene within the A59 genome is sufficient to produce the hepatitis phenotype of the strain from which the spike is derived. This is not surprising, since the spike is responsible for viral attachment to the receptor, entry, and cell-to-cell fusion, and thus it may be expected to play a crucial role in initiation of infection as well as in spread of the virus. It is noteworthy that the S protein of MHV has recently been exchanged by targeted RNA recombination for the S protein of feline infectious peritonitis virus. The resulting chimeric virus acquired the ability to infect feline cells and lost the ability to infect murine cells, demonstrating that receptor utilization is a major factor in determining the host range of coronavirus infections (26).
It is likely that interaction of the spike and the viral receptor may play a role in the outcome of infection in the liver. We have previously shown that a one-amino-acid substitution, Q159L, within the amino-terminal region of the MHV-A59 spike, a region that has been demonstrated to bind to receptor in an vitro assay, results in the inability of the virus to replicate in the liver and induce hepatitis (32). This suggests that the ability to induce hepatitis may be regulated at the receptor level. Indeed, there are differences in the interaction of the MHV-A59 and MHV-4 spikes with viral receptors (46). The primary cellular receptor for MHV is a transmembrane glycoprotein of the murine biliary glycoprotein (Bgp) subfamily of the carcinoembryonic antigen family, also known as MHVR or Bgpla. Bgpla is found on epithelial and endothelial cells as well as on B lymphocytes and macrophages. Godfraind et al. (16) recently found that in the liver, Bgpla expression correlates with infection of hepatocytes and endothelial cells, leading to the development of hepatitis.
Both parenchyma cells (mostly hepatocytes) and sinusoidal lining cells (endothelial and/or Kupfer cells) express viral antigen after infection with all three viruses, and similar patterns of labeled sinusoidal lining vessel cells are found. The major difference among the strains lies in the hepatocellular degeneration and necrosis induced by each virus. Consistent with this is the staining of the hepatocytes. In liver sections from S4R21-infected mice, a few isolated labeled hepatocytes are observed. In the case of SA59R13-infected mice, there are many more labeled hepatocytes as well as antigen-positive necrotic foci. With Penn98-1, there are nearly confluent antigen-positive necrotic foci. These results suggest that endothelial and/or Kupfer cells may be infected by all three viruses but that spread into the parenchymal hepatocytes is greatly reduced with S4R21. We have previously observed that both MHV-A59 (19) and a very weakly hepatotropic strain (C12) (unpublished results) replicate to very similar low titers, suggesting that the hepatocytes are not a major site of replication for MHV-A59. By comparing the highly hepatotropic MHV-3 with MHV-4, it has been also demonstrated that replication in hepatic endothelial cells but not hepatocytes correlated with hepatotropism (22, 34, 37, 38). Our data are consistent with the notion that all three strains may infect the vessel cells but that replication and/or spread from these cells into hepatocytes might be much less efficient with MHV-4.
Belyarsky et al. (2) found that MHV-3, a strain that results in lethal fulminant hepatic necrosis in susceptible mice (9), is capable of inducing apoptosis in primary macrophage cultures. Schwartz et al. (40) have demonstrated that acute infection with MHV-A59, MHV-2, and the recombinant Penn98-1 induces apoptosis in hepatocytes and inflammatory cells. It is also known that the murine coronavirus spike contains both B-cell and T-cell epitopes (3, 4); thus, differences in spike sequences might have a major effect on the immune response. Interestingly, recent data obtained in various mouse hepatitis models for fulminant hepatitis indicate that Fas-mediated apoptosis plays an important role in the cytotoxicity induced by hepatitis virus-specific cytotoxic T lymphocytes against virus-infected hepatocytes (1, 24, 27). Taking all these data together, it may be argued that both apoptosis and the immune response play a role in MHV-induced hepatitis.
Finally, it should be noted that there is a noticeable difference in the pathogenesis of MHV in the liver and the CNS in that the extent of replication of the recombinant viruses in the CNS did not predict the amount of inflammation and disease or the virulence of the recombinant viruses. In the CNS, while S4R21 (and other MHV-4 spike-containing recombinant viruses) are approximately 1,000-fold more virulent than SA59R13 and other A59 spike-containing wild-type recombinant viruses and induce more inflammation and significantly more viral antigen, both viruses replicate to similar levels (39). In the liver, the amount of replication correlates with the extent of hepatitis. Thus, S4R21 and its parent MHV-4 replicate minimally in the liver and induce minimal hepatitis, while SA59R13 and its parent A59 replicate to a significant degree, causing an intermediate level of viral antigen expression and necrosis in the liver. Finally, Penn98-1 and its parent MHV-2 replicate to the highest levels in the liver and induce extensive hepatocellular necrosis.
Future studies will be directed at elucidating the mechanisms which control the ability of the spike to induce various levels of hepatitis. We are starting by mapping the regions of the spike protein that control hepatotropism.
Acknowledgments
We thank Julian L. Leibowitz for providing MAb clone 1-16-1.
This work was supported by Public Health Service grants NS-21954 and NS30606 (S.R.W.) and National Multiple Sclerosis Society grant RG-2615A1/2 (E.L.).
REFERENCES
- 1.Ando K, Moriyama T, Guidotti L G, Wirth S, Schreiber R D, Schlicht H J, Huang S N, Chisari F V. Mechanisms of class I restricted immunopathology: a transgenic mouse model of fulminant hepatitis. J Exp Med. 1993;178:1541–1554. doi: 10.1084/jem.178.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belyavskyi M, Belyavskaya E, Levy G A, Leibowitz J L. Coronavirus MHV-3-induced apoptosis in macrophages. Virology. 1998;250:41–49. doi: 10.1006/viro.1998.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann C C, Yao Q, Lin M, Stohlman S A. The JHM strain of mouse hepatitis virus induces a spike protein-specific Db-restricted cytotoxic T cell response. J Gen Virol. 1996;77:315–325. doi: 10.1099/0022-1317-77-2-315. [DOI] [PubMed] [Google Scholar]
- 4.Castro R F, Perlman S. CD8+ T-cell epitopes within the surface glycoprotein of a neurotropic coronavirus and correlation with pathogenicity. J Virol. 1995;69:8127–8131. doi: 10.1128/jvi.69.12.8127-8131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers P, Pringle C R, Easton A J. Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. J Gen Virol. 1990;71:3075–3080. doi: 10.1099/0022-1317-71-12-3075. [DOI] [PubMed] [Google Scholar]
- 6.Dalziel R G, Lampert P W, Talbot P J, Buchmeier M J. Site-specific alteration of murine hepatitis virus type 4 peplomer glycoprotein E2 results in reduced neurovirulence. J Virol. 1986;59:463–471. doi: 10.1128/jvi.59.2.463-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das Sarma J, Fu L, Tsai J C, Weiss S R, Lavi E. Demyelination determinants map to the spike glycoprotein gene of coronavirus mouse hepatitis virus. J Virol. 2000;74:9206–9213. doi: 10.1128/jvi.74.19.9206-9213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Groot R J, Luytjes W, Horzinek M C, van der Zeijst B A, Spaan W J, Lenstra J A. Evidence for a coiled-coil structure in the spike proteins of coronaviruses. J Mol Biol. 1987;196:963–966. doi: 10.1016/0022-2836(87)90422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding J W, Ning Q, Liu M F, Lai A, Leibowitz J, Peltekian K M, Cole E H, Fung L S, Holloway C, Marsden P A, Yeger H, Phillips M J, Levy G A. Fulminant hepatic failure in murine hepatitis virus strain 3 infection: tissue-specific expression of a novel fgl2 prothrombinase. J Virol. 1997;71:9223–9230. doi: 10.1128/jvi.71.12.9223-9230.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fazakerley J K, Parker S E, Bloom F, Buchmeier M J. The V5A13.1 envelope glycoprotein deletion mutant of mouse hepatitis virus type-4 is neuroattenuated by its reduced rate of spread in the central nervous system. Virology. 1992;187:178–188. doi: 10.1016/0042-6822(92)90306-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer F, Peng D, Hingley S T, Weiss S R, Masters P S. The internal open reading frame within the nucleocapsid gene of mouse hepatitis virus encodes a structural protein that is not essential for viral replication. J Virol. 1997;71:996–1003. doi: 10.1128/jvi.71.2.996-1003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer F, Stegen C F, Koetzner C A, Masters P S. Analysis of a recombinant mouse hepatitis virus expressing a foreign gene reveals a novel aspect of coronavirus transcription. J Virol. 1997;71:5148–5160. doi: 10.1128/jvi.71.7.5148-5160.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming J O, Trousdale M D, el-Zaatari F A, Stohlman S A, Weiner L P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J Virol. 1986;58:869–875. doi: 10.1128/jvi.58.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frana M F, Behnke J N, Sturman L S, Holmes K V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: host-dependent differences in proteolytic cleavage and cell fusion. J Virol. 1985;56:912–920. doi: 10.1128/jvi.56.3.912-920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher T M, Parker S E, Buchmeier M J. Neutralization-resistant variants of a neurotropic coronavirus are generated by deletions within the amino-terminal half of the spike glycoprotein. J Virol. 1990;64:731–741. doi: 10.1128/jvi.64.2.731-741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godfraind C, Coutelier J P. Morphological analysis of mouse hepatitis virus A59-induced pathology with regard to viral receptor expression. Histol Histopathol. 1998;13:181–199. doi: 10.14670/HH-13.181. [DOI] [PubMed] [Google Scholar]
- 17.Hingley S T, Gombold J L, Lavi E, Weiss S R. MHV-A59 fusion mutants are attenuated and display altered hepatotropism. Virology. 1994;200:1–10. doi: 10.1006/viro.1994.1156. [DOI] [PubMed] [Google Scholar]
- 18.Hingley S T, Gombold J L, Lavi E, Weiss S R. Hepatitis mutants of mouse hepatitis virus strain A59. Adv Exp Med Biol. 1995;380:577–582. doi: 10.1007/978-1-4615-1899-0_92. [DOI] [PubMed] [Google Scholar]
- 19.Hingley S T, Lepare-Goffart I, Weiss S R. The spike protein of murine coronavirus mouse hepatitis virus strain A59 is not cleaved in primary glial cells and primary hepatocytes. J Virol. 1998;72:1606–1609. doi: 10.1128/jvi.72.2.1606-1609.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirano N, Murakami T, Taguchi F, Fujiwara K, Matumoto M. Comparison of mouse hepatitis virus strains for pathogenicity in weanling mice infected by various routes. Arch Virol. 1981;70:69–73. doi: 10.1007/BF01320795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houtman J J, Fleming J O. Pathogenesis of mouse hepatitis virus-induced demyelination. J Neurovirol. 1996;2:361–376. doi: 10.3109/13550289609146902. [DOI] [PubMed] [Google Scholar]
- 22.Joseph J, Kim R, Siebert K, Lublin F D, Offenbach C, Knobler R L. Organ specific endothelial cell heterogeneity influences differential replication and cytopathogenicity of MHV-3 and MHV-4: implications in viral tropism. Adv Exp Med Biol. 1995;380:43–50. doi: 10.1007/978-1-4615-1899-0_6. [DOI] [PubMed] [Google Scholar]
- 23.Knobler R L, Haspel M V, Oldstone M B. Mouse hepatitis virus type 4 (JHM strains). induced fatal central nervous system disease. I. Genetic control and murine neuron as the susceptible site of disease. J Exp Med. 1981;153:832–843. doi: 10.1084/jem.153.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat Med. 1997;3:409–413. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 25.Kubo H, Yamada Y K, Taguchi F. Localization of neutralizing epitopes and the receptor-binding site within the amino-terminal 330 amino acids of the murine coronavirus spike protein. J Virol. 1994;68:5403–5410. doi: 10.1128/jvi.68.9.5403-5410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo L, Godeke G J, Raamsman M J, Masters P S, Rottier P J. Retargeting of coronavirus by substitution of the spike glycoprotein ectodomain: crossing the host cell species barrier. J Virol. 2000;74:1393–1406. doi: 10.1128/jvi.74.3.1393-1406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, Henrion A, Bouscary D, Varlet P, Joulin V, Kahn A. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med. 1996;2:80–86. doi: 10.1038/nm0196-80. [DOI] [PubMed] [Google Scholar]
- 28.Lamontagne L, Page C, Martin J P. T cell immunodeficiency involved in pathogenicity of attenuated MHV-3 mutants. Adv Exp Med Biol. 1995;380:189–191. doi: 10.1007/978-1-4615-1899-0_32. [DOI] [PubMed] [Google Scholar]
- 29.Lavi E, Gilden D H, Wroblewska Z, Rorke L B, Weiss S R. Experimental demyelination produced by the A59 strain of mouse hepatitis virus. Neurology. 1984;34:597–603. doi: 10.1212/wnl.34.5.597. [DOI] [PubMed] [Google Scholar]
- 30.Lavi E, Gilden D H, Highkin M K, Weiss S R. The organ tropism of mouse hepatitis virus A59 in mice is dependent on dose and route of inoculation. Lab Anim Sci. 1986;36:130–135. [PubMed] [Google Scholar]
- 31.Lepare-Goffart I, Hingley S T, Chua M M, Jiang X, Lavi E, Weiss S R. Altered pathogenesis of a mutant of the murine coronavirus MHV-A59 is associated with a Q159L amino acid substitution in the spike protein. Virology. 1997;239:1–10. doi: 10.1006/viro.1997.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lepare-Goffart I, Hingley S T, Chua M M, Phillips J J, Lavi E, Weiss S R. Targeted recombination within the spike gene of murine coronavirus mouse hepatitis virus-A59: Q159 is a determinant of hepatotropism. J Virol. 1998;72:9628–9636. doi: 10.1128/jvi.72.12.9628-9636.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luytjes W, Sturman L S, Bredenbeek P J, Charite J, van der Zeijst B A, Horzinek M C, Spaan W J. Primary structure of the glycoprotein E2 of coronavirus MHV-A59 and identification of the trypsin cleavage site. Virology. 1987;161:479–487. doi: 10.1016/0042-6822(87)90142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin J P, Chen W, Obert G, Koehren F. Characterization of attenuated mutants of MHV-3: importance of the E2 protein in organ tropism and infection of isolated liver cells. Adv Exp Med Biol. 1990;276:403–410. doi: 10.1007/978-1-4684-5823-7_55. [DOI] [PubMed] [Google Scholar]
- 35.Masters P S, Koetzner C A, Peng D, Parker M M, Ricard C S, Sturman L S. Site-directed mutagenesis of the genome of mouse hepatitis virus by targeted RNA recombination. Adv Exp Med Biol. 1993;342:143–148. doi: 10.1007/978-1-4615-2996-5_23. [DOI] [PubMed] [Google Scholar]
- 36.Parker S E, Gallagher T M, Buchmeier M J. Sequence analysis reveals extensive polymorphism and evidence of deletions within the E2 glycoprotein gene of several strains of murine hepatitis virus. Virology. 1989;173:664–673. doi: 10.1016/0042-6822(89)90579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira C A, Steffan A M, Kirn A. Kupffer and endothelial liver cell damage renders A/J mice susceptible to mouse hepatitis virus type 3. Virus Res. 1984;1:557–563. doi: 10.1016/0168-1702(84)90013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pereira C A, Steffan A M, Kirn A. Interaction between mouse hepatitis viruses and primary cultures of Kupffer and endothelial liver cells from resistant and susceptible inbred mouse strains. J Gen Virol. 1984;65:1617–1620. doi: 10.1099/0022-1317-65-9-1617. [DOI] [PubMed] [Google Scholar]
- 39.Phillips J J, Chua M M, Lavi E, Weiss S R. Pathogenesis of chimeric MHV4/MHV-A59 recombinant viruses: the murine coronavirus spike protein is a major determinant of neurovirulence. J Virol. 1999;73:7752–7760. doi: 10.1128/jvi.73.9.7752-7760.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz, T., L. Fu, and E. Lavi. Programmed cell death in MHV-induced demyelination. Adv. Exp. Med. Biol., in press. [DOI] [PubMed]
- 41.Suzuki H, Taguchi F. Analysis of the receptor-binding site of murine coronavirus spike protein. J Virol. 1996;70:2632–2636. doi: 10.1128/jvi.70.4.2632-2636.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wege H, Stephenson J R, Koga M, Wege H, ter Meulen V. Genetic variation of neurotropic and non-neurotropic murine coronaviruses. J Gen Virol. 1981;54:67–74. doi: 10.1099/0022-1317-54-1-67. [DOI] [PubMed] [Google Scholar]
- 43.Wege H, Winter J, Meyermann R. The peplomer protein E2 of coronavirus JHM as a determinant of neurovirulence: definition of critical epitopes by variant analysis. J Gen Virol. 1988;69:87–98. doi: 10.1099/0022-1317-69-1-87. [DOI] [PubMed] [Google Scholar]
- 44.Yamada Y K, Takimoto K, Yabe M, Taguchi F. Requirement of proteolytic cleavage of the murine coronavirus MHV-2 spike protein for fusion activity. Adv Exp Med Biol. 1998;440:89–93. doi: 10.1007/978-1-4615-5331-1_12. [DOI] [PubMed] [Google Scholar]
- 45.Yamada Y K, Takimoto K, Yabe M, Taguchi F. Acquired fusion activity of a murine coronavirus MHV-2 variant with mutations in the proteolytic cleavage site and the signal sequence of the S protein. Virology. 1997;227:215–219. doi: 10.1006/viro.1996.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zelus B D, Wessner D R, Williams R K, Pensiero M N, Phibbs F T, deSouza M, Dveksler G S, Holmes K V. Purified, soluble recombinant mouse hepatitis virus receptor, Bgp1(b), and Bgp2 murine coronavirus receptors differ in mouse hepatitis virus binding and neutralizing activities. J Virol. 1998;72:7237–7244. doi: 10.1128/jvi.72.9.7237-7244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]