Abstract
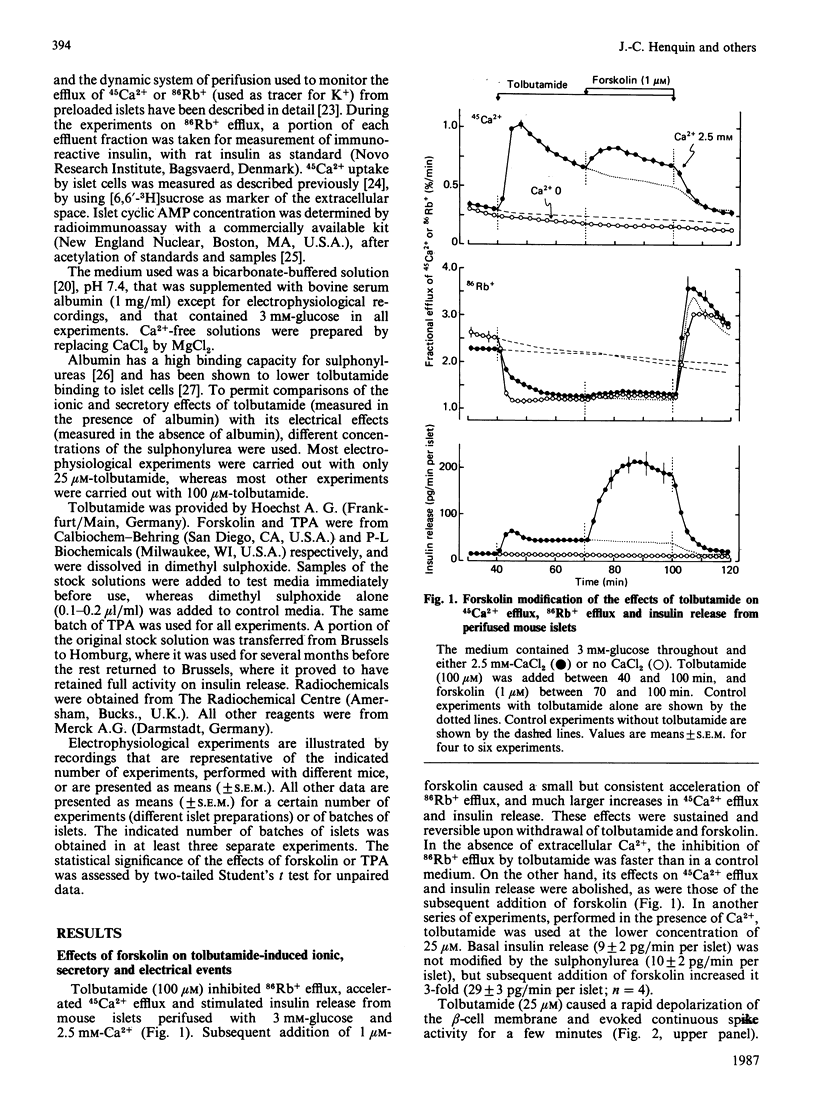
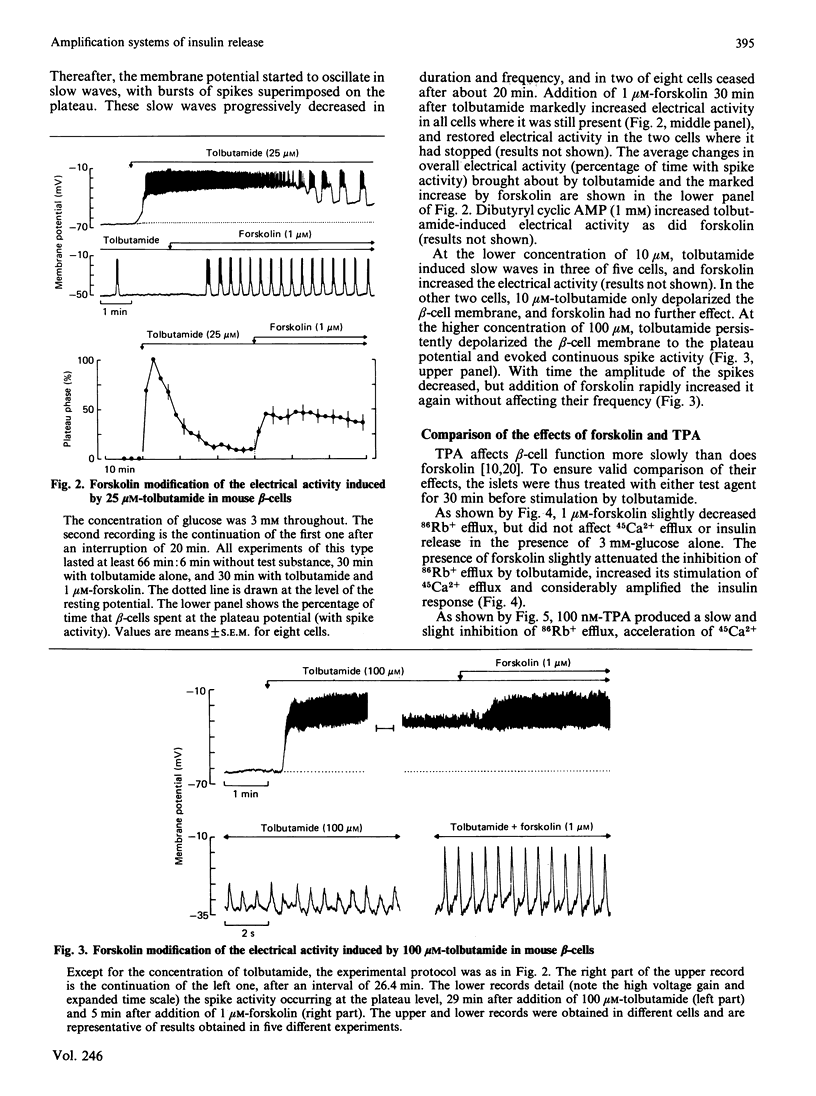
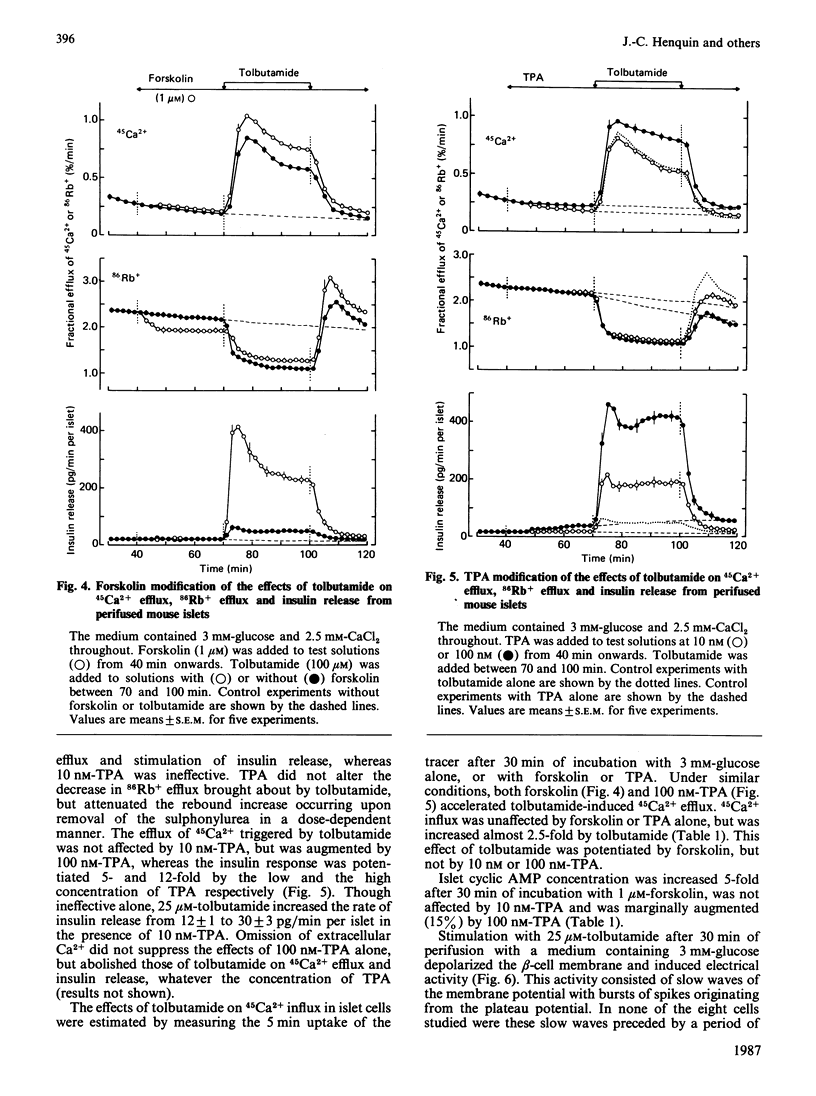
The mechanisms whereby activation of the cyclic AMP-dependent protein kinase A or the Ca2+-phospholipid-dependent protein kinase C amplifies insulin release were studied with mouse islets. Forskolin and the phorbol ester 12-O-tetradecanoylphorbol 13-acetate (TPA) were used to stimulate adenylate cyclase and protein kinase C respectively. The sulphonylurea tolbutamide was used to initiate insulin release in the presence of 3 mM-glucose. Tolbutamide alone inhibited 86Rb+ efflux, depolarized beta-cell membrane, triggered electrical activity, accelerated 45Ca2+ influx and efflux and stimulated insulin release. Forskolin alone only slightly inhibited 86Rb+ efflux, but markedly increased the effects of tolbutamide on electrical activity, 45Ca2+ influx and efflux, and insulin release. In the absence of Ca2+, only the inhibition of 86Rb+ efflux persisted. TPA (100 nM) alone slightly accelerated 45Ca2+ efflux and insulin release without affecting 45Ca2+ influx or beta-cell membrane potential. It increased the effects of tolbutamide on 45Ca2+ efflux and insulin release without changing 86Rb+ efflux, 45Ca2+ influx or electrical activity. Omission of extracellular Ca2+ suppressed all effects due to the combination of TPA and tolbutamide, but not those of TPA alone. Though ineffective alone, 10 nM-TPA amplified the releasing action of tolbutamide without affecting its ionic and electrical effects. In conclusion, the two amplification systems of insulin release involve at least partially distinct mechanisms. The cyclic AMP but not the protein kinase C system initiating signal (Ca2+ influx) triggered by the primary secretagogue.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkhammar P., Nilsson T., Berggren P. O. Stimulation of insulin release by the phorbol ester 12-O-tetradecanoylphorbol 13-acetate in the clonal cell line RINm5F despite a lowering of the free cytoplasmic Ca2+ concentration. Biochim Biophys Acta. 1986 Jul 11;887(2):236–241. doi: 10.1016/0167-4889(86)90060-1. [DOI] [PubMed] [Google Scholar]
- Christie M. R., Ashcroft S. J. Substrates for cyclic AMP-dependent protein kinase in islets of Langerhans. Studies with forskolin and catalytic subunit. Biochem J. 1985 May 1;227(3):727–736. doi: 10.1042/bj2270727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry D. L. Glucagon potentiation of insulin secretion by the perfused rat pancreas. Diabetes. 1970 Jun;19(6):420–428. doi: 10.2337/diab.19.6.420. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Pozzan T., Wollheim C. B., Vicentini L. M., Meldolesi J. Tumor promoter phorbol myristate acetate inhibits Ca2+ influx through voltage-gated Ca2+ channels in two secretory cell lines, PC12 and RINm5F. J Biol Chem. 1986 Jan 5;261(1):32–35. [PubMed] [Google Scholar]
- Eddlestone G. T., Oldham S. B., Lipson L. G., Premdas F. H., Beigelman P. M. Electrical activity, cAMP concentration, and insulin release in mouse islets of Langerhans. Am J Physiol. 1985 Jan;248(1 Pt 1):C145–C153. doi: 10.1152/ajpcell.1985.248.1.C145. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Ashcroft S. J., Christie M. R., Lord J. M. Protein phosphorylation in the pancreatic B-cell. Experientia. 1984 Oct 15;40(10):1075–1084. doi: 10.1007/BF01971454. [DOI] [PubMed] [Google Scholar]
- Hellman B. Calcium transport in pancreatic beta-cells: implications for glucose regulation of insulin release. Diabetes Metab Rev. 1986;2(3-4):215–241. doi: 10.1002/dmr.5610020302. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Effects of glucose and other modifiers of insulin release on the oxidative metabolism of amino acids in micro-dissected pancreatic islets. Biochem J. 1971 Jul;123(4):513–521. doi: 10.1042/bj1230513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Glibenclamide is exceptional among hypoglycaemic sulphonylureas in accumulating progressively in beta-cell-rich pancreatic islets. Acta Endocrinol (Copenh) 1984 Mar;105(3):385–390. doi: 10.1530/acta.0.1050385. [DOI] [PubMed] [Google Scholar]
- Hellman B. Tolbutamide stimulation of 45Ca fluxes in microdissected pancreatic islets rich in beta-cells. Mol Pharmacol. 1981 Jul;20(1):83–88. [PubMed] [Google Scholar]
- Henquin J. C. Effects of trifluoperazine and pimozide on stimulus-secretion coupling in pancreatic B-cells. Suggestion for a role of calmodulin? Biochem J. 1981 Jun 15;196(3):771–780. doi: 10.1042/bj1960771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C., Lambert A. E. Cobalt inhibition of insulin secretion and calcium uptake by isolated rat islets. Am J Physiol. 1975 Jun;228(6):1669–1677. doi: 10.1152/ajplegacy.1975.228.6.1669. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Cyclic adenosine monophosphate differently affects the response of mouse pancreatic beta-cells to various amino acids. J Physiol. 1986 Dec;381:77–93. doi: 10.1113/jphysiol.1986.sp016314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Effects of amino acids on membrane potential and 86Rb+ fluxes in pancreatic beta-cells. Am J Physiol. 1981 Mar;240(3):E245–E252. doi: 10.1152/ajpendo.1981.240.3.E245. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982 Apr 1;31(7):1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia. 1984 Oct 15;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. The ionic, electrical, and secretory effects of endogenous cyclic adenosine monophosphate in mouse pancreatic B cells: studies with forskolin. Endocrinology. 1984 Sep;115(3):1125–1134. doi: 10.1210/endo-115-3-1125. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Opposite effects of intracellular Ca2+ and glucose on K+ permeability of pancreatic islet cells. Nature. 1979 Jul 5;280(5717):66–68. doi: 10.1038/280066a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. The interplay between cyclic AMP and ions in the stimulus-secretion coupling in pancreatic B-cells. Arch Int Physiol Biochim. 1985 May;93(1):37–48. doi: 10.3109/13813458509104514. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Tolbutamide stimulation and inhibition of insulin release: studies of the underlying ionic mechanisms in isolated rat islets. Diabetologia. 1980;18(2):151–160. doi: 10.1007/BF00290493. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M., Cook D. L. Glucagon and forskolin have dual effects upon islet cell electrical activity. Life Sci. 1984 Aug 6;35(6):685–691. doi: 10.1016/0024-3205(84)90264-9. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Fyles J. M., Howell S. L. Regulation of insulin secretion by cAMP in rat islets of Langerhans permeabilised by high-voltage discharge. FEBS Lett. 1986 Sep 15;205(2):205–209. doi: 10.1016/0014-5793(86)80898-5. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Stutchfield J., Howell S. L. Effects of Ca2+ and a phorbol ester on insulin secretion from islets of Langerhans permeabilised by high-voltage discharge. FEBS Lett. 1985 Oct 21;191(1):102–106. doi: 10.1016/0014-5793(85)81002-4. [DOI] [PubMed] [Google Scholar]
- Judis J. Binding of sulfonylureas to serum proteins. J Pharm Sci. 1972 Jan;61(1):89–93. doi: 10.1002/jps.2600610116. [DOI] [PubMed] [Google Scholar]
- Lambert A. E., Jeanrenaud B., Junod A., Renold A. E. Organ culture of fetal rat pancreas. II. Insulin release induced by amino and organic acids, by hormonal peptides, by cationic alterations of the medium and by other agents. Biochim Biophys Acta. 1969 Sep 2;184(3):540–553. doi: 10.1016/0304-4165(69)90268-2. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Ashcroft S. J. Identification and characterization of Ca2+-phospholipid-dependent protein kinase in rat islets and hamster beta-cells. Biochem J. 1984 Apr 15;219(2):547–551. doi: 10.1042/bj2190547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J., Lebrun P., Herchuelz A., Sener A., Malaisse-Lagae F. Synergistic effect of a tumor-promoting phorbol ester and a hypoglycemic sulfonylurea upon insulin release. Endocrinology. 1983 Nov;113(5):1870–1877. doi: 10.1210/endo-113-5-1870. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F. The role of cyclic AMP in insulin release. Experientia. 1984 Oct 15;40(10):1068–1074. doi: 10.1007/BF01971453. [DOI] [PubMed] [Google Scholar]
- Meissner H. P. Electrical characteristics of the beta-cells in pancreatic islets. J Physiol (Paris) 1976 Nov;72(6):757–767. [PubMed] [Google Scholar]
- Meissner H. P., Schmelz H. Membrane potential of beta-cells in pancreatic islets. Pflugers Arch. 1974;351(3):195–206. doi: 10.1007/BF00586918. [DOI] [PubMed] [Google Scholar]
- Montague W., Howell S. L. Cyclic AMP and the physiology of the islets of Langerhans. Adv Cyclic Nucleotide Res. 1975;6:201–243. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Pace C. S., Goldsmith K. T. Action of a phorbol ester on B-cells: potentiation of stimulant-induced electrical activity. Am J Physiol. 1985 May;248(5 Pt 1):C527–C534. doi: 10.1152/ajpcell.1985.248.5.C527. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Abrahamsson H. Cyclic AMP potentiates glucose-induced insulin release from mouse pancreatic islets without increasing cytosolic free Ca2+. Acta Physiol Scand. 1985 Dec;125(4):639–647. doi: 10.1111/j.1748-1716.1985.tb07766.x. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Sharp G. W. The adenylate cyclase-cyclic AMP system in islets of Langerhans and its role in the control of insulin release. Diabetologia. 1979 May;16(5):287–296. doi: 10.1007/BF01223617. [DOI] [PubMed] [Google Scholar]
- Stutchfield J., Jones P. M., Howell S. L. The effects of polymyxin B, a protein kinase C inhibitor, on insulin secretion from intact and permeabilized islets of Langerhans. Biochem Biophys Res Commun. 1986 May 14;136(3):1001–1006. doi: 10.1016/0006-291x(86)90432-8. [DOI] [PubMed] [Google Scholar]
- Tamagawa T., Niki H., Niki A. Insulin release independent of a rise in cytosolic free Ca2+ by forskolin and phorbol ester. FEBS Lett. 1985 Apr 22;183(2):430–432. doi: 10.1016/0014-5793(85)80825-5. [DOI] [PubMed] [Google Scholar]
- Thams P., Capito K., Hedeskov C. J. Endogenous substrate proteins for Ca2+-calmodulin-dependent, Ca2+-phospholipid-dependent and cyclic AMP-dependent protein kinases in mouse pancreatic islets. Biochem J. 1984 Jul 1;221(1):247–253. doi: 10.1042/bj2210247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Ullrich S., Pozzan T. Glyceraldehyde, but not cyclic AMP-stimulated insulin release is preceded by a rise in cytosolic free Ca2+. FEBS Lett. 1984 Nov 5;177(1):17–22. doi: 10.1016/0014-5793(84)80972-2. [DOI] [PubMed] [Google Scholar]
- Zawalich W., Zawalich K., Rasmussen H. Insulin secretion: combined tolbutamide, forskolin and TPA mimic action of glucose. Cell Calcium. 1984 Dec;5(6):551–558. doi: 10.1016/0143-4160(84)90031-9. [DOI] [PubMed] [Google Scholar]


