Abstract
In an attempt to determine why high frequencies of circulating virus-specific CD8+ T cells are unable to control human immunodeficiency virus and simian immunodeficiency virus (SIV) replication, we assessed the functional nature of SIV-specific CD8+ lymphocytes. After vaccination and early after infection, nearly all tetramer-staining CD8+ cells produced gamma interferon in response to their specific stimulus. However, by 4 months postinfection with pathogenic SIVmac239, signs of functional impairment in the CD8+ T-cell compartment were detected which might prevent these T cells from efficiently controlling the infection during the chronic phase.
It is still unclear why the immune system is not able to clear an infection with immunodeficiency viruses. These lentiviruses appear to have devised multiple strategies to evade the immune response (reviewed in reference 26), including major histocompatibility complex class I downregulation (10) and escape from cytotoxic T lymphocyte (CTL) responses (1, 7, 9, 13, 17, 27). However, the maintenance of high frequencies of virus-specific cells against certain viral epitopes in human immunodeficiency virus (HIV)-infected humans (4, 23, 30) and simian immunodeficiency virus (SIV)-infected monkeys (19, 20) indicates that these epitopes are still being recognized and have not mutated. While the invention of tetramers (4) made it possible to detect these high frequencies of virus-specific cells in HIV and SIV infection, tetramer staining simply identifies antigen-specific lymphocytes but does not provide information about the functional nature of these virus-specific cells. Functionally impaired CD8+ T-cell responses have been previously described by Zajac et al. in chronic lymphocytic choriomeningitis virus (LCMV) infection (31) and by Lee et al. in a tumor system (22), where CTL were unable to directly lyse their specific target cells and produce cytokines in response to mitogens. We therefore were interested in investigating whether similar functional defects could account for the inability of CD8+ T cells to control HIV and SIV infections. To address this question we combined tetramer-staining technology (4) and the intracellular cytokine assay (18, 25) to determine whether SIV-specific CD8+ T cells manifest any functional defects.
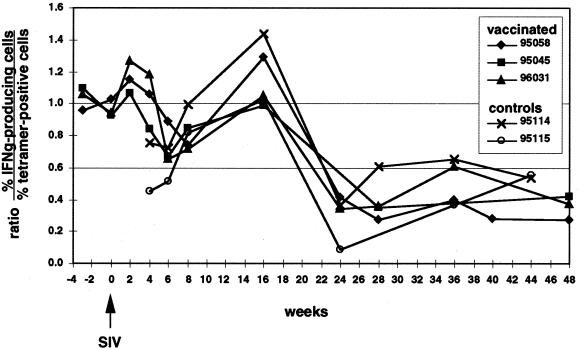
The majority of tetramer-positive cells produce IFN-γ after peptide-specific stimulation in vaccinated animals and early after infection with pathogenic SIVmac239.
Using an epitope-based DNA prime-modified vaccinia virus Ankara boost vaccine, we induced Mamu-A*01-restricted, p11C,C-M (CM9)-specific CTL (2) in three Mamu-A*01-positive rhesus macaques (95058, 95045, and 96031), as previously described (3). We followed the levels of Mamu-A*01/CM9-specific cells in these animals before and after intrarectal infection with SIVmac239 (molecular clone) by tetramer staining and investigated their ability to produce gamma interferon (IFN-γ) after antigen-specific stimulation using the intracellular cytokine assay. Fresh or thawed peripheral blood mononuclear cells (PBMCs) were stimulated for 6.5 h with mitogen (50 ng of phorbol myristate acetate/ml and 1 μg of ionomycin/ml) or with 5 μM CM9 peptide in the presence of B-lymphoblastoid cell line cells (B-LCL) as antigen-presenting cells. Brefeldin A (10 μg/ml) was present for the last 5 h to inhibit the secretion of any produced cytokines. The cells were then surface stained with anti-CD8 antibodies (conjugated to peridinin chlorophyll protein; Becton-Dickinson) alone or together with the Mamu-A*01/CM9 tetramer (labeled with phycoerythrin) for 40 min at room temperature. The cells were then washed with flow buffer (2% fetal calf serum in phosphate-buffered saline), fixed with paraformaldehyde (2% in phosphate-buffered saline) overnight, permeabilized with 0.1% saponin, and stained intracellularly with anti-IFN-γ antibodies (labeled with fluoroscein isothiocyanate; Pharmingen), as described previously (3). As the T-cell receptor is downregulated after antigen-specific stimulation (29), the tetramer staining of CM9-specific cells nearly completely disappeared following CM9-specific stimulation. Therefore, we were not able to express the percentage of tetramer-positive cells able to produce IFN-γ. However, we observed that in immunized animals and early after infection, the percentage of CD8+ cells expressing IFN-γ after peptide-specific stimulation correlated with the levels of tetramer staining in unstimulated samples (Fig. 1; see also reference 3). All tetramer-positive lymphocytes produced IFN-γ at weeks −3, 0, and 2 postchallenge (the ratio of IFN-γ-producing cells to tetramer-positive cells was approximately 1). We also observed this correlation between the intracellular cytokine assay and tetramer staining in other immunized animals and for another Mamu-A*01-restricted epitope (data not shown). Between weeks 6 to 8 postchallenge, some tetramer-positive cells from all three animals (95058, 95045, and 96031) were unable to produce IFN-γ (ratios below 1). However, by week 16 the ratios came back up to values around or higher than 1. As this drop was only temporary, it is not clear if this represents a significant change in phenotype and could indicate a functional impairment of tetramer-positive cells shortly after the primary peak of viremia reached its high point in week 3 (Allen et al., unpublished observations). However, the two naive control animals (95114 and 95115), which were infected with the same virus at the same time as the immunized animals (Allen et al., unpublished), also evidenced a reduced ratio during the time of primary viremia, which peaked at week 4 postchallenge. This may indicate an impaired ability of some tetramer-positive cells to produce IFN-γ shortly after peak viral replication was resolved in these animals. Nevertheless, the ratios returned to values of approximately 1 in week 16 in these two control animals (Fig. 1). These results indicate that the majority of tetramer-positive cells produce IFN-γ in response to stimulation with their cognate peptide up to 4 months after infection with pathogenic SIV.
FIG. 1.
Functional nature of tetramer-positive, CD8+ T cells. PBMCs were stimulated with the CM9 peptide for 6.5 h, with the last 5 h in the presence of BFA. The percentage of CD8+ cells producing IFN-γ (IFNg), as detected in the cytokine assay, was divided by the percentage of CD8-positive cells staining with the Mamu-A*01/CM9-tetramer before stimulation, to obtain the ratios plotted. Only values of >0.1% (above background) of CD8+ cells were considered positive for both the tetramer staining and the intracellular cytokine assay (see Fig. 2 legend). Ratios below 1 indicate that some tetramer-positive cells are unable to produce IFN-γ in response to specific peptide stimulation. Weeks −3, 6, 24, 28, 40, 44, and 48 were tested using thawed PBMCs, and samples from week 6, 28, 44, and 48 were all tested in one assay.
Discrepancy between tetramer staining and intracellular cytokine production after 4 months of infection with pathogenic SIVmac239.
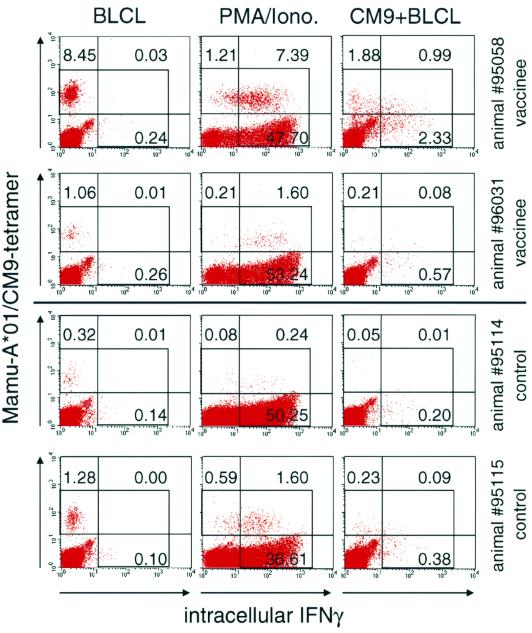
Unexpectedly, the number of tetramer-positive cells that produced IFN-γ in response to peptide-specific stimulation dropped dramatically after 4 months of infection (Fig. 1). The ratio of IFN-γ-positive cells to tetramer-positive cells dropped below 0.66 in all animals and remained relatively stable at these lower values thereafter. There was no obvious difference between previously immunized and naive control animals. The possibility that the reduced cytokine production is caused by a destruction of antigen-presenting cells was excluded, because B-LCL were added as antigen-presenting cells in the intracellular cytokine assay. It is, therefore, likely that the virus-specific cells during chronic SIV infection have defects in cytokine production in response to stimulation by their cognate peptide. Interestingly, we were able to demonstrate that most of the tetramer-positive cells in our SIV-infected macaques were still able to produce IFN-γ after mitogen stimulation at week 24 (data not shown) and at week 36 postchallenge (Fig. 2). This contrasts with the “silent” phenotype described by Zajac et al. in chronic LCMV infection (31) and by Lee et al. in a tumor system (22). Therefore, the defect of the virus-specific cells in our monkeys is not as prominent, at least at this early stage of infection with SIV, as the defect observed by Zajac and colleagues in LCMV-infected mice (31). It is interesting that Donahoe and colleagues found a good correlation of intracellular cytokine production after peptide-specific stimulation, in this case tumor necrosis factor alpha, and Mamu-A*01/CM9-tetramer staining in monkeys infected with an attenuated, nonpathogenic SIV, SIVmac239delta nef (11). It is possible that tetramer-positive cells in animals infected with an apathogenic SIV might not demonstrate a reduced ability of cytokine production as found in our animals, but this needs to be investigated further.
FIG. 2.
IFN-γ production in response to mitogen or peptide-specific stimulation at week 36 postchallenge. Tetramer-positive cells are still able to respond to mitogen stimulation, although they demonstrate reduced ability to respond to specific peptide stimulation at week 36 postchallenge. PBMCs were either stimulated with autologous B-LCL in the presence of a control peptide (B-LCL), with PMA/ionomycin (PMA/Iono.), or with autologous B-LCL in the presence of CM9 peptide (CM9+BLCL). Between 100,000 and 150,000 events were acquired, gated on small lymphocytes. Numbers within graphs indicate the percentage of CD8+ cells in each quadrant. The background for the tetramer staining, using samples from Mamu-A*01-positive, uninfected or Mamu-A*01-negative, infected animals, was below 0.08%. The gate for IFN-γ staining was set using an isotype-stained control, so that less than 0.1% of CD8+ cells fell into the positive gate.
Similarities to functional defects of LCMV- and HIV-specific immune responses.
Zajac and colleagues also showed that the silent phenotype of virus-specific CTL was especially prevalent when there was inadequate CD4 help. Infection with SIV or HIV does impair CD4 help (5, 12, 21, 24), and it may therefore not only result in a loss of virus-specific CD8 T-cell response over time (8, 15) but also result in a functional impairment of the virus-specific CTL response. Although the absolute numbers of CD4-positive cells per microliter of blood in our SIV-infected monkeys were continuously decreasing over time (data not shown), there was no obvious correlation between the drop in IFN-γ production in tetramer-positive cells with CD4 counts. A recent study by Gea-Banacloche and colleagues described an inability of 30 to 50% of tetramer-positive cells to produce IFN-γ in response to stimulation with recombinant vaccinia virus-infected B-LCL, which expressed HIV genes, in two HIV-infected patients (14), which is very similar to the defect of SIV-specific CD8+ T cells described here. In addition, Goepfert and colleagues have described a 10-fold difference between the number of peptide-specific cells as measured by tetramer staining or ELISPOT assay in HIV-infected patients (16). Therefore, as we have demonstrated here, SIV infection seems to cause a functional impairment of the specific immune response that is very similar to that which has been described for HIV infection (6, 14, 16, 28). The SIV/rhesus monkey model is well suited for investigating the mechanism behind the impairment of the virus-specific immune response. Finally, our finding that not all antigen-specific CD8+ lymphocytes effectively produce IFN-γ during the chronic phase of infection has implications for the measurement of antigen-specific CD8+ lymphocytes during chronic SIV infection. Intracellular staining for IFN-γ might result in the underestimation of antigen-specific CD8+ lymphocytes in the chronic phase of SIV infection if this is the sole assay employed. Multiple assays, therefore, need to be employed when assessing antiviral immune responses in chronically infected macaques and humans.
Acknowledgments
This work was supported by grants AI42512, AI41913, and RR00167. D.I.W. is a recipient of an Elizabeth Glaser Scientist award.
REFERENCES
- 1.Allen T M, O'Connor D H, Peicheng J, Dzuris J L, Mothé B R, Vogel T U, Dunphy E, Liebl M E, Emerson C, Wilson N, Kunstman K J, Wang X, Allison D B, Hughes A L, Desrosiers R C, Altman J D, Wolinsky S M, Sette A, Watkins D I. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viremia. Nature. 2000;407:386–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- 2.Allen T M, Sidney J, del Guercio M F, Glickman R L, Lensmeyer G L, Wiebe D A, DeMars R, Pauza C D, Johnson R P, Sette A, Watkins D I. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J Immunol. 1998;160:6062–6071. [PubMed] [Google Scholar]
- 3.Allen T M, Vogel T U, Fuller D H, Mothe B R, Steffen S, Boyson J E, Shipley T, Fuller J, Hanke T, Sette A, Altman J D, Moss B, McMichael A J, Watkins D I. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J Immunol. 2000;164:4968–4978. doi: 10.4049/jimmunol.164.9.4968. [DOI] [PubMed] [Google Scholar]
- 4.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 5.Ameisen J C, Capron A. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol Today. 1991;12:102–105. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 6.Appay V, Nixon D F, Donahoe S M, Gillespie G M, Dong T, King A, Ogg G S, Spiegel H M, Conlon C, Spina C A, Havlir D V, Richman D D, Waters A, Easterbrook P, McMichael A J, Rowland-Jones S L. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–76. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 8.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z W, Craiu A, Shen L, Kuroda M J, Iroku U C, Watkins D I, Voss G, Letvin N L. Simian immunodeficiency virus evades a dominant epitope-specific cytotoxic T lymphocyte response through a mutation resulting in the accelerated dissociation of viral peptide and MHC class I. J Immunol. 2000;164:6474–6479. doi: 10.4049/jimmunol.164.12.6474. [DOI] [PubMed] [Google Scholar]
- 10.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 11.Donahoe S M, Moretto W J, Samuel R V, Marx P A, Hanke T, Connor R I, Nixon D F. Direct measurement of CD8+ T cell responses in macaques infected with simian immunodeficiency virus. Virology. 2000;272:347–356. doi: 10.1006/viro.2000.0404. [DOI] [PubMed] [Google Scholar]
- 12.Epstein J S, Frederick W R, Rook A H, Jackson L, Manischewitz J F, Mayner R E, Masur H, Enterline J C, Djeu J Y, Quinnan G V., Jr Selective defects in cytomegalovirus- and mitogen-induced lymphocyte proliferation and interferon release in patients with acquired immunodeficiency syndrome. J Infect Dis. 1985;152:727–733. doi: 10.1093/infdis/152.4.727. [DOI] [PubMed] [Google Scholar]
- 13.Evans D T, O'Connor D H, Jing P, Dzuris J L, Sidney J, da Silva J, Allen T M, Horton H, Venham J E, Rudersdorf R A, Vogel T, Pauza C D, Bontrop R E, DeMars R, Sette A, Hughes A L, Watkins D I. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat Med. 1999;5:1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- 14.Gea-Banacloche J C, Migueles S A, Martino L, Shupert W L, McNeil A C, Sabbaghian M S, Ehler L, Prussin C, Stevens R, Lambert L, Altman J, Hallahan C W, de Quiros J C, Connors M. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J Immunol. 2000;165:1082–1092. doi: 10.4049/jimmunol.165.2.1082. [DOI] [PubMed] [Google Scholar]
- 15.Geretti A M, Dings M E, van Els C A, van Baalen C A, Wijnholds F J, Borleffs J C, Osterhaus A D. Human immunodeficiency virus type 1 (HIV-1) and Epstein-Barr virus-specific cytotoxic T lymphocyte precursors exhibit different kinetics in HIV-1-infected persons. J Infect Dis. 1996;174:34–45. doi: 10.1093/infdis/174.1.34. [DOI] [PubMed] [Google Scholar]
- 16.Goepfert P A, Bansal A, Edwards B H, Ritter G D, Jr, Tellez I, McPherson S A, Sabbaj S, Mulligan M J. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J Virol. 2000;74:10249–10255. doi: 10.1128/jvi.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goulder P J, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 18.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda M J, Schmitz J E, Barouch D H, Craiu A, Allen T M, Sette A, Watkins D I, Forman M A, Letvin N L. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J Exp Med. 1998;187:1373–1381. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuroda M J, Schmitz J E, Charini W A, Nickerson C E, Lord C I, Forman M A, Letvin N L. Comparative analysis of cytotoxic T lymphocytes in lymph nodes and peripheral blood of simian immunodeficiency virus-infected rhesus monkeys. J Virol. 1999;73:1573–1579. doi: 10.1128/jvi.73.2.1573-1579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane H C, Depper J M, Greene W C, Whalen G, Waldmann T A, Fauci A S. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985;313:79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- 22.Lee P P, Yee C, Savage P A, Fong L, Brockstedt D, Weber J S, Johnson D, Swetter S, Thompson J, Greenberg P D, Roederer M, Davis M M. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 23.Moss P A, Rowland-Jones S L, Frodsham P M, McAdam S, Giangrande P, McMichael A J, Bell J I. Persistent high frequency of human immunodeficiency virus-specific cytotoxic T cells in peripheral blood of infected donors. Proc Natl Acad Sci USA. 1995;92:5773–5777. doi: 10.1073/pnas.92.13.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray H W, Rubin B Y, Masur H, Roberts R B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- 25.Picker L J, Singh M K, Zdraveski Z, Treer J R, Waldrop S L, Bergstresser P R, Maino V C. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–1419. [PubMed] [Google Scholar]
- 26.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 27.Price D A, Goulder P J, Klenerman P, Sewell A K, Easterbrook P J, Troop M, Bangham C R, Phillips R E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trimble L A, Lieberman J. Circulating CD8 T lymphocytes in human immunodeficiency virus-infected individuals have impaired function and downmodulate CD3 zeta, the signaling chain of the T-cell receptor complex. Blood. 1998;91:585–594. [PubMed] [Google Scholar]
- 29.Valitutti S, Muller S, Dessing M, Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J Exp Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson J D, Ogg G S, Allen R L, Goulder P J, Kelleher A, Sewell A K, O'Callaghan C A, Rowland-Jones S L, Callan M F, McMichael A J. Oligoclonal expansions of CD8(+) T cells in chronic HIV infection are antigen specific. J Exp Med. 1998;188:785–790. doi: 10.1084/jem.188.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J, Suresh M, Altman J D, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]