Abstract
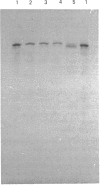
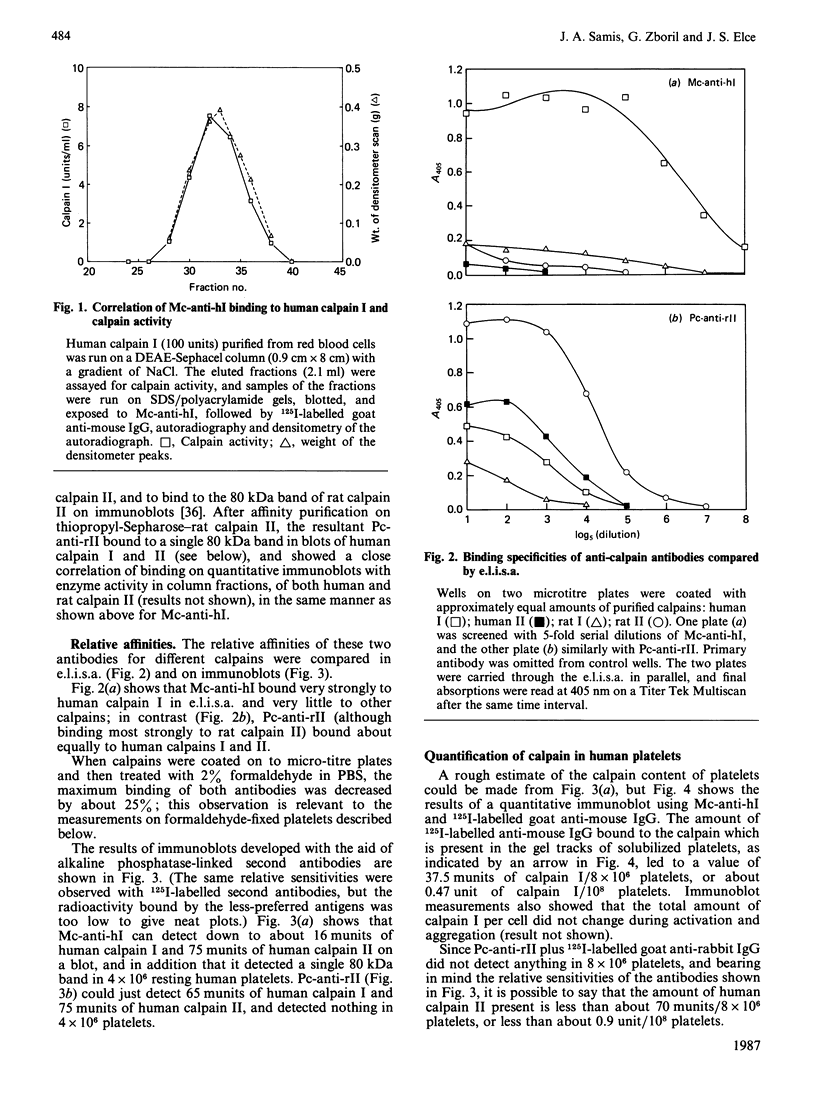
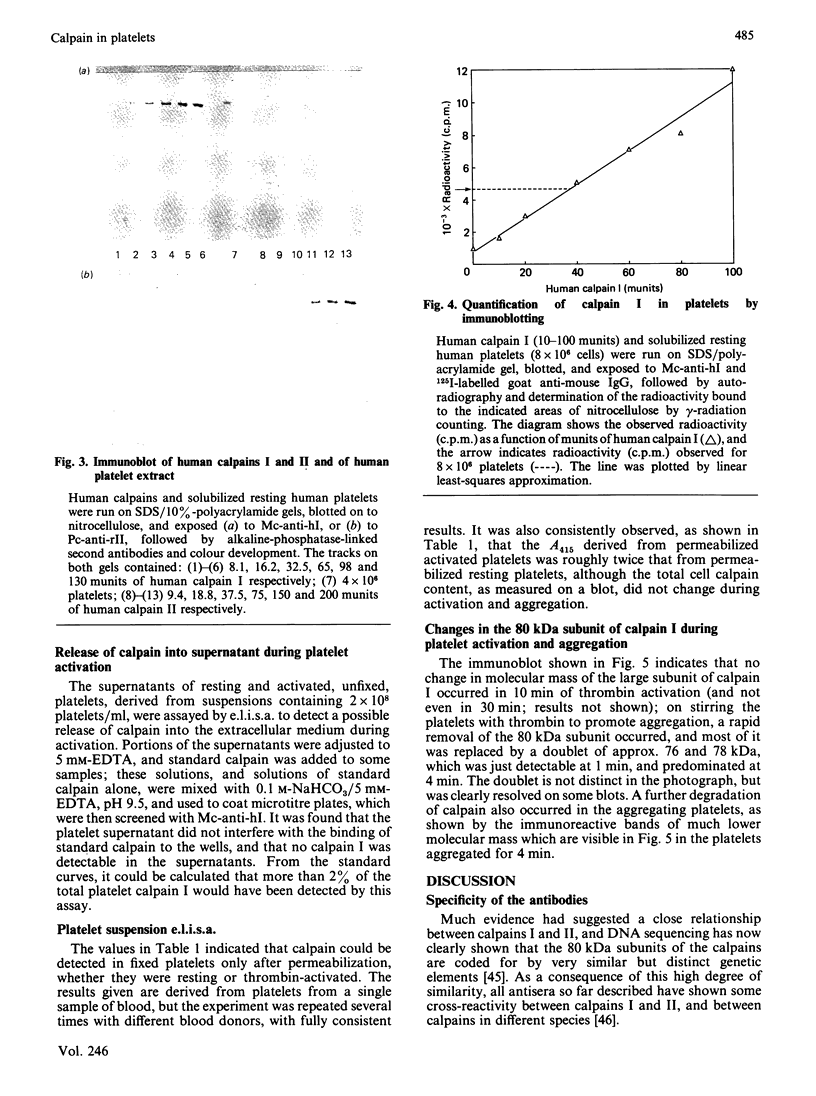
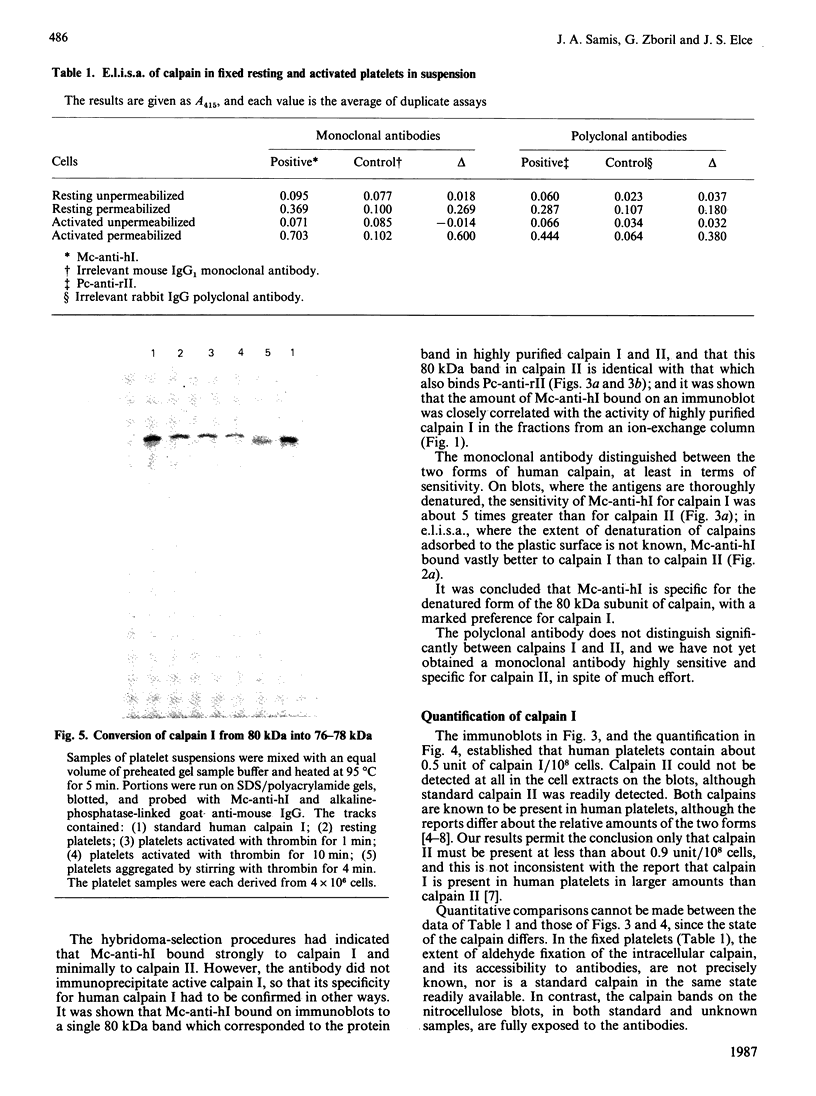
As a step towards understanding the physiological function of calpain (Ca2+-activated neutral proteinase, EC 3.4.22.17) in blood platelets, and in view of some suggestions that calpain is transferred to the platelet external surface during platelet activation, the enzyme was studied with immunochemical methods in resting and thrombin-activated cells. (1) A mouse IgG1 monoclonal antibody was prepared which binds strongly only to the denatured large subunit of human calpain I, and weakly to that of human calpain II. A polyclonal antibody raised against rat calpain II was available which, apart from binding strongly to rat calpain II, binds to the large subunits of human calpain I and II about equally. (2) With these antibodies, it was found that calpain could be detected in fixed platelets in suspension only after permeabilization with 0.1% saponin, and could not be detected on the exterior surface of resting or of activated platelets, or in the supernatant media of these platelets. It was concluded that calpain is not significantly externalized during platelet activation. (3) Immunoblotting showed that conversion of the larger calpain I subunit from 80 kDa into 76-78 kDa occurred only when thrombin-activated platelets were stirred to permit aggregation, and did not occur during unstirred thrombin activation. Although an action of calpain in the 80 kDa form on possible platelet substrates such as cytoskeletal proteins cannot be excluded, calpain is certainly not present as the 76-78 kDa form, which is assumed to be its active form, until aggregation is initiated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldassare J. J., Bakshian S., Knipp M. A., Fisher G. J. Inhibition of fibrinogen receptor expression and serotonin release by leupeptin and antipain. J Biol Chem. 1985 Sep 5;260(19):10531–10535. [PubMed] [Google Scholar]
- Collier N. C., Wang K. Purification and properties of human platelet P235. A high molecular weight protein substrate of endogenous calcium-activated protease(s). J Biol Chem. 1982 Jun 25;257(12):6937–6943. [PubMed] [Google Scholar]
- Coolican S. A., Haiech J., Hathaway D. R. The role of subunit autolysis in activation of smooth muscle Ca2+-dependent proteases. J Biol Chem. 1986 Mar 25;261(9):4170–4176. [PubMed] [Google Scholar]
- Coolican S. A., Hathaway D. R. Effect of L-alpha-phosphatidylinositol on a vascular smooth muscle Ca2+-dependent protease. Reduction of the Ca2+ requirement for autolysis. J Biol Chem. 1984 Oct 10;259(19):11627–11630. [PubMed] [Google Scholar]
- Dayton W. R. Comparison of low- and high-calcium-requiring forms of the calcium-activated protease with their autocatalytic breakdown products. Biochim Biophys Acta. 1982 Dec 20;709(2):166–172. doi: 10.1016/0167-4838(82)90457-5. [DOI] [PubMed] [Google Scholar]
- DeMartino G. N., Huff C. A., Croall D. E. Autoproteolysis of the small subunit of calcium-dependent protease II activates and regulates protease activity. J Biol Chem. 1986 Sep 15;261(26):12047–12052. [PubMed] [Google Scholar]
- Elce J. S., Baenziger J. E., Young D. C. Ca2+-activated proteinase in the rat. Quantification by immunoassay in the uterus during pregnancy and involution, and in other tissues. Biochem J. 1984 Jun 1;220(2):507–512. doi: 10.1042/bj2200507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emori Y., Kawasaki H., Sugihara H., Imajoh S., Kawashima S., Suzuki K. Isolation and sequence analyses of cDNA clones for the large subunits of two isozymes of rabbit calcium-dependent protease. J Biol Chem. 1986 Jul 15;261(20):9465–9471. [PubMed] [Google Scholar]
- Feinstein M. B. Release of intracellular membrane-bound calcium precedes the onset of stimulus-induced exocytosis in platelets. Biochem Biophys Res Commun. 1980 Mar 28;93(2):593–600. doi: 10.1016/0006-291x(80)91119-5. [DOI] [PubMed] [Google Scholar]
- Fisher G. J., Bakshian S., Baldassare J. J. Activation of human platelets by ADP causes a rapid rise in cytosolic free calcium without hydrolysis of phosphatidylinositol-4,5-bisphosphate. Biochem Biophys Res Commun. 1985 Jun 28;129(3):958–964. doi: 10.1016/0006-291x(85)91984-9. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Goll D. E., Reynolds C. C., Phillips D. R. Identification of two proteins (actin-binding protein and P235) that are hydrolyzed by endogenous Ca2+-dependent protease during platelet aggregation. J Biol Chem. 1985 Jan 25;260(2):1060–1066. [PubMed] [Google Scholar]
- Fox J. E., Reynolds C. C., Phillips D. R. Calcium-dependent proteolysis occurs during platelet aggregation. J Biol Chem. 1983 Aug 25;258(16):9973–9981. [PubMed] [Google Scholar]
- Gache Y., Landon F., Touitou H., Olomucki A. Susceptibility of platelet alpha-actinin to a Ca2+-activated neutral protease. Biochem Biophys Res Commun. 1984 Nov 14;124(3):877–881. doi: 10.1016/0006-291x(84)91039-8. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Barsky S. H. Quantitation of tissue calpain activity after isolation by hydrophobic chromatography. Anal Biochem. 1985 Aug 1;148(2):413–423. doi: 10.1016/0003-2697(85)90247-7. [DOI] [PubMed] [Google Scholar]
- Kamakura K., Ishiura S., Sugita H. Mu-type calcium-activated neutral protease in the rat peripheral nerve. J Neurosci Res. 1986;15(2):167–173. doi: 10.1002/jnr.490150206. [DOI] [PubMed] [Google Scholar]
- Karlsson J. O., Gustavsson S., Hall C., Nilsson E. A simple one-step procedure for the separation of calpain I, calpain II and calpastatin. Biochem J. 1985 Oct 1;231(1):201–204. doi: 10.1042/bj2310201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlero de Rosbo N., Carnegie P. R., Bernard C. C. Quantitative electroimmunoblotting study of the calcium-activated neutral protease in human myelin. J Neurochem. 1986 Oct;47(4):1007–1012. doi: 10.1111/j.1471-4159.1986.tb00713.x. [DOI] [PubMed] [Google Scholar]
- Kunicki T. J., Montgomery R. R., Schullek J. Cleavage of human von Willebrand factor by platelet calcium-activated protease. Blood. 1985 Feb;65(2):352–356. [PubMed] [Google Scholar]
- Kunicki T. J., Mosesson M. W., Pidard D. Cleavage of fibrinogen by human platelet calcium-activated protease. Thromb Res. 1984 Jul 15;35(2):169–182. doi: 10.1016/0049-3848(84)90212-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lämmle B., Berrettini M., Schwarz H. P., Heeb M. J., Griffin J. H. Quantitative immunoblotting assay of blood coagulation factor XII. Thromb Res. 1986 Mar 15;41(6):747–759. doi: 10.1016/0049-3848(86)90373-7. [DOI] [PubMed] [Google Scholar]
- McGowan E. B., Yeo K. T., Detwiler T. C. The action of calcium-dependent protease on platelet surface glycoproteins. Arch Biochem Biophys. 1983 Nov;227(1):287–301. doi: 10.1016/0003-9861(83)90373-9. [DOI] [PubMed] [Google Scholar]
- Murachi T. The proteolytic system involving calpains. Biochem Soc Trans. 1985 Dec;13(6):1015–1018. doi: 10.1042/bst0131015. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Ardlie N. G., Packham M. A. Preparation of suspensions of washed platelets from humans. Br J Haematol. 1972 Feb;22(2):193–204. doi: 10.1111/j.1365-2141.1972.tb08800.x. [DOI] [PubMed] [Google Scholar]
- O'Halloran T., Beckerle M. C., Burridge K. Identification of talin as a major cytoplasmic protein implicated in platelet activation. Nature. 1985 Oct 3;317(6036):449–451. doi: 10.1038/317449a0. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Jakábová M. Ca2+-dependent protease in human platelets. Specific cleavage of platelet polypeptides in the presence of added Ca2+. J Biol Chem. 1977 Aug 25;252(16):5602–5605. [PubMed] [Google Scholar]
- Rink T. J., Smith S. W., Tsien R. Y. Cytoplasmic free Ca2+ in human platelets: Ca2+ thresholds and Ca-independent activation for shape-change and secretion. FEBS Lett. 1982 Nov 1;148(1):21–26. doi: 10.1016/0014-5793(82)81234-9. [DOI] [PubMed] [Google Scholar]
- Schmaier A. H., Bradford H., Silver L. D., Farber A., Scott C. F., Schutsky D., Colman R. W. High molecular weight kininogen is an inhibitor of platelet calpain. J Clin Invest. 1986 May;77(5):1565–1573. doi: 10.1172/JCI112472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaier A. H., Smith P. M., Purdon A. D., White J. G., Colman R. W. High molecular weight kininogen: localization in the unstimulated and activated platelet and activation by a platelet calpain(s). Blood. 1986 Jan;67(1):119–130. [PubMed] [Google Scholar]
- Schollmeyer J. E. Role of Ca2+ and Ca2+-activated protease in myoblast fusion. Exp Cell Res. 1986 Feb;162(2):411–422. doi: 10.1016/0014-4827(86)90346-0. [DOI] [PubMed] [Google Scholar]
- Solum N. O., Olsen T. M. Effects of diamide and dibucaine on platelet glycoprotein Ib, actin-binding protein and cytoskeleton. Biochim Biophys Acta. 1985 Jul 25;817(2):249–260. doi: 10.1016/0005-2736(85)90026-4. [DOI] [PubMed] [Google Scholar]
- Stenberg P. E., Shuman M. A., Levine S. P., Bainton D. F. Redistribution of alpha-granules and their contents in thrombin-stimulated platelets. J Cell Biol. 1984 Feb;98(2):748–760. doi: 10.1083/jcb.98.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy P. B., Nesheim M. E., Mann K. G. Proteolytic alterations of factor Va bound to platelets. J Biol Chem. 1983 Jan 10;258(1):662–669. [PubMed] [Google Scholar]
- Truglia J. A., Stracher A. Purification and characterization of a calcium dependent sulfhydryl protease from human platelets. Biochem Biophys Res Commun. 1981 May 29;100(2):814–822. doi: 10.1016/s0006-291x(81)80247-1. [DOI] [PubMed] [Google Scholar]
- Tsujinaka T., Sakon M., Kambayashi J., Kosaki G. Cleavage of cytoskeletal proteins by two forms of Ca2+ activated neutral proteases in human platelets. Thromb Res. 1982 Oct 15;28(2):149–156. doi: 10.1016/0049-3848(82)90257-2. [DOI] [PubMed] [Google Scholar]
- Tsujinaka T., Shiba E., Kambayashi J., Kosaki G. Purification and characterization of a low calcium requiring form of Ca2+-activated neutral protease from human platelets. Biochem Int. 1983 Jan;6(1):71–80. [PubMed] [Google Scholar]
- White G. C., 2nd Calcium-dependent proteins in platelets: response of calcium-activated protease in normal and thrombasthenic platelets to aggregating agents. Biochim Biophys Acta. 1980 Aug 1;631(1):130–138. doi: 10.1016/0304-4165(80)90061-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Kosaki G., Suzuki K., Tanoue K., Yamazaki H. Cleavage site of calcium-dependent protease in human platelet membrane glycoprotein Ib. Thromb Res. 1986 Jul 1;43(1):41–55. doi: 10.1016/0049-3848(86)90043-5. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Weksler B., Nachman R. Purification of human platelet calcium-activated protease. Effect on platelet and endothelial function. J Biol Chem. 1983 Jun 10;258(11):7168–7174. [PubMed] [Google Scholar]