ABSTRACT
The decline in bone mineral density (BMD) poses a significant concern for postmenopausal women with obesity. Research indicates that aerobic exercises show potential for enhancing bone health. However, there remains no consensus regarding their effects on BMD. This study aimed to evaluate the effect of various exercise interventions on BMD and overall health among postmenopausal women with obesity, with particular attention to caloric restriction (CR). Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines, we performed a comprehensive literature search on PubMed, targeting studies published up to August 2023. Our search focused on aerobic exercise, resistance training, and combined exercise modalities, examining their impact on BMD, body composition, and physical fitness in postmenopausal women with obesity. We reviewed 11 studies, predominantly on aerobic exercise, involving women who are overweight and sedentary, nine of which were randomized trials. Our findings suggest that aerobic exercise has a mild protective effect on BMD and can significantly reduce fat mass. Notably, when combined with CR, aerobic exercise not only enhances the reduction of fat tissue mass but also potentially offers a certain level of protection for BMD. Additionally, the intervention combining aerobic exercise with resistance training emerges as a key promoter of bone health, underscoring the importance of tailored exercise programs for this population. Consequently, balanced dietary patterns (like the Mediterranean diet), combined with exercise, are recommended for optimal health outcomes. Tailored exercise programs integrating both aerobic and resistance training are crucial for sustaining overall health and bone density in this population.
KEYWORDS: Bone density, Dietary carbohydrate restriction, Exercise, Obesity, Postmenopause
INTRODUCTION
Aerobic exercises, such as walking, jogging, cycling, and swimming, are widely acknowledged for their cardiovascular benefits and positive impact on bone health [1]. During physical activities, muscular contractions exert mechanical stress on the skeletal structure they are attached to, resulting in profound effects on bone tissue at various molecular levels. For instance, this mechanical stimulus triggers an increase in the secretion of osteoblastic interleukin-11, promoting enhanced osteogenesis and mitigating the detrimental effects of various etiological agents precipitating bone demineralization [2]. Moreover, given the inherent interconnection between physical activity and skeletal integrity, exercise assumes heightened significance in the preservation of bone health [3]. Different types of exercise have varying effects on bone health. Specifically, weight-bearing aerobic activities have been found to benefit bone density, especially in patients with osteoporosis, whereas walking alone, without additional physical activity interventions, may not increase bone mass but shows the potential to slow its decline [4]. Additionally, the intensity of physical activities plays a crucial role in improving bone health. Previous studies have shown that high-intensity exercise is a more effective stimulus for lumbar spine bone mineral density (BMD) than low or moderate intensity [5,6]. Furthermore, another study suggests that a higher frequency of exercise (≥2 sessions per week) is more effective in increasing lumbar spine BMD compared to a lower training frequency (between 1 and <2 sessions per week) [6]. Thus, this indicates that exercises varying in intensity and type play a critical role in mitigating bone mass loss and enhancing muscle strength and balance [4]. Extensive research, including a comprehensive review of 108 randomized controlled trials (RCTs), supports this, demonstrating the effectiveness of balance and functional exercise programs in reducing falls among the older population [7]. Moreover, investigations focusing on postmenopausal women have revealed a relationship between physical activity and the risk of fractures, highlighting the need for further research into sedentary behaviors and their effects on bone health [8].
Furthermore, an exercise regimen not only contributes to the BMD of postmenopausal women but also offers numerous health benefits for premenopausal women, encompassing both bone-related and non-bone-related benefits. Previous studies have suggested that moderate-to-vigorous aerobic exercise can influence estrogen metabolism, potentially reducing the risk of breast cancer [9]. Similarly, research indicates that exercise during childhood and adolescence is vital for maximizing peak bone mass and delaying the onset of osteoporosis, emphasizing the importance of early-life interventions [10].
Despite the benefits of exercise regimens, obesity and sedentary lifestyles are of significant concern among women in modern developed nations. The impact of obesity and weight-reducing dietary practices on bone health has garnered considerable attention in recent years. Recent research challenges the notion that fat mass is beneficial for bone health, pointing to an increased risk of fractures in individuals with obesity, possibly due to the detrimental effects of adipose tissue, particularly visceral adipose tissue [11]. As a result, the intricate link between obesity, chronic conditions, and bone health underscores the need for in-depth research to decipher their complex interactions [11,12]. Additionally, the influence of diet, specifically ketogenic diets and caloric restriction (CR) diets, on bone health and body composition has been extensively investigated [13,14,15]. While ketogenic diets are associated with weight loss and improved serum Vitamin D and parathyroid hormone levels, their impact on bone turnover remains unclear [13], thus highlighting the critical need to understand the subtle effects of caloric intake on body composition and BMD. Regarding CR diets, there is currently no consensus on their impact on BMD. While some literature suggests that these diets effectively reduce body weight and visceral adipose tissue, others suggest that they may also result in the loss of bone mass and other bodily constituents [14,15]. Likewise, another study [16] argues that CR diets do not significantly affect BMD, further highlighting the importance of investigating the interactive relationship between CR diets and exercise regimens in relation to BMD.
Aerobic exercise, commonly utilized for weight loss, raises significant concerns regarding bone health, especially when combined with CR. However, there remains a lack of consensus on the overall impact of aerobic exercise on BMD, particularly for postmenopausal women with obesity undergoing weight loss, who face a higher risk of substantial bone loss. This study aimed to perform a narrative review of existing literature to assess the impact of aerobic exercise and CR on BMD, body composition, and associated physical performance in postmenopausal women who are overweight and sedentary.
METHODS
Search strategy
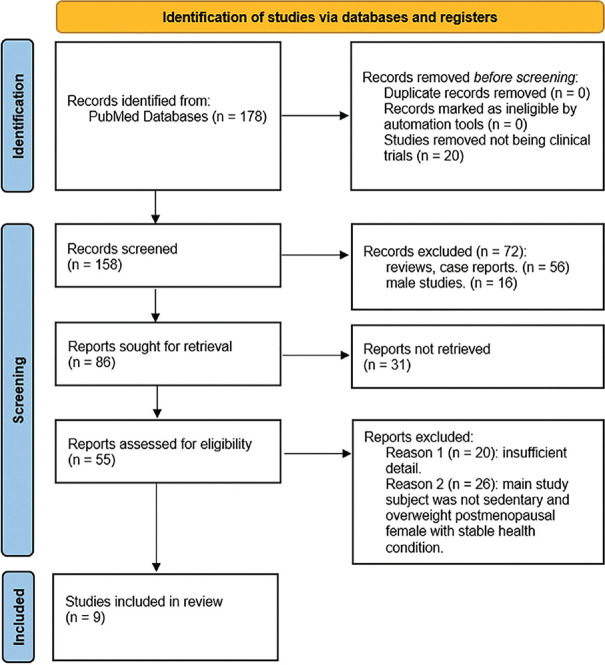
The methodology of this narrative review adhered closely to the PRISMA 2020 guidelines [Figure 1]. We conducted a comprehensive literature search using PubMed, a prominent electronic database for biomedical literature. To ensure the inclusivity of relevant clinical trials on aerobic exercise, we employed specific search terms designed to yield targeted results. The search string utilized was: “(bone mineral density [Title/Abstract]) AND (aerobic [Title/Abstract]).” This approach aimed to identify studies directly associating aerobic exercise with changes in BMD.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for this study
Eligibility criteria
The eligibility criteria for this review were rigorously defined to capture the most recent and relevant experimental evidence on aerobic exercise in women who are overweight and sedentary. The inclusion criteria comprised: (1) participants with postmenopausal status, (2) studies comprising ≥50% females, (3) those identified as overweight (body mass index [BMI] ≥25 kg/m2) or living a sedentary lifestyle (regular exercise <1 h/week) [17,18], (4) those on stable medication regimens, and (5) those undergoing primary intervention of aerobic exercise, with or without dietary CR. Additionally, only studies published between January 2013 and August 2023 were included, and it was essential for the included studies to provide experimental BMD data both before and after the intervention. The exclusion criteria encompassed case reports, meta-analyses, guidelines, reviews (narrative or systematic), studies exclusively involving males, participants who are not in postmenopausal status, those with severe diseases affecting bone metabolism (e.g. cancer), or studies primarily focusing on resistance training.
Study selection
The literature selection process adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 flowchart guidelines [Figure 1]. Duplicate records were eliminated using EndNote. Following this, 20 animal studies were excluded via manual inspection, and titles and abstracts screening based on the predefined inclusion and exclusion criteria resulted in the removal of 147 articles. Exclusion reasons were categorized into (1) study type (n = 56), (2) study participants (n = 42), and (3) insufficient detail (n = 20).
Data extraction
The data extraction process was thorough, encompassing: (1) the number of female participants; (2) participants’ demographics; (3) details of the intervention, such as frequency and duration; (4) changes in body mass or BMI; (5) changes in BMD; and (6) positive and negative outcomes postexercise, including body composition and physical capabilities. These data were collected to provide a comprehensive understanding of the effects of aerobic exercise, facilitating a balanced evaluation of its benefits and drawbacks.
RESULTS
Study characteristics
In this review, we analyzed 11 articles, predominantly focusing on aerobic exercise as the main intervention. Among these, nine were RCTs [Figure 1]. The interventions varied and included aerobic exercise alone, combination exercise (aerobic plus resistance), CR, CR combined with aerobic or resistance exercises, and CR with combination exercise [Table 1] [17,18,19,20,21,22,23,24,25]. Notably, two studies reported on different phases of the same experiment involving identical participant groups, which we combined in our analysis [17,18]. Another study utilized pre–post comparison methodologies to evaluate the impacts of the interventions [19]. The primary demographic across these studies was adult women who were overweight and sedentary. Dropout rates varied significantly, ranging from 0% to 48.3%, with common reasons being incomplete BMD data due to personal and medical factors.
Table 1.
Review summary of the included articles
| Article | Subject of female | Introduction of subject | Intervention frequency and duration | Change in body mass or BMI | Highlighted results (change in BMD) | Related results (bright side) | Related results (dark side) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Control group: Usual life | |||||||
| Villareal et al., 2017 [17] and Armamento-Villareal et al., 2020 [18] | 28 | 65 or older, BMI ≥30, sedentary, stable medication, mild to moderate frailty | Healthy diet educational session monthly for 26 weeks | −0.9±0.5 ↓(kg) | Femur neck ↓, the other sites (total hip, trochanter, intertrochanter, etc.) ↑ | Body weight and fat mass ↓ | No significant change in strength, gait, balance, adiponectin ↓, bone turnover markers (CTX, P1NP, osteocalcin) ↓ |
| Rossi et al., 2017 [20] | 15 | Menopause for at least 1 year, sedentary for at least 6 months | Maintaining usual lives without participating in regular physical activity for 16 weeks | 60.5±10.1 → 60.9±11.7 ↑ (kg) | ↑(1.042±0.18→1.035±0.17) (evaluated site wasn’t mentioned) | No related obvious result | No related obvious result (stable) |
|
| |||||||
| Exercise intervention | |||||||
|
| |||||||
| Gonzalo-Encabo et al., 2019 [21] (aerobic exercise) | 330 | 50–74 years old, BMI 22–40, postmenopausal women, sedentary | Two groups: High group: 60 min × 5 days/week for 12 months Moderate group: 30 min×5 days/week for 12 months | Moderate-volume group: −1.79 ↓↓ (kg), −0.70 in BMI ↓↓ (kg/m2) High-volume group: −2.52 ↓↓ (kg), −1.05 in BMI ↓↓ (kg/m2) | BMD decreases in both groups. Higher volume of exercise leads to smaller decline | Higher volume of exercise leads to greater decline of fat mass and increase in VO2 max | No related obvious result |
| Monleón et al., 2014 [19] (aerobic exercise) | 25 | Aged 50.43 in average, BMI 38.37±4.82, sedentary | Rhythmic and choreographic exercise 60 min × 3 times/week for 8 months | No related result reported | Calcaneus ↑↑ (T-score: −0.79±0.75→−0.61±0.86) | Balance, leg flexibility, strength, BQI ↑↑ | No related obvious result |
| Weiss et al., 2017 [22] (aerobic exercise) | 11 | Aged 45–65, BMI: 25.0–29.9, postmenopausal women, sedentary, weight stable | Cardiovascular exercise to 60 min (to create 20% energy deficit to reduce 6%–8% body weight) every day for 12–14 week | 78.1±2.3 → 72.6±2.3 ↓↓ (kg) | Wrist ↓↓ (0.906±0.029→0.883±0.032) | Fat mass ↓↓, VO2 max increased, perseverance resting metabolism when energy deficit | No related obvious result |
| Rossi et al., 2017 [20] (aerobic exercise) | 8 | Menopause for at least 1 year, sedentary for at least 6 months | Aerobic training 50 min × 3 days/week for 16 weeks | 66.4±7.6 → 63.0±8.0 ↓ (kg) | ↓(1.040±0.06→1.037±0.06) | Better metabolic ability (blood glucose and triacylglycerol) after detraining for 1 year. Percentage of fat decrease over time | No related obvious result |
| Rossi et al., 2017 [20] (aerobic + resistance exercise) | 15 | Menopause for at least 1 year, sedentary for at least 6 months | Aerobic training 30 min+30 min strength training ×3 days/week for 16 weeks | 61.9±8.7 → 60.9±8.9 ↓ (kg) | ↑ (1.093±0.10→1.102±0.01) | Trunk lean mass, arm lean mass ↑↑ | Leg lean mass ↑ but the benefits couldn’t maintain after 6 months-detraining |
|
| |||||||
| Caloric restriction intervention | |||||||
|
| |||||||
| Serra et al., 2013 [23] | 45 | Aged 45–80, obese, postmenopausal, sedentary, stable medical condition | Heart healthy diet (low sodium intake, more fruits) 250–350 kcal deficit/day×6 month | Weight difference (African American; Caucasian): −6.6±0.65 ↓↓; −7.0±0.7 ↓↓ (kg) BMI difference (African American; Caucasian): −2.5±0.3 ↓↓; −2.7±0.3↓↓ (kg/m2) | Femoral neck ↓↓, total femur ↓ | Fat mass ↓↓ | Lean mass ↓↓ |
| Weiss et al., 2017 [22] | 13 | Aged 45–65, BMI: 25.0–29.9, postmenopausal women, sedentary, weight stable | Decrease dietary intake to create 20% energy deficit to reduce 6%–8% body weight for 12–14 week | 77.9±2.4 → 72.5±2.3 ↓↓ (kg) | No significant change in whole body, spine, hip, forearm BMD | Fat mass ↓↓ | Lower extremity and total appendicular lean mass ↓ (The author considered it preparation for function transformation instead of negative effect.) |
| Serra and Ryan, 2021 [24] | 26 | Aged 45–80, BMI ≧25, postmenopausal women, sedentary, weight stable | Two-phase trial: 0~6 month (weight loss phase): 250~300 kcal deficit/day 7~12 month (weight regain phase): Optional monthly nutrition refresher class | Lost~9% body weight ↓↓ (weight-loss phase); regained 2% ↑↑ (weight-regain phase) | Total body and total femur ↓↓ | No related obvious finding | BMD loss continues following weight loss despite weight regain |
|
| |||||||
| Caloric restriction + exercise intervention | |||||||
|
| |||||||
| Villareal et al., 2017 [17] and Armamento-Villareal et al., 2020 [18] (aerobic exercise) | 26 | 65 or older, BMI ≥30, sedentary, stable medication, mild to moderate frailty | Energy deficit of 500~750 kcal/day and aerobic training 60 min×3 times/week for 26 weeks | −9.0±0.6 ↓↓ (kg) | Total hip, femur neck, trochanter and intertrochanter ↓↓ | Balance and gait ↑↑, body weight & fat mass ↓↓, VO2 max increase 18% and 1RM-strength increase 4% | Significantly accelerate BMD loss |
| Serra et al., 2013 [23] (aerobic exercise) | 52 | Aged 45–80, obese, postmenopausal, sedentary, stable medical condition | Healthy diet (low Na+, more fruits) calorie deficit 250–350 kcal/day, treadmill walking 1 h×3 times/week for 6 months | Weight difference (African American; Caucasian): −6.2±0.6 ↓↓; −7.3±0.6 ↓↓ (kg) BMI difference (African American; Caucasian): −2.4±0.2 ↓↓; −2.8±0.2 ↓↓ (kg/m2) | Femoral neck and total femur ↓ | Fat mass ↓↓, VO2 max ↑↑ | Lean mass of Caucasian ↓↓ but lesser than weight loss (alone) group |
| Weiss et al., 2017 [22] (aerobic exercise) | 15 | Aged 45–65, BMI: 25.0–29.9, postmenopausal women, sedentary, weight stable | 10% energy deficit/day from diet and the other 10% from cardiovascular exercise for 12–14 week | 82.4±2.9 → 76.2±2.8 in body weight ↓↓ (kg) | No significant change in whole body, spine, hip, forearm BMD | Fat mass↓↓, VO2 max↑, perseverance resting metabolism ability when energy deficit | No related obvious result |
|
| |||||||
| Caloric restriction + exercise intervention | |||||||
|
| |||||||
| Serra and Ryan, 2021 [24] (aerobic exercise) | 28 | Aged 45–80, BMI ≧25, postmenopausal women, sedentary, weight stable | Two-phase trial: 0~6 month (weight loss phase): 250~300kcal deficit/day + 45 min × 3 days/week aerobic exercise 7~12month (weight regain phase): Optional monthly nutrition refresher class + optional exercise facility | Lost~9% body weight ↓↓ (weight-loss phase); regained 2% ↑↑ (weight-regain phase) | Total body and total femur↓↓ | More VO2 max increase and less appendicular lean mass loss during weight-loss phase were associated with less BMD loss when weight-regain | BMD loss continues following weight loss despite weight regain.VO2 max didn’t maintain as aerobic exercise program termination |
| Beavers et al., 2017 [25] (aerobic exercise) | 37 | Aged 65–79, BMI 30–45, sedentary, weight stable | 250 kcal deficit/day + 30 min × 4 days/week for 5 months | −8.2% of body weight in average ↓↓ | Total hip and femur neck ↓, lumbar spine ↑ | No related obvious finding | No related obvious finding |
| Villareal et al., 2017 [17] and Armamento-Villareal et al., 2020 [18] (resistance exercise) | 25 | 65 or older, BMI ≥30, sedentary, stable medication, mild to moderate frailty | Energy deficit of 500~750 kcal/day and resistance training 60 min × 3 times/week for 26 week | −8.5±0.5 ↓↓ (kg) | Lumbar spine and whole body ↑, the other sites (total hip, femur neck, trochanter) ↓ | Balance, strength and gait ↑↑, body weight and fat mass ↓↓, VO2 max increase 8% and 1RM-strength increase 19% | Estradiol ↓↓ followed by weight loss |
| Beavers et al., 2017 [25] | 45 | Aged 65–79, BMI 27–35, sedentary, weight stable | 600 kcal deficit/day + 3 day/week for 5 months | −5.7% of body weight in average ↓↓ | Total hip and femur neck↑and lumbar spine ↑↑ | No related obvious finding | No related obvious finding |
| Villareal et al., 2017 [17] and Armamento-Villareal et al., 2020 [18] (aerobic + resistance exercise) | 24 | 65 or older, BMI ≥30, sedentary, stable medication, mild to moderate frailty | Energy deficit of 500~750 kcal/day and resistance training 75~90 min × 3 times/week for 26 weeks | −8.5±0.5 ↓↓ (kg) | Total hip, trochanter and intertrochanter ↓↓ | Balance, strength and gait ↑↑, body weight and fat mass ↓↓, VO2 max increase 17% and 1RM-strength increase 18% | Significantly accelerate BMD loss, estradiol ↓↓ followed by weight loss |
↓: For decrease insignificantly, ↓↓: For decrease significantly, ↑: For increase insignificantly, ↑↑: For increase significantly, BMI: Body mass index, BMD: Bone mineral density, CTX: C-terminal telopeptide of type 1 collagen, BQI: Bone quality index
Exercise intervention effects on sedentary female adults who are overweight
Classification 1: Control intervention
Among the selected articles, three studies [17,18,20] included a control group that did not engage in weight management or exercise programs. Notably, these studies involved 43 postmenopausal women who experienced no significant weight changes during the trials. In two studies, participants in the control group underwent a 26-week control intervention without participating in weight management or exercise programs, followed by a BMD assessment [17,18]. The results revealed no substantial changes in BMD across various body regions, with a minimal to no decrease observed. However, the other study offered a contrasting perspective, indicating a slight trend toward a reduction in whole-body BMD over 1 year (baseline: 1.042 ± 0.18 vs. postintervention: 1.033 ± 0.16) [20].
All studies suggested that physical inactivity could lead to reduced exercise capacity and balance, especially with aging. Additionally, the control group also exhibited decreased levels of adiponectin, a hormone known for its protective role against type 2 diabetes. Furthermore, bone turnover markers, including the C-terminal telopeptide of type 1 collagen (CTX), procollagen type I N-terminal propeptide, and osteocalcin, showed a slight decline in the absence of physical activity [17,18].
Classification 2: Aerobic intervention
Only four articles [19,20,21,22] among the selected studies implemented interventions focusing on aerobic exercise exclusively, without any dietary modifications. These studies incorporated various aerobic exercises, including treadmill workouts and rhythmically coordinated aerobic programs. Collectively, these studies involved 374 postmenopausal women with obesity and sedentary lifestyles, with the majority of participants experiencing weight reduction due to the aerobic exercise regimen. The results of these studies found that aerobic exercise can effectively reduce fat mass and may have a slight protective effect on BMD. Monleón et al. [19] observed an improvement in calcaneal BMD following aerobic dance training. In contrast, Rossi et al. reported a slight decrease in BMD after a 16-week training program, followed by a subsequent increase [20]. Gonzalo-Encabo et al. found that while a higher volume of aerobic exercise did not enhance BMD in postmenopausal women, it contributed to a lesser decline in total BMD [21]. Weiss et al. [22] reported a significant reduction in wrist BMD.
Additionally, the literature highlights several benefits of aerobic exercise, such as increased muscle density, reduced body fat [22], enhanced exercise capacity [21,22], and improved metabolic efficiency [20]. However, these studies also raise a concern: despite the overall health benefits, aerobic exercise may not uniformly prevent the deterioration of BMD, indicating the need for further investigation.
Classification 3: Combination (resistance + aerobic) intervention
This review examined a single study [20] that implemented a regimen of aerobic exercise and resistance training for 15 sedentary postmenopausal women. This combined approach appeared to arrest the decline in BMD and even facilitated a slight, though not statistically significant, increase in BMD, alongside a modest reduction in body weight. Importantly, the benefits of this training persisted for several months after cessation. Furthermore, an increase in lean mass across various body regions was observed, although the sustainability of this outcome remains uncertain.
Additionally, we noted that combining resistance training with aerobic exercise enhances the protective effects of BMD [20], as this regimen not only preserved BMD but also resulted in muscle mass gains despite weight loss, emphasizing the importance of long-term maintenance posttraining [20].
Classification 4: Caloric restriction intervention
The impact of CR was assessed in three studies [22,23,24] involving 84 postmenopausal women aged 45–80 years, with most participants experiencing significant weight loss. Serra et al. reported that both African American and Caucasian women were prone to BMD loss following weight loss, and this deterioration persisted even after weight regain [23]. However, the remaining two studies [22] suggested that CR may not significantly compromise BMD. All three studies indicated that calorie reduction resulted in weight and body fat loss, raising concerns about potential declines in BMD and lean mass [22,23,24].
Classification 5: Caloric restriction with aerobic intervention
The effects of combining diet with aerobic exercise were investigated in six studies [17,18,22,23,24,25] involving 158 postmenopausal females aged 45 years or older who were overweight. While Serra et al. [23] found that aerobic exercise could mitigate BMD decline despite weight loss, another study observed no significant changes in BMD [22]. Notably, one study [25] indicated that this regimen can slightly increase lumbar spine BMD, but slightly decrease femoral neck and hip bone BMD, and three studies [17,18,24] expressed concerns regarding potential reductions in BMD, particularly in the total hip and femoral neck regions. Reported health benefits included reductions in body fat [17,18,22,23] and improvements in balance [17,18], VO2 max [17,18,22], and maximal strength [17,18], although the sustainability of these benefits was questioned [24].
Classification 6: Caloric restriction with resistance intervention
Three studies [17,18,25] investigated the combination of diet control with resistance training in 70 women aged 65 and older with obesity. Following these interventions, there was a notable reduction in participants’ body weight. Interestingly, while the effects on BMD varied, this intervention appears to have a protective effect on BMD. Additionally, improvements in balance, gait, VO2 max, and muscular strength were observed, and a significant decrease in estradiol following weight loss emerged as a concerning finding [17,18,25].
Classification 7: Caloric restriction with combination exercise (resistance + aerobic)
Two studies [17,18] involving 24 women aged >65 examined the impact of a combined exercise regimen during dieting. While significant weight loss was observed, the preservation of BMD could not be fully achieved. Nevertheless, the combined exercise regimen demonstrated a relatively better ability to mitigate BMD decline compared to aerobic exercise alone. Improvements in balance, gait, VO2 max, and muscular strength were noted; however, the substantial decrease in BMD and estradiol levels remained a significant concern [17,18].
In summary, our analysis indicates that pure aerobic exercise interventions effectively reduce fat mass and have a slight protective effect on BMD. In contrast, CR interventions primarily lead to significant reductions in body weight and body fat, with a more complex effect on BMD, where some studies indicated potential reductions. When aerobic exercise is combined with CR, it not only further promotes fat mass reduction but also mitigates the negative impact on BMD to some extent. Moreover, the intervention combining aerobic exercise and resistance training significantly protected BMD while promoting an increase in muscle mass. These findings underscore the nuanced interplay between different types of exercise, dietary interventions, and their combined effects on bone health and body composition among postmenopausal women with obesity, highlighting the importance of tailored approaches to maximize health benefits while minimizing potential risks.
DISCUSSION
Control versus caloric restriction (diet control on bone mineral density)
This review underscores the complex interplay between CR and bone health in postmenopausal women with obesity. Our findings suggest that while CR can lead to significant weight loss and reduced body fat, it may also increase the risk of bone density loss, particularly in the femur, compared to maintaining usual dietary habits [23,24].
Notably, the control group not engaging in CR exhibited a decline in physical activity and lower adiponectin levels, potentially increasing the risk of type 2 diabetes, and showed reduced bone turnover markers, indicating potential long-term implications for bone health [17,18]. A pivotal meta-analysis of 41 studies highlighted the association between diet-induced weight loss and reduced hip BMD, with no significant impact on lumbar spine BMD [26]. Seimon et al.’s [14] dose–response clinical trial demonstrated that greater CR resulted in more substantial bone density losses, regardless of a standardized protein intake, highlighting the insufficiency of protein alone in mitigating BMD reduction [14]. The role of dietary patterns in preserving BMD during CR is vital, with the Mediterranean diet emerging as a beneficial option. Studies have shown its positive effects on BMD, such as a decrease in femoral neck bone loss and higher BMD in postmenopausal women adhering to this diet [27,28,29]. A comprehensive review also emphasized the significance of fruits and dairy in reducing fracture risks [30]. Thus, the nutritional aspects of dieting, particularly the balance of essential nutrients for bone health, are critical in dietary planning for postmenopausal women. Future research should delve into the intricate relationships between dietary components and their collective impact on bone health, especially in the context of weight management strategies.
Control versus aerobic exercise (aerobic exercise on bone mineral density)
In comparing the effects of regular daily activities versus aerobic exercise on BMD, our findings reveal that typical daily activities alone do not markedly influence body weight, BMI, or BMD in adult females. However, the impact of aerobic exercise on these health metrics may be more complex and varied. Aerobic exercise, unlike routine activities, has the potential to influence these parameters in significant ways, demonstrating a range of outcomes on body composition and bone health [19,20,21,22]. This variability underscores the importance of considering aerobic exercise as a multifaceted intervention that can provide benefits beyond general physical activity, particularly in enhancing bone density and overall metabolic health. A notable finding is the association between weight loss or caloric deficit and a decline in BMD, highlighting the intricate relationship between aerobic exercise, weight management, and bone health. A dose–response relationship was observed, where higher volumes of aerobic exercise were associated with less BMD loss, particularly evident in rhythmic aerobic exercises increasing heel BMD in middle-aged women with obesity [21]. Additionally, while aerobic exercise may not consistently increase bone density, it significantly enhances overall health, as evidenced by studies reporting improved bone metabolic markers, muscle strength, and cardiovascular endurance [31,32,33]. Moreover, early engagement in high-impact sports has been linked to increased bone mass, underscoring the importance of initiating physical activity early to mitigate the risk of osteoporosis in the future [34]. Therefore, we recommend the early adoption of regular aerobic exercise as a key strategy for females to maintain bone health throughout their lifespan.
Aerobic exercise versus combined exercise interventions
This review elucidates the intricate effects of exercise interventions on body weight, BMD, and overall physical fitness during CR. While both aerobic and combined exercise interventions generally result in weight reduction [12,13,14,15,16,17,18,19,20,21,22,23,24,25], their impact on BMD varies. Aerobic exercise alone offers modest protection for BMD [19,21], but this effect seems diminished when paired with CR. Among studies exploring this combination, findings range from significant bone loss to negligible changes in BMD [17,18,22,23,24,25]. Additionally, aerobic exercise enhances VO2 max, reduces body fat, and helps maintain muscle mass [17,18,22,23,24]. In comparison, resistance training combined with CR shows a more pronounced protective effect on BMD, maintaining muscle mass and increasing maximal strength, although improvements in VO2 max and balance are less evident compared to aerobic exercise alone [17,18,25].
Combination training, which involves both aerobic and resistance exercises, exhibits a decline in BMD but offers balanced improvements in physical capacity and strength [17,18]. Consistent with the findings above, this study underscores the importance of resistance training for older women with obesity, given its substantial benefits for muscle strength and bone health. Research indicates that resistance training enhances BMD, muscle strength, and functional fitness in this demographic [35]. On the other hand, aerobic exercise, which is more effective in increasing VO2 max and total energy expenditure, also supports cardiovascular health by reducing arterial stiffness [36,37,38]. These findings highlight the complexity of exercise interventions with CR, particularly for postmenopausal women. Thus, balancing weight loss, muscle preservation, and bone health requires personalized exercise regimens, emphasizing the need for future research to determine optimal combinations of exercise and dietary strategies for maximizing health benefits while minimizing negative impacts on bone density.
Interplay between aerobic exercise and diet in balancing bone health and weight management
CR can adversely affect BMD [39], yet avoiding weight reduction may increase the risk of type 2 diabetes [17,18]. While protein supplementation is crucial, it cannot solely preserve BMD [24]. To overcome this limitation, a balanced diet, such as the Mediterranean diet, is key for effective weight loss [40]. Aerobic exercise, while not significantly protective of BMD, has been shown to be beneficial for bone health, as indicated by serum bone turnover markers. Early initiation of aerobic exercise can lead to long-term skeletal advantages [34,41], and when combined with resistance training, the protective effect on BMD is enhanced [17,18]. For postmenopausal women with obesity undergoing CR, the impact of aerobic exercise on BMD varies; in contrast, resistance training promotes BMD preservation [17,18,25]. Thus, resistance training is important in maintaining BMD. Nevertheless, the various benefits of aerobic exercise, including enhanced energy metabolism and cardiovascular health, underscore its vital role in bone health for postmenopausal women with obesity [42].
This study has some limitations, including the lack of studies focusing solely on resistance training (without CR) in obese women, potential variability due to heterogeneous study designs and populations, and the focus on studies primarily sourced from PubMed, resulting in insufficient data. Additionally, the gender ratio variation across selected studies may have led to some inaccuracies in our conclusions. Furthermore, the absence of long-term follow-up in the studies limits the understanding of the sustained effects of exercise interventions, highlighting the need for more longitudinal research. Despite these limitations, this review systematically examines the effects of different exercise interventions on BMD and health outcomes in postmenopausal women with obesity, illustrating the complex interplay between exercise, body composition, and bone health in the context of CR.
CONCLUSION
This study highlights the importance of aerobic exercise, CR, and their combined interventions in improving body composition and bone health among postmenopausal women with obesity. Aerobic exercise not only reduces fat mass but also has a protective effect on BMD. CR mainly affects body composition but requires careful consideration of its potential negative impact on BMD, especially during weight loss. The combined intervention of aerobic and resistance training, especially under CR, offers the greatest protection for bone health while promoting muscle mass increase. Therefore, for postmenopausal women with obesity, developing personalized exercise programs that include both aerobic and resistance training is key to maintaining bone health and improving body composition.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Ing-Ho Chen, an editorial board member at Tzu Chi Medical Journal, had no role in the peer review process or the decision to publish this article. The other authors declared no conflicts of interest in writing this paper.
REFERENCES
- 1.DiPietro L, Buchner DM, Marquez DX, Pate RR, Pescatello LS, Whitt-Glover MC. New scientific basis for the 2018 U. S. Physical activity guidelines. J Sport Health Sci. 2019;8:197–200. doi: 10.1016/j.jshs.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong B, Hiasa M, Higa Y, Ohnishi Y, Endo I, Kondo T, et al. Osteoblast/osteocyte-derived interleukin-11 regulates osteogenesis and systemic adipogenesis. Nat Commun. 2022;13:7194. doi: 10.1038/s41467-022-34869-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suiko M, Mizukami S, Arima K, Nakashima H, Nishimura T, Tomita Y, et al. Association between physical performance and bone mass in community-dwelling postmenopausal Japanese women: The Unzen study. PLoS One. 2024;19:e0296457. doi: 10.1371/journal.pone.0296457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedetti MG, Furlini G, Zati A, Letizia Mauro G. The effectiveness of physical exercise on bone density in osteoporotic patients. Biomed Res Int 2018. 2018 doi: 10.1155/2018/4840531. 4840531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kistler-Fischbacher M, Weeks BK, Beck BR. The effect of exercise intensity on bone in postmenopausal women (part 2): A meta-analysis. Bone. 2021;143:115697. doi: 10.1016/j.bone.2020.115697. [DOI] [PubMed] [Google Scholar]
- 6.Zitzmann AL, Shojaa M, Kast S, Kohl M, von Stengel S, Borucki D, et al. The effect of different training frequency on bone mineral density in older adults. A comparative systematic review and meta-analysis. Bone. 2022;154:116230. doi: 10.1016/j.bone.2021.116230. [DOI] [PubMed] [Google Scholar]
- 7.Sherrington C, Fairhall NJ, Wallbank GK, Tiedemann A, Michaleff ZA, Howard K, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1 doi: 10.1002/14651858.CD012424.pub2. CD012424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaMonte MJ, Wactawski-Wende J, Larson JC, Mai X, Robbins JA, LeBoff MS, et al. Association of physical activity and fracture risk among postmenopausal women. JAMA Netw Open. 2019;2:e1914084. doi: 10.1001/jamanetworkopen.2019.14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AJ, Phipps WR, Thomas W, Schmitz KH, Kurzer MS. The effects of aerobic exercise on estrogen metabolism in healthy premenopausal women. Cancer Epidemiol Biomarkers Prev. 2013;22:756–64. doi: 10.1158/1055-9965.EPI-12-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savvidis C, Tournis S, Dede AD. Obesity and bone metabolism. Hormones (Athens) 2018;17:205–17. doi: 10.1007/s42000-018-0018-4. [DOI] [PubMed] [Google Scholar]
- 11.Palermo A, Tuccinardi D, Defeudis G, Watanabe M, D’Onofrio L, Lauria Pantano A, et al. BMI and BMD: The potential interplay between obesity and bone fragility. Int J Environ Res Public Health. 2016;13:544. doi: 10.3390/ijerph13060544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gkastaris K, Goulis DG, Potoupnis M, Anastasilakis AD, Kapetanos G. Obesity, osteoporosis and bone metabolism. J Musculoskelet Neuronal Interact. 2020;20:372–81. [PMC free article] [PubMed] [Google Scholar]
- 13.Garofalo V, Barbagallo F, Cannarella R, Calogero AE, La Vignera S, Condorelli RA. Effects of the ketogenic diet on bone health: A systematic review. Front Endocrinol (Lausanne) 2023;14:1042744. doi: 10.3389/fendo.2023.1042744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seimon RV, Wild-Taylor AL, Keating SE, McClintock S, Harper C, Gibson AA, et al. Effect of weight loss via severe versus moderate energy restriction on lean mass and body composition among postmenopausal women with obesity: The TEMPO diet randomized clinical trial. JAMA Netw Open. 2019;2:e1913733. doi: 10.1001/jamanetworkopen.2019.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph RP, Casazza K, Durant NH. The effect of a 3-month moderate-intensity physical activity program on body composition in overweight and obese African American college females. Osteoporos Int. 2014;25:2485–91. doi: 10.1007/s00198-014-2825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam SS, Sunoo S, Park HY, Moon HW. The effects of long-term whole-body vibration and aerobic exercise on body composition and bone mineral density in obese middle-aged women. J Exerc Nutrition Biochem. 2016;20:19–27. doi: 10.20463/jenb.2016.06.20.2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. 2017;376:1943–55. doi: 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armamento-Villareal R, Aguirre L, Waters DL, Napoli N, Qualls C, Villareal DT. Effect of aerobic or resistance exercise, or both, on bone mineral density and bone metabolism in obese older adults while dieting: A randomized controlled trial. J Bone Miner Res. 2020;35:430–9. doi: 10.1002/jbmr.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monleón C, Pablos A, Carnide F, Martín M, Pablos C. Effects of a rhythmic and choreographic program in obese and overweight participants. Nutr Hosp. 2014;30:622–8. doi: 10.3305/nh.2014.30.3.7365. [DOI] [PubMed] [Google Scholar]
- 20.Rossi FE, Diniz TA, Neves LM, Fortaleza AC, Gerosa-Neto J, Inoue DS, et al. The beneficial effects of aerobic and concurrent training on metabolic profile and body composition after detraining: A 1-year follow-up in postmenopausal women. Eur J Clin Nutr. 2017;71:638–45. doi: 10.1038/ejcn.2016.263. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalo-Encabo P, McNeil J, Boyne DJ, Courneya KS, Friedenreich CM. Dose-response effects of exercise on bone mineral density and content in post-menopausal women. Scand J Med Sci Sports. 2019;29:1121–9. doi: 10.1111/sms.13443. [DOI] [PubMed] [Google Scholar]
- 22.Weiss EP, Jordan RC, Frese EM, Albert SG, Villareal DT. Effects of weight loss on lean mass, strength, bone, and aerobic capacity. Med Sci Sports Exerc. 2017;49:206–17. doi: 10.1249/MSS.0000000000001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serra MC, Blumenthal JB, Ryan AS. Impact of weight loss and aerobic exercise on nutrition and bone mineral density in African American and caucasian postmenopausal women. J Aging Res Clin Pract. 2013;2:11–6. [PMC free article] [PubMed] [Google Scholar]
- 24.Serra MC, Ryan AS. Bone mineral density changes during weight regain following weight loss with and without exercise. Nutrients. 2021;13:2848. doi: 10.3390/nu13082848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beavers KM, Beavers DP, Martin SB, Marsh AP, Lyles MF, Lenchik L, et al. Change in bone mineral density during weight loss with resistance versus aerobic exercise training in older adults. J Gerontol A Biol Sci Med Sci. 2017;72:1582–5. doi: 10.1093/gerona/glx048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zibellini J, Seimon RV, Lee CM, Gibson AA, Hsu MS, Shapses SA, et al. Does diet-induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta-analysis of clinical trials. J Bone Miner Res. 2015;30:2168–78. doi: 10.1002/jbmr.2564. [DOI] [PubMed] [Google Scholar]
- 27.Jennings A, Cashman KD, Gillings R, Cassidy A, Tang J, Fraser W, et al. A mediterranean-like dietary pattern with Vitamin D3 (10 µg/d) supplements reduced the rate of bone loss in older Europeans with osteoporosis at baseline: Results of a 1-y randomized controlled trial. Am J Clin Nutr. 2018;108:633–40. doi: 10.1093/ajcn/nqy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva TR, Martins CC, Ferreira LL, Spritzer PM. Mediterranean diet is associated with bone mineral density and muscle mass in postmenopausal women. Climacteric. 2019;22:162–8. doi: 10.1080/13697137.2018.1529747. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Rey J, Roncero-Martín R, Rico-Martín S, Rey-Sánchez P, Pedrera-Zamorano JD, Pedrera-Canal M, et al. Adherence to a mediterranean diet and bone mineral density in Spanish premenopausal women. Nutrients. 2019;11:555. doi: 10.3390/nu11030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzoli R, Biver E, Brennan-Speranza TC. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021;9:606–21. doi: 10.1016/S2213-8587(21)00119-4. [DOI] [PubMed] [Google Scholar]
- 31.Wen HJ, Huang TH, Li TL, Chong PN, Ang BS. Effects of short-term step aerobics exercise on bone metabolism and functional fitness in postmenopausal women with low bone mass. Osteoporos Int. 2017;28:539–47. doi: 10.1007/s00198-016-3759-4. [DOI] [PubMed] [Google Scholar]
- 32.Al Dahamsheh Z, Al Rashdan K, Al Hadid A, Jaradat R, Al Bakheet M, Bataineh ZS. The impact of aerobic exercise on female bone health indicators. Med Arch. 2019;73:35–8. doi: 10.5455/medarh.2019.73.35-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wochna K, Nowak A, Huta-Osiecka A, Sobczak K, Kasprzak Z, Leszczyński P. Bone mineral density and bone turnover markers in postmenopausal women subjected to an aqua fitness training program. Int J Environ Res Public Health. 2019;16:2505. doi: 10.3390/ijerph16142505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hara S, Yanagi H, Amagai H, Endoh K, Tsuchiya S, Tomura S. Effect of physical activity during teenage years, based on type of sport and duration of exercise, on bone mineral density of young, premenopausal Japanese women. Calcif Tissue Int. 2001;68:23–30. doi: 10.1007/BF02684999. [DOI] [PubMed] [Google Scholar]
- 35.Kim SW, Park HY, Jung WS, Lim K. Effects of twenty-four weeks of resistance exercise training on body composition, bone mineral density, functional fitness and isokinetic muscle strength in obese older women: A randomized controlled trial. Int J Environ Res Public Health. 2022;19:14554. doi: 10.3390/ijerph192114554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, et al. Aerobic versus resistance exercise in non-alcoholic fatty liver disease: A systematic review. J Hepatol. 2017;66:142–52. doi: 10.1016/j.jhep.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 37.Hunter GR, Byrne NM, Gower BA, Sirikul B, Hills AP. Increased resting energy expenditure after 40 minutes of aerobic but not resistance exercise. Obesity (Silver Spring) 2006;14:2018–25. doi: 10.1038/oby.2006.236. [DOI] [PubMed] [Google Scholar]
- 38.Lopes S, Afreixo V, Teixeira M, Garcia C, Leitão C, Gouveia M, et al. Exercise training reduces arterial stiffness in adults with hypertension: A systematic review and meta-analysis. J Hypertens. 2021;39:214–22. doi: 10.1097/HJH.0000000000002619. [DOI] [PubMed] [Google Scholar]
- 39.Radak TL. Caloric restriction and calcium's effect on bone metabolism and body composition in overweight and obese premenopausal women. Nutr Rev. 2004;62:468–81. doi: 10.1111/j.1753-4887.2004.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 40.Lupsa BC, Insogna K. Bone health and osteoporosis. Endocrinol Metab Clin North Am. 2015;44:517–30. doi: 10.1016/j.ecl.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Banfi G, Lombardi G, Colombini A, Lippi G. Bone metabolism markers in sports medicine. Sports Med. 2010;40:697–714. doi: 10.2165/11533090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Murray KO, Mahoney SA, Venkatasubramanian R, Seals DR, Clayton ZS. Aging, aerobic exercise, and cardiovascular health: Barriers, alternative strategies and future directions. Exp Gerontol. 2023;173:112105. doi: 10.1016/j.exger.2023.112105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.