ABSTRACT
Objectives:
Laparoscopic hepatectomy (LH) for hepatocellular carcinoma (HCC) has been well known for its advantages in the past 10 years, but little is known regarding its oncologic outcomes while the technique is being developed at an institution. This study aimed to evaluate the safety and effectiveness of LH for patients with primary HCC at favorable locations, focusing on postoperative short- and long-term outcomes during the development period.
Materials and Methods:
We retrospectively reviewed patients diagnosed with primary HCC who underwent hepatectomy between January 2013 and December 2019 at Hualien Tzu Chi Hospital. Patients with HCC at favorable locations (anterolateral segments) were collected and divided into laparoscopic and open hepatectomy (OH) groups. The data for long-term outcomes, as the primary endpoint, and postoperative outcomes, as the secondary endpoint, were collected.
Results:
The review included 159 patients, among which 42 and 44 patients in favorable locations underwent open and laparoscopic hepatectomies, respectively. There were no significant differences in intraoperative blood loss, major complication rate, and 90-day mortality rate between the two groups. The laparoscopic group had a lower transfusion rate, shorter postoperative hospital stay, and lower 90-day readmission rate. There were no significant differences in 12-, 36-, and 60-month overall survival and disease-free survival.
Conclusion:
LH for favorably located HCC is the preferred surgical approach compared to OH due to the decreased transfusion rate, shorter postoperative hospital stay, and lower 90-day readmission rate. LH did not compromise the 90-day mortality rate with sustained long-term overall and disease-free survival. LH for favorably located HCC is a safe and effective surgical approach even during the development period.
KEYWORDS: Favorable location, Hepatocellular carcinoma, Laparoscopic hepatectomy
INTRODUCTION
Hepatocellular carcinoma (HCC) was the sixth most common neoplasm and the third leading cause of cancer death worldwide in 2020, with 905,677 diagnosed cases and 830,180 deaths [1]. The treatment guidelines established by the Barcelona Clinic Liver Cancer strategy underscore hepatectomy as a pivotal therapeutic avenue for managing HCC [2]. Since the inaugural description of laparoscopic hepatectomy (LH) in 1991 [3], its evolution into a viable surgical alternative has been gradual yet significant. Following the landmark feasibility study of LH in 2000 [4], numerous investigations have amalgamated to ascertain the safety and efficacy of the laparoscopic approach across diverse clinical scenarios. These investigations have encompassed variations in tumor size [5], prior abdominal surgeries [6], patients with cirrhosis [7], and the elderly population [8]. Furthermore, LH offers a plethora of advantages, including a smaller incision size, reduced operation duration, diminished transfusion requirements, lower incidence of major complications, shorter hospitalization periods, and comparable overall survival and disease-free survival rates when juxtaposed with open hepatectomy (OH) [9,10,11,12].
A myriad of challenges, encompassing liver mobilization, hemorrhage management, diminished tactile feedback, a deeper surgical field, and intraoperative risks, pose impediments to the acquisition of proficiency in LH [13,14]. Recent investigations evaluating the feasibility and safety of LH have predominantly emanated from well-established medical centers, thus lacking comprehensive data from developing institutions or their formative stages. Additionally, the influence of tumor location on LH outcomes remains inadequately elucidated.
The aim of this study was to assess the safety and efficacy of our preliminary encounters with LH for HCC situated in favorable anatomical sites. Specifically, we aimed to scrutinize the short-term and long-term outcomes of LH during its developmental phase.
MATERIALS AND METHODS
Patient characteristics
We conducted a retrospective analysis on a series of sequential cases involving patients newly diagnosed with HCC who underwent hepatectomy at Hualien Tzu Chi General Hospital, a tertiary referral center in eastern Taiwan, between January 2013 and December 2019. The preoperative identification of HCC relied upon outcomes derived from two sets of noninvasive dynamic imaging within high-risk cohorts afflicted with chronic hepatitis B, chronic hepatitis C, or cirrhosis, irrespective of alpha-fetoprotein elevation.
Postoperative HCC was histopathologically confirmed through examination of resected specimens across all patients. Patients with prior HCC treatment, synchronous malignancy, or a recurrent diagnosis of HCC were excluded from the study cohort. The patient cohort was stratified into two groups: LH and OH. Further stratification was conducted within the subgroup of patients with tumors located in favorable positions, categorized as the favorable location group. Within this subset, patients were further divided into either the open group (F-OH) or laparoscopic group (F-LH). The assignment of patients to the open or laparoscopic group was determined by the surgeon’s discretion, considering factors such as patient age, liver function, tumor size, location, and proximity to major vessels. This decision-making process was influenced by the surgeon’s proficiency in laparoscopic techniques. Volumetric assessment of the tumor was not standard practice due to lack of coverage by the National Health Insurance of Taiwan, rendering it financially unfeasible for the majority of patients. Resection volume was determined using the Makuuchi criteria [15]. A retrospective review of medical records was conducted to gather demographic characteristics, perioperative variables, and follow-up outcomes. The primary endpoint focused on long-term oncologic outcomes, encompassing overall and disease-free survival. Perioperative outcomes constituted the secondary endpoint. The median follow-up duration for the cohort was 48 months.
Definitions and surgical technique
Definitions for liver anatomy were derived from the Brisbane 2000 Guidelines [16]. Major resection was characterized by the removal of ≥3 segments, whereas minor resection involved the excision of <3 segments. The determination between major and minor resections hinged upon factors such as tumor size, location, and the feasibility of achieving adequate surgical margins. Postoperative complications were graded according to the Clavien–Dindo classification system, with major complications defined as those categorized as class 3 or higher [17]. The definitions for bile leakage and posthepatectomy liver failure were standardized based on the criteria established by the International Study Group of Liver Surgery [18,19]. Tumor locations were classified as favorable if situated within segments S2, S3, S4b, S5, and S6, while locations within segments S1, S4a, S7, and S8 were considered unfavorable. Details regarding the surgical techniques employed in OH and LH were previously described in our prior study [9].
Statistical analysis
The Chi-square test was used to analyze categorical variables, presented as numbers and percentages. The Kolmogorov–Smirnov test was used to check the normality of continuous variables. Normally distributed continuous variables are presented as means with standard deviations and were analyzed with Student’s t-test. Nonnormally distributed continuous variables are presented as medians with interquartile ranges and were analyzed using the Mann–Whitney U-test. The Kaplan–Meier curve with the log-rank test was used for the survival analysis. SPSS for MAC ver. 26 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Statistical significance was set at a P < 0.05.
Ethic declaration
Ethical approval for this study (Research Ethics Committee, REC No. IRB 109-074-B) was provided by the Research Ethics Committee of Hualien Tzu Chi Hospital, the Buddhist Tzu Chi Medical Foundation, on April 16, 2020. Informed written consent was waived by the IRB because the study was a retrospective data analysis. This study was conducted in accordance with the Declaration of Helsinki.
RESULTS
Figures 1 and 2 depict the evolution of LH from 2013 to 2019. Over this developmental period, the ratio of LH [Figure 1] demonstrated a gradual increase, surpassing 50% post-2017. Similarly, the proportion of favorable location laparoscopic hepatectomies (F-LH) [Figure 2] exhibited a concurrent rise, exceeding 50% post-2017, aligning with the overall trend observed in LH development.
Figure 1.
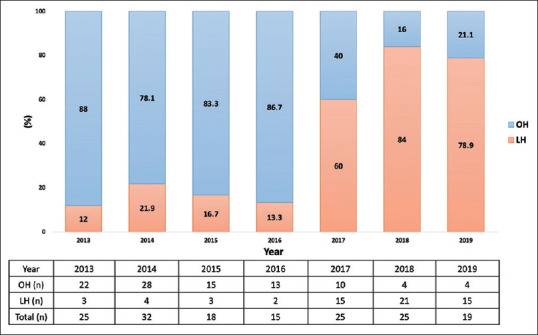
Distribution of hepatectomy. LH: Laparoscopic hepatectomy, OH: Open hepatectomy
Figure 2.
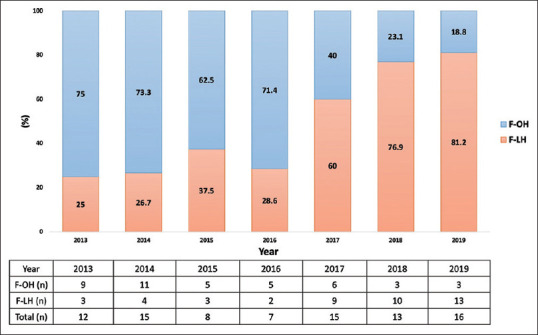
Distribution of hepatectomy of favorable location by year. F-OH: Open hepatectomy in the favorable location group, F-LH: Laparoscopic hepatectomy in the favorable location group
Baseline characteristics
The baseline characteristics of the patients are summarized in Table 1. A total of 159 patients were included, of which 86 (54.1%) exhibited tumors in favorable locations. The OH group comprised 96 patients (60.3%), while the LH group consisted of 63 patients (39.6%). Both the groups predominantly comprised male patients, with no significant disparity observed in the prevalence of comorbidities such as diabetes mellitus, hypertension, and coronary artery disease between the OH and LH groups. Notably, a higher proportion of patients in the LH group were classified as Child–Pugh class A compared to the OH group (98.4% vs. 88.9%, P = 0.026), although the Model for End-Stage Liver Disease-Na (MELD-Na) scores were comparable between the two groups (8 vs. 7, P = 0.229). The preoperative 15-min retention rate for indocyanine green was 13.3% in the LH group and 9.1% in the OH group, with no statistically significant difference observed (P = 0.748).
Table 1.
Patient characteristics
| Characteristics | Whole cohort (n=159) | OH (n=96) | LH (n=63) | P | Favorable location subgroup (n=86) | F-OH (n=42) | LH (n=44) | P |
|---|---|---|---|---|---|---|---|---|
| Age (years)* | 65±10 | 64±11 | 67±9 | 0.156 | 65±10 | 64±11 | 66±9 | 0.148 |
| Sex (male), n (%) | 120/159 (75.5) | 70/96 (72.9) | 50/63 (79.4) | 0.452 | 65/86 (75.6) | 33/42 (78.6) | 32/44 (72.7) | 0.619 |
| BMI (kg/m2)* | 25.2±3.6 | 25.0±3.7 | 25.7±3.6 | 0.687 | 25.6±3.7 | 25.7±4.0 | 25.5±3.4 | 0.379 |
| Comorbidity, n (%) | ||||||||
| DM | 57/159 (35.8) | 28/96 (29.2) | 29/63 (46.3) | 0.042 | 21/86 (36.0) | 10/42 (23.8) | 21/44 (47.7) | 0.026 |
| HTN | 72/159 (45.3) | 40/96 (41.7) | 32/63 (51.0) | 0.329 | 42/86 (48.8) | 21/42 (50.0) | 21/44 (47.7) | 1.00 |
| CAD | 10/159 (6.3) | 6/96 (6.2) | 4/63 (6.3) | 0.98 | 6/86 (7.0) | 3/42 (7.1) | 3/44 (6.8) | 0.953 |
| FEV1/FVC§ | 79.3 (72.4–83.5) | 78.8 (70.3–82.7) | 77.9 (73.2–83.0) | 0.244 | 78.7 (72.0–82.6) | 79.1 (71.1–84.1) | 77.8 (74.1–82.4) | 0.554 |
| LVEF (%)§ | 75.3 (69.3–79.8) | 73.9 (66.1–77.8) | 75.4 (69.8–79.7) | 0.047 | 75.4 (67.0–80.3) | 74.5 (66.1–78.5) | 76.0 (69.1–83.1) | 0.345 |
| Child–Pugh classification stage A, n (%) | 141/152 (92.8) | 80/90 (88.9) | 61/62 (98.4) | 0.026 | 78/82 (95.1) | 35/39 (89.7) | 43/43 (100.0) | 0.031 |
| MELD-Na score§ | 8 (7–9) | 7 (7–8) | 8 (10–11) | 0.229 | 7 (7–9) | 7 (7–10) | 8 (7–9) | 0.604 |
| ICG-15 (%)§ | 10.6 (5.2–20.1) | 9.1 (4.4–16.3) | 13.3 (8.1–16.0) | 0.748 | 10.7 (4.2–22.9) | 10.6 (4.5–22.9) | 12.2 (3.8–24.9) | 0.871 |
| Viral hepatitis, n (%) | 139/159 (87.4) | 86/96 (89.6) | 53/63 (84.1) | 0.273 | 76/86 (88.4) | 38/42 (90.5) | 38/44 (86.4) | 0.602 |
*Normal distribution, §Nonnormal distribution. OH: Open hepatectomy, LH: Laparoscopic hepatectomy, F-OH: Open hepatectomy in favorable location group, DM: Diabetes mellitus, HTN: Hypertension, CAD: Coronary artery disease, FEV1: Forced expiratory volume in 1 s, FVC: Forced vital capacity, LVEF: Left ventricular ejection fraction, MELD-Na: Model for End-Stage Liver Disease-Na, ICG: Indocyanine green, BMI: Body mass index
In the subgroup analysis focusing on the favorable location group, 42 patients (48.8%) were categorized into the F-OH group, while 44 patients (51.2%) belonged to the F-LH group. Significantly, a higher proportion of patients in the F-LH group compared to the F-OH group were classified as Child–Pugh class A (100% vs. 89.7%, P = 0.031), while the MELD-Na scores remained similar between both the groups (7 vs. 8, P = 0.604). The preoperative 15-min retention rate of indocyanine green was 10.6% in the F-OH group and 12.2% in the F-LH group, with no statistically significant difference observed (P = 0.871). Viral hepatitis was identified as the primary risk factor for HCC in both the groups of patients, with rates of 90.5% in the F-OH group and 86.4% in the F-LH group, demonstrating no significant difference (P = 0.602).
Perioperative outcomes and histopathology
When comparing the OH versus LH groups, a higher rate of major hepatectomy was observed in the OH group compared to the LH group (38.5% vs. 11.1%, P < 0.001) [Table 2]. Conversely, the LH group exhibited significantly shorter median operation time (208 vs. 255 min, P = 0.025) and lower intraoperative blood loss (250.0 vs. 355.0 mL, P = 0.005) compared to the OH group. In the favorable location subgroup analysis, a greater proportion of patients in the F-OH group underwent major hepatectomy compared to the F-LH group (38.1% vs. 4.5%, P < 0.001). However, the median operation time and intraoperative blood loss were similar in both the groups. Notably, the intraoperative transfusion rate was significantly lower in the F-LH group compared to the F-OH group (2.3% vs. 14.3%, P = 0.042).
Table 2.
Perioperative outcomes and pathology findings
| Characteristics | Whole cohort (n=159) | OH (n=96) | LH (n=63) | P | Favorable location subgroup (n=86) | F-OH (n=42) | F-LH (n=44) | P |
|---|---|---|---|---|---|---|---|---|
| IWATE core | 7 (5–10) | 9 (6–10) | 5 (3–7) | <0.001 | 3 (6–7) | 7 (6–8) | 4 (3–6) | 0.001 |
| Major resection, n (%) | 44/159 (27.7) | 37/96 (38.5) | 7/63 (11.1) | <0.001 | 18/86 (20.9) | 16/42 (38.1) | 2/44 (4.5) | <0.001 |
| Operative time (min)§ | 248 (181–340) | 255 (195–348) | 208 (167–372) | 0.025 | 229 (170–296) | 242 (175–307) | 198 (159–267) | 0.101 |
| Pringle maneuver, n (%) | 122/159 (76.7) | 78/96 (81.3) | 44/63 (69.8) | 0.125 | 59/86 (68.6) | 32/42 (76.2) | 27/44 (61.4) | 0.167 |
| Pringle duration (min)§ | 72.0 (44.8–101.0) | 72.9 (55.0–100) | 59.0 (31–93.5) | 0.021 | 60.0 (35.0–85.0) | 65.0 (49.2–82.5) | 55.0 (27.0–88.0) | 0.322 |
| Blood loss (mL)§ | 300 (100–900) | 355 (150–730) | 250 (125–815) | 0.005 | 200 (100–448) | 300 (138–700) | 150 (50–388) | 0.072 |
| Intraoperative transfusion, n (%) | 15/159 (9.4) | 10/96 (10.4) | 5/63 (7.9) | 0.783 | 7/86 (8.1) | 6/42 (14.3) | 1/44 (2.3) | 0.042 |
| Solitary tumor, n (%) | 130/159 (81.8) | 73/96 (76.0) | 57/63 (90.5) | 0.022 | 75/86 (87.2) | 36/42 (85.7) | 39/44 (88.6) | 0.755 |
| Multiple tumors, n (%) | 29/159 (18.2) | 23/96 (24.0) | 6/63 (9.5) | 11/86 (12.8) | 6/42 (14.3) | 5/44 (11.4) | ||
| 2 | 24/159 (15.1) | 20/96 (20.8) | 4/63 (6.3) | 8/86 (9.3) | 5/42 (11.9) | 3/44 (6.8) | ||
| 3 | 5/159 (3.1) | 3/96 (3.2) | 2/63 (3.2) | 3/86 (3.5) | 1/42 (2.4) | 2/44 (4.6) | ||
| Size (cm)§ | 3.5 (2.5–5.5) | 4.0 (3.0–7.5) | 3.0 (2.5–4.2) | 0.001 | 3.2 (2.5–5.0) | 4.3 (2.7–6.1) | 3.0 (2.3–3.8) | 0.005 |
| Margin (cm)§ | 0.7 (0.2–1.2) | 1.0 (0.2–1.5) | 0.5 (0.2–0.9) | 0.049 | 0.8 (0.2–1.2) | 1.0 (0.2–1.4) | 0.7 (0.2–1.2) | 0.640 |
| Ishak score§ | 4 (1–6) | 3 (0–6) | 5 (3–5) | 0.436 | 4 (2–6) | 4 (0–6) | 4 (2–6) | 0.346 |
§Nonnormal distribution. OH: Open hepatectomy, LH: Laparoscopic hepatectomy, F-OH: Open hepatectomy in favorable location group, F-LH: Laparoscopic hepatectomy in favorable location group
In comparison to the OH group, the LH group exhibited a higher proportion of patients diagnosed with solitary HCC (90.5% vs. 76.0%, P = 0.022) and a smaller median tumor diameter (3.0 vs. 4.0 cm, P = 0.001) [Table 2]. In the favorable location subgroup analysis, the majority of patients in both the groups underwent hepatectomy for solitary tumors (85.7% in the F-OH group vs. 88.6% in the F-LH group, P = 0.755). However, the median tumor diameter was significantly smaller in the F-LH group compared to the F-OH group (3.0 vs. 4.3 cm, P = 0.005). Notably, no significant differences were observed in resection margin status or Ishak score between the two groups.
Postoperative short-term outcomes
The overall complication rate was lower in the LH group than in the OH group (33.3% vs. 50.0%, P = 0.050) [Table 3]. In the favorable location subgroup analysis, the F-LH group had a lower rate of major complications rate than the F-OH group (12.0% vs. 2.3%, P = 0.080). In the F-OH group, two patients experienced grade IIIa complications (both had pleural effusion), one patient experienced a grade IIIb complication (grade C bile leakage), one patient experienced a grade IVa complication (posthepatectomy liver failure), and one patient experienced a grade IVb complications (acute kidney injury and posthepatectomy liver failure). In the F-LH group, one patient experienced a grade IIIa complication (superficial surgical site infection). No grade V complications developed in either group.
Table 3.
Postoperative outcomes
| Characteristics | Whole cohort (n=159) | OH (n=96) | LH (n=63) | P | Favorable location subgroup (n=86) | F-OH (n=42) | F-LH (n=44) | P |
|---|---|---|---|---|---|---|---|---|
| Complications, n (%) | 69/159 (43.4) | 48/96 (50.0) | 21/63 (33.3) | 0.050 | 40/86 (46.6) | 24/42 (57.2) | 16/44 (36.4) | 0.083 |
| Major complications, n (%) | 12/159 (7.5) | 9/16 (9.4) | 3/63 (4.8) | 0.281 | 6/86 (7.0) | 5/42 (11.9) | 1/44 (2.3) | 0.080 |
| Minor complications, n (%) | 57/159 (35.8) | 39/96 (40.7) | 18/63 (28.5) | 0.132 | 34/86 (39.5) | 19/42 (45.2) | 15/44 (34.1) | 0.378 |
| Postoperative hospital stay (days)§ | 8 (7–12) | 11 (9–14) | 7 (6–10) | <0.001 | 8 (6–10) | 10 (8–17) | 7 (6–8) | <0.001 |
| 90-day readmission rate, n (%) | 14/159 (8.8) | 12/96 (12.5) | 2/63 (3.2) | 0.048 | 7/86 (8.1) | 6/42 (14.3) | 1/44 (2.3) | 0.042 |
| 90-day mortality rate, n (%) | 6/159 (3.8) | 5/96 (5.2) | 1/63 (1.6) | 0.241 | 3/86 (3.5) | 3/42 (7.1) | 0 | 0.071 |
§Nonnormal distribution. OH: Open hepatectomy, LH: Laparoscopic hepatectomy, F-OH: Open hepatectomy in favorable location group, F-LH: Laparoscopic hepatectomy in favorable location group
The postoperative hospital stay was significantly shorter in the LH group compared to the OH group (7 vs. 11 days, P < 0.001) [Table 3]. Furthermore, the 90-day readmission rate was significantly lower in the LH group than in the OH group (3.2% vs. 12.5%, P = 0.048) [Table 3]. However, there was no significant difference in 90-day mortality between both the groups (1.6% vs. 5.2%, P = 0.241). In the favorable location subgroup analysis, the F-LH group exhibited a shorter postoperative hospital stay compared to the F-OH group (7 vs. 10 days, P < 0.001) and a significantly lower 90-day readmission rate (2.3% vs. 14.3%, P = 0.042). Nevertheless, no significant difference was observed in the 90-day mortality rate between the F-LH and F-OH groups (0.0% vs. 7.1%, P = 0.071).
Long-term outcomes
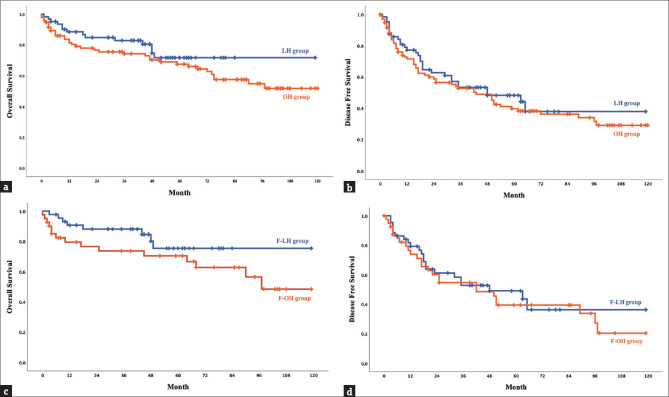
Between the LH and OH groups, no significant difference was observed in 12-, 36-, and 60-month overall survival rates (88.5%, 82.9%, and 71.8% vs. 83.7%, 74.3%, and 67.6%, respectively, P = 0.257) and disease-free survival rates (77.2%, 53.3%, and 48.3% vs. 71.5%, 52.8%, and 39.7%, respectively, P = 0.509) [Figure 3a and b]. In the OH group, intrahepatic recurrence occurred in 35 patients (94.6%), while 2 patients (5.4%) experienced both intrahepatic and extrahepatic recurrence. In contrast, in the LH group, intrahepatic recurrence was observed in 19 patients (95.0%), with 1 patient (5.0%) experiencing extrahepatic recurrence. In the favorable location subgroup analysis, the F-LH and F-OH groups exhibited no significant difference in 12-, 36-, and 60-month overall survival rates (respectively, 90.7%, 88.1%, and 75.4% vs. 79.5%, 76.6%, and 70.4%, P = 0.184) and disease-free survival rates (respectively, 79.2%, 52.9%, and 49.1% vs. 73.9%, 54.7%, and 39.5%, P = 0.683) [Figure 3c and d]. In the F-OH group, intrahepatic recurrence occurred in all 13 patients (100%), whereas in the F-LH group, 16 patients (94.1%) experienced intrahepatic recurrence and 1 patient (5.9%) experienced extrahepatic recurrence.
Figure 3.
Kaplan–Meier curve of the overall survival and disease-free survival in the LH, OH, F-LH, and F-OH groups. (a and b) The overall survival and disease-free survival rates in LH and OH groups. (c and d) The overall survival and disease-free survival rates in F-LH and F-OH groups. LH: Laparoscopic hepatectomy, OH: Open hepatectomy, F-LH: Laparoscopic hepatectomy in the favorable location group, Open hepatectomy in the favorable location group
DISCUSSION
The first feasibility study for LH in 2000 concluded that LH was both feasible and safe for patients with left- and right-sided peripheral lesions requiring limited resection [4]. Subsequently, numerous feasibility studies for LH have been conducted under various circumstances, including considerations such as tumor size [5], previous abdominal surgery [6], cirrhotic patients [7], and elderly patients [8]. These studies consistently indicated that LH does not compromise perioperative outcomes, short-term outcomes, or long-term survival. Throughout this period, the indications for LH have evolved, initially outlined in the Louisville statement of 2008 [20], which delineated specific criteria for LH, and later refined in the Morioka consensus of 2014 [21], which found no definitive indications for LH. Consequently, at our center, the implementation of LH began with smaller tumors and minor resections, gradually progressing to encompass major resections during the developmental phase. Adhering to the principles outlined in the Louisville statement, we primarily targeted tumors located in peripheral segments, specifically segments 2–6, which are considered favorable locations for LH.
Several retrospective studies have investigated the feasibility and safety of LH in patients with HCC [22,23,24]. In the study conducted by Kim et al. demonstrated desirable outcomes associated with LH, including reduced postoperative ascites (0.0% vs. 17.2%, P = 0.025), shorter hospital stays (7.69 ± 2.94 vs. 13.38 ± 7.37 days, P < 0.001), and preserved long-term survival rates (12-, 36-, and 60-monthsurvival rates of 100%, 100%, and 92.2%, respectively; 12-, 36-, and 60-month disease-free survival rates of 81.7%, 61.7%, and 54.0%, respectively) [22]. Similarly, Memeo et al. [23] reported significantly shorter operative times (80 vs. 140 min, P = 0.02), reduced hospital stays (7 vs. 12 days, P < 0.0001), and lower morbidity rates (20% vs. 4%, P = 0.01) associated with LH, without compromising long-term survival rates (1-, 5-, and 10-year rates of 88%, 59%, and 12%, respectively). In our series comparing F-LH and F-OH, the F-LH group exhibited a significantly shorter postoperative hospital stay (7 vs. 10 days, P < 0.001), with a trend toward a lower major complication rate (2.3% vs. 11.9%, P = 0.080), consistent with findings from previous studies. Regarding long-term outcomes, there were no significant differences observed in 12-, 36-, and 60-month overall survival rates (respectively, 90.7%, 88.1%, and 75.4% vs. 79.5%, 76.6%, and 70.4%, P = 0.184) and disease-free survival rates (respectively, 79.2%, 52.9%, and 49.1% vs. 73.9%, 54.7%, and 39.5%, P = 0.683), aligning with previous research. These results suggest that the utilization of laparoscopic techniques alone may not directly influence outcomes; instead, factors such as intraoperative hemorrhage-related blood transfusion, tumor exposure, and surgical margin, which are affected by the laparoscopic approach, may impact tumor recurrence and survival in HCC patients [25,26,27]. Proficiency in laparoscopic techniques and the learning curve associated with hepatectomy are crucial prerequisites for successful LH. Additionally, when implementing LH, patient selection plays a pivotal role in determining both short-term and long-term outcomes. Among various selection criteria, the tumor’s location emerges as one of the most critical factors influencing the difficulty of LH [28].
Regarding tumor location, the majority of studies investigating the feasibility of LH have primarily focused on tumors situated in unfavorable locations. For instance, in a comparative analysis by Kwon et al., tumors in unfavorable locations were found to have similar median blood loss (500 vs. 400 mL, P = 0.214), rates of intraoperative transfusion (39% vs. 19%, P = 0.061), median postoperative hospital stay (10 vs. 8 days, P = 0.166), and complication rates (21% vs. 11%, P = 0.148) when compared to tumors in more favorable locations [29]. Similarly, the INSTALL-2 study reported that tumors located at segments S7 and S8 were associated with a median operative time of 315 min, a postoperative hospital stay of 7 days, and a major complication rate of 11.9% [30]. It is important to note that these studies were conducted by well-developed and experienced centers. The evidence provided by studies focusing on tumor location suggests that LH may be feasible even in unfavorable locations when performed in high-quality centers with expertise in the procedure.
Unfortunately, there is currently insufficient evidence available for centers seeking to initiate the development of LH. In our present study, we analyzed cases of HCC located in favorable locations who underwent LH during the developmental phase. Following the indications outlined in the Louisville statement, LH was initiated for tumors situated in favorable locations and those of smaller sizes, with minor resections performed initially before advancing to more complex procedures during the developmental phase at our institution. Consequently, the ratio of major resections was significantly higher in the F-OH group compared to the F-LH group. Throughout the developmental phase of our study, the utilization of laparoscopic techniques gradually increased, eventually becoming the predominant intervention in our series as proficiency in the technique improved. Importantly, during this developmental period, short-term outcomes such as intraoperative blood loss, major complication rate, postoperative hospital stay, 90-day readmission rate, and 90-day mortality rate were not compromised under the laparoscopic approach. Furthermore, long-term outcomes, including overall and disease-free survival rates, remained unaffected by the use of the laparoscopic method. Our study provides evidence to suggest that centers embarking on the development of LH, even those with lower surgical volumes (<20–50 liver resections per year), may benefit from implementing this technique for favorably located tumors without significantly impacting short- and long-term outcomes, even without being classified as high-volume centers [31].
Only a limited number of studies have focused on the experience of developing LH using cases of favorably located tumors. Our study successfully demonstrates the feasibility and safety of LH during its developmental phase within a single center. These findings hold promise for institutions aspiring to initiate the development of this procedure. However, it is important to acknowledge several limitations of our study. First, this was a retrospective, nonrandomized study, which may have introduced observation bias. Second, there was a lack of data on short- and long-term quality of life outcomes, such as the presence of incisional abdominal wall hernia and assessments of pain scale. Third, the study had a relatively small sample size and was conducted at a single center. As the technique of LH becomes more mature, we plan to gradually expand its application to more challenging cases. Subsequent studies will aim to provide further insights into the outcomes associated with LH in broader patient populations and settings.
CONCLUSIONS
LH emerges as a viable alternative to OH for primary HCC with favorably located tumors. Our study highlights several advantages of LH, including reduced intraoperative transfusion rates, shorter postoperative hospital stays, and lower 90-day readmission rates compared to OH. Importantly, LH does not compromise outcomes related to intraoperative blood loss, complication rates, morbidity, 90-day mortality rates, or long-term overall and disease-free survival. These findings underscore the feasibility and safety of LH as a surgical approach for primary HCC located at favorable sites during the developmental phase. Institutions contemplating the adoption of LH can draw confidence from our study, as it provides evidence supporting the efficacy and benefits of LH in this patient population.
Data availability statement
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Ming-Che Lee, an editorial board member at Tzu Chi Medical Journal, had no role in the peer review process of or decision to publish this article. The other authors declared no conflicts of interest in writing this paper.
REFERENCES
- 1.International Agency for Research on Cancer Liver. World Health Organization. 2020. [[Last accessed on 2022 Feb 28]]. Available from: https://www.gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf .
- 2.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681–93. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 4.Cherqui D, Husson E, Hammoud R, Malassagne B, Stéphan F, Bensaid S, et al. Laparoscopic liver resections: A feasibility study in 30 patients. Ann Surg. 2000;232:753–62. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ai JH, Li JW, Chen J, Bie P, Wang SG, Zheng SG. Feasibility and safety of laparoscopic liver resection for hepatocellular carcinoma with a tumor size of 5-10 cm. PLoS One. 2013;8:e72328. doi: 10.1371/journal.pone.0072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng L, Cao J, Hu X, Xiao W, Zhou Z, Mao S. Safety and feasibility of laparoscopic liver resection for patients with previous upper abdominal surgery: A systematic review and meta-analysis. Int J Surg. 2019;65:96–106. doi: 10.1016/j.ijsu.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Pan Y, Xia S, Cai J, Chen K, Cai X. Efficacy of laparoscopic hepatectomy versus open surgery for hepatocellular carcinoma with cirrhosis: A meta-analysis of case-matched studies. Front Oncol. 2021;11:652272. doi: 10.3389/fonc.2021.652272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amato B, Aprea G, De Rosa D, Milone M, di Domenico L, Amato M, et al. Laparoscopic hepatectomy for HCC in elderly patients: Risks and feasibility. Aging Clin Exp Res. 2017;29:179–83. doi: 10.1007/s40520-016-0675-6. [DOI] [PubMed] [Google Scholar]
- 9.Lee YH, Huang YT, Kuo TL, Lee MC, Chen YC. Laparoscopic hepatectomy is a feasible and safe choice for primary hepatocellular carcinoma. Surg Insights [Internet] 2022. [[Last cited on 2024 Apr 28]]. Available from: https://mediterraneanjournals.com/index.php/si/article/view/687 .
- 10.Chen J, Li H, Liu F, Li B, Wei Y. Surgical outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma for various resection extent. Medicine (Baltimore) 2017;96:e6460. doi: 10.1097/MD.0000000000006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831–41. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- 12.Ho KM, Cheng KC, Chan FK, Yeung YP. Laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: A propensity case-matched analysis of the long-term survival. Ann Hepatobiliary Pancreat Surg. 2021;25:1–7. doi: 10.14701/ahbps.2021.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buell JF, Thomas MJ, Doty TC, Gersin KS, Merchen TD, Gupta M, et al. An initial experience and evolution of laparoscopic hepatic resectional surgery. Surgery. 2004;136:804–11. doi: 10.1016/j.surg.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67–72. doi: 10.1002/bjs.5150. [DOI] [PubMed] [Google Scholar]
- 15.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 16.Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;2:333–9. [Google Scholar]
- 17.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann Surg. 2009;250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 18.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: A definition and grading of severity by the international study group of liver surgery. Surgery. 2011;149:680–8. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: A definition and grading by the international study group of liver surgery (ISGLS) Surgery. 2011;149:713–24. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: The Louisville statement, 2008. Ann Surg. 2009;250:825–30. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 21.Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619–29. doi: 10.1097/SLA.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Suh KS, Lee KW, Yi NJ, Hong G, Suh SW, et al. Long-term outcome of laparoscopic versus open liver resection for hepatocellular carcinoma: A case-controlled study with propensity score matching. Surg Endosc. 2014;28:950–60. doi: 10.1007/s00464-013-3254-3. [DOI] [PubMed] [Google Scholar]
- 23.Memeo R, de’Angelis N, Compagnon P, Salloum C, Cherqui D, Laurent A, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma of cirrhotic liver: A case-control study. World J Surg. 2014;38:2919–26. doi: 10.1007/s00268-014-2659-z. [DOI] [PubMed] [Google Scholar]
- 24.Lee DH, Kim D, Park YH, Yoon J, Kim JS. Long-term surgical outcomes in patients with hepatocellular carcinoma undergoing laparoscopic versus open liver resection: A retrospective and propensity score-matched study. Asian J Surg. 2021;44:206–12. doi: 10.1016/j.asjsur.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Lu Q, Zhang N, Wang F, Chen X, Chen Z. Surgical and oncological outcomes after laparoscopic versus open major hepatectomy for hepatocellular carcinoma: A systematic review and meta-analysis. Transl Cancer Res. 2020;9:3324–38. doi: 10.21037/tcr.2020.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harada N, Shirabe K, Maeda T, Kayashima H, Ishida T, Maehara Y. Blood transfusion is associated with recurrence of hepatocellular carcinoma after hepatectomy in Child-Pugh class A patients. World J Surg. 2015;39:1044–51. doi: 10.1007/s00268-014-2891-6. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Xu J, Hong J, Si Y, He Y, Zhang J. Prognostic impact of surgical margin in hepatectomy on patients with hepatocellular carcinoma: A meta-analysis of observational studies. Front Surg. 2022;9:810479. doi: 10.3389/fsurg.2022.810479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakabayashi G. What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg Nutr. 2016;5:281–9. doi: 10.21037/hbsn.2016.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon Y, Lee B, Cho JY, Han HS, Yoon YS, Lee HW, et al. Acase-matched analysis of laparoscopic liver resection for hepatocellular carcinoma located in posterosuperior segments of the liver according to adaption of developed techniques. Medicina (Kaunas) 2022;58:543. doi: 10.3390/medicina58040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibuki S, Hibi T, Tanabe M, Geller DA, Cherqui D, Wakabayashi G, et al. Short-term outcomes of “difficult” laparoscopic liver resection at specialized centers: Report from INSTALL (international survey on technical aspects of laparoscopic liver resection)-2 on 4478 patients. Ann Surg. 2022;275:940–6. doi: 10.1097/SLA.0000000000004434. [DOI] [PubMed] [Google Scholar]
- 31.Eppsteiner RW, Csikesz NG, Simons JP, Tseng JF, Shah SA. High volume and outcome after liver resection: Surgeon or center? J Gastrointest Surg. 2008;12:1709–16. doi: 10.1007/s11605-008-0627-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.