Abstract
Background
In Indonesia, heart failure has become a major community problem because of the high cost of care, low quality of life, and premature death. Until now, loop diuretics are still the main therapy in patients with acute decompensated heart failure with clinical congestion. Diuretic responsiveness can be assessed objectively by measuring sodium urine. This study aimed to determine the response of natriuresis 2 h after loop diuretic administration and its relationship to length of stay and readmission within 30 days in daily clinical practice.
Methods
This is a prospective cohort study conducted at the National Cardiovascular Center Harapan Kita Hospital in acute decompensated heart failure patients. Patient characteristics were collected from medical records. Response to intravenous (IV) loop diuretics was assessed using urinary sodium laboratory panels. The primary outcomes of interest in this study were length of stay and rehospitalization. Analyses were conducted between the outcome of interests and patient characteristics.
Results
There were 51 acute decompensated heart failure patients in this study with 78.4% males. The mean age was 52.47 ± 13.62. The mean ejection fraction was 37.53±17.95%, with the majority of patients having a left ventricular ejection fraction less than 40% (62.7% of study subjects). The average glomerular filtration rate of subjects in this study was 57.29 ± 27.25 mL/min. Pearson correlation test between pre- and post-loop diuretic urinary sodium showed trends of significant correlation (r = -0.238, P = 0.093) and (r = -0.308, P = 0.028), respectively. Patients with lower pre-loop diuretic urinary sodium were shown to have a shorter length of stay (8.57 ± 6.161 vs. 5.30 ± 4.01, P = 0.04), while patients with lower post-loop diuretic urinary sodium showed trends of longer length of stay (8.67 ± 4.14 vs. 6.03 ± 5.39, P = 0.126).
Conclusions
In this study, we observe lower rehospitalization in patients with higher pre-loop diuretic urinary sodium levels. Post-loop diuretic urinary sodium level was shown to be inversely related to length of stay in acute decompensated heart failure patients.
Keywords: Acute decompensated heart failure, Loop diuretic, Urine sodium
Introduction
Due to the high mortality and morbidity, heart failure remains a challenge in developed and developing countries with high rates of rehospitalization [1]. Globally it is predicted that the incidence of heart failure in patients age 60 and older will increase twofold by 2025 and threefold in 2050, while the incidence in developing countries will increase from 1.5-4% to 6.7-9% [2].
In Indonesia, heart failure treatment remains a challenge due to the high cost of treatment, poor patient quality of life, and high mortality, with the incidence of heart failure on the steady rise in the past decade. Contrary to developed countries, Indonesia has seen a higher incidence of heart failure in younger patients. A study from the National Cardiovascular Center in Indonesia found the post-treatment mortality rate to be 12% and the rehospitalization rate to be 26% [1].
Current treatment of acute decompensated heart failure still emphasizes a safe decongestive regimen that should be implemented as early and effectively as possible to ameliorate congestion, and fluid overload and decrease the length of hospitalization of patients, with loop diuretics being one of the most commonly used decongestive agent in acute decompensated heart failure. Although loop diuretics are heavily utilized in the early decongestion of acute decompensated heart failure patients, assessing its treatment effect in terms of congestion status has proven to be challenging [3].
Impaired natriuresis is a phenomenon seen in all patients with subclinical and clinical systolic and diastolic heart failure. This phenomenon might be a factor that drives the progression of heart failure, as opposed to only being a consequence of elevated renin angiotensin aldosterone system (RAAS) activation caused by heart dysfunction [4]. Amongst other factors, impaired heart function and renal venous hypertension have been implicated as the contributing factors to this phenomenon [5].
Alterations in urinary sodium levels have been recently studied as a predictor of long-term adverse events in acute decompensated heart failure patients receiving loop diuretics. One study showed that impaired natriuretic response to loop diuretics is associated with worsening renal function in the long run [6].
In this study, we aim to investigate the association between post-loop diuretic urinary sodium levels with the length of stay and rehospitalization in acutely decompensated heart failure patients.
Materials and Methods
Study design
This is a prospective cohort study of acutely decompensated heart failure patients who were hospitalized at the National Cardiovascular Center Harapan Kita, Jakarta, Indonesia, between March and April 2021.
The inclusion criteria for this study were: 1) adult male or female (age > 18); 2) acutely decompensated heart failure patients with a minimum of one congestive symptom (edema, dyspnea, or orthopnea) and a minimum of one physical sign of congestion (edema, rales, ascites, elevated jugular venous pressure, vascular congestion on chest X-ray). The exclusion criteria for this study were: 1) patient without previous history of heart failure; 2) referred patients that have received loop diuretics in the preceding 24 h. This study was approved by the Ethical Institutional Review Board of the National Cardiovascular Center Harapan Kita, Jakarta, Indonesia (ethical clearance No. LB 01.02/VII/KEP 016/2021). Prospective collection of patients’ characteristics and clinical parameters were collected from medical records and hospital information systems. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Intervention protocol
Patients were asked to urinate in a collection pot before the administration of the intravenous loop diuretic (furosemide). Patients who were unable to urinate were assisted using a urinary catheter to ensure bladder emptying. Palpation was performed to assess bladder filling status post urination. Intravenous furosemide was then administered in daily doses of one to two times, as directed by the managing physician. The second urine collection was performed 2 h after the administration of furosemide. Samples were taken to the Laboratory of National Cardiovascular Center Harapan Kita, to be analyzed using a selective ion electrode-based device (ISE indirect Na-K-Cl for Gen.2; COBAS; Roche/Hitachi, Japan) with a normal urinary sodium reference range of 40 - 220 mmol/24 h. Aggressive diuretic strategy is defined as diuretic dosing in acute decompensated heart failure management as per the European Society of Cardiology guideline in the management of acute decompensated heart failure [7], while nonaggressive diuretic strategy is defined as diuretic dosing in acute decompensated heart failure management at the clinician’s discretion. Patients were monitored in the ward during treatment. Patients were discharged from the hospital when signs of clinical decongestion were achieved (absence of pulmonary rales, absence of Lower Extremity Edema, absence of orthopnea).
Data analysis
The baseline characteristics of study subjects were presented using tables. Categorical data were presented using proportion and frequency, while numerical data were presented with mean ± standard deviation or interquartile ranges with minimum or maximum values depending on data distribution. We assessed data distribution using the Shapiro-Wilk test with a threshold of P < 0.05. Urinary sodium thresholds of 50 mmol/L were adopted based on the results of previous studies [6, 8, 9].
Urinary sodium level was predefined as urinary sodium level obtained from measurement 2 h post administration of loop diuretic of furosemide that was twice the pre-admission routine dosage. Dichotomized urinary sodium levels were defined as urinary sodium levels that have been dichotomized into 1) below 50 mmol/L; and 2) beyond > 50 mmol/L. This threshold was selected based on previous publications on this matter [6, 8, 9]. There were two patterns of loop diuretic administration in this study. Aggressive diuretic administration refers to diuretic administration according to the latest European Society of Cardiology guideline for the diagnosis and treatment of acute heart failure [7]. Meanwhile, a nonaggressive strategy refers to a loop diuretic administration strategy based on previous guidelines.
Bivariate analysis between urinary sodium and the outcome of interest was performed using a Chi-square test. Independent t-test and Mann-Whitney test were performed on numerical outcomes based on data distribution. Pearson correlation tests were performed between continuous variables.
Interaction between confounding variables and categorical outcomes is also analyzed using the Chi-square test for categorical variables and an independent t-test or Mann-Whitney test for numerical data. Numerical outcomes are analyzed using the independent t-test or Mann-Whitney test and Spearman or Pearson correlative tests. Confounding variables with a P value < 0.05 will be included in multivariate analysis to assess their influence on study outcomes. Multivariate analyses in this study are performed using logistic regression. Data were analyzed with SPSS software version 21.
Results
There were 51 acute decompensated heart failure patients in this study with 78.4% males. The mean age was 52.47 ± 13.62 years old. The mean left ventricular ejection fraction was 37.53±17.95%, with the majority of patients having less than 40% (62.7% of study subjects). The average glomerular filtration rate of subjects in this study was 57.29 ± 27.25 mL/min. The majority of patients in this study were administered an aggressive diuretic treatment strategy as per the European Society of Cardiology guideline on the management of acute decompensated heart failure (58.8%). Patients with type 2 diabetes mellitus were not excluded from this study, with baseline random blood sugar of 134.43 ± 46.83 mg/dL. Most of the patients in this study already received heart failure medication regimens of angiotensin-converting enzyme inhibitors or angiotensin receptor blocker (78.4%) and beta-blockers (70.6%). The mean serum sodium level between pre- and post-loop diuretic was 134.18 ± 4.55 vs. 135.53 ± 1.33 mmol/L, and urinary sodium of pre- and post-loop diuretic was 67.69 ± 40.1 vs. 87.78 ± 36.37 mmol/L. Most of the study subjects showed urinary sodium levels > 60 mmol/L (70.6%) with only 29.4% of patients showing otherwise (Table 1).
Table 1. Baseline Characteristics of Study Subjects (N = 51).
| Gender, n (%) | |
| Male | 40 (78.4) |
| Female | 11 (21.6) |
| Age (years), mean ± SD | 52.47 ± 13.62 |
| Left ventricular ejection fraction (%), mean ± SD | 37.53 ± 17.95 |
| Left ventricular ejection fraction category, n (%) | |
| > 50% | 14 (27.4) |
| 40-50% | 5 (9.8) |
| < 40% | 32 (62.7) |
| Systolic blood pressure (mm Hg), mean ± SD | 124.22 ± 26.35 |
| Diastolic blood pressure (mm Hg), mean ± SD | 74.76 ± 17.45 |
| Glomerular filtration rate (mL/min/1.73 m2), mean ± SD | 57.29 ± 27.25 |
| Random blood glucose (mg/dL), mean ± SD | 134.43 ± 46.83 |
| Diuretic administration strategy | |
| Nonaggressive | 21 (41.2) |
| Aggressive | 30 (58.8) |
| Angiotensin-converting enzyme inhibitors/ARB, n (%) | 40 (78.4) |
| Beta-blockers, n (%) | 36 (70.6) |
| Adherence to medication regiments, n (%) | 11 (21.6) |
| Baseline urinary and sodium levels | |
| Serum sodium (mmol/L) | |
| Pre-loop diuretic (mean ± SD) | 134.18 ± 4.55 |
| Post-loop diuretic (mean ± SD) | 135.53 ± 4.33 |
| Urinary sodium (mmol/L) | |
| Pre-loop diuretic (mean ± SD) | 67.69 ± 40.1 |
| Post-loop diuretic (mean ± SD) | 87.78 ± 36.37 |
| Pre-loop diuretic urinary sodium, n (% of subjects) | |
| < 50 mmol/L | 21(41.2) |
| > 50 mmol/L | 30 (58.8) |
| Post-loop diuretic urinary sodium, n (% of subjects) | |
| < 50 mmol/L | 12 (23.5) |
| > 50 mmol/L | 39 (76.5) |
SD: standard deviation; ARB: angiotensin receptor blocker.
The Pearson correlation test between pre- and post-loop diuretic urinary sodium showed trends of significant correlation (r = -0.238, P = 0.093) and (r = -0.308, P = 0.028), respectively (Figs. 1, 2).
Figure 1.
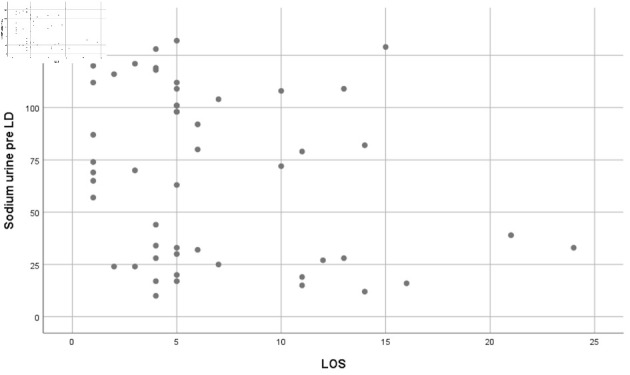
Pearson correlation result showing trends of significant correlation between pre-loop diuretic (LD) sodium urine and length of stay (LOS) (r = -0.238, P = 0.093).
Figure 2.
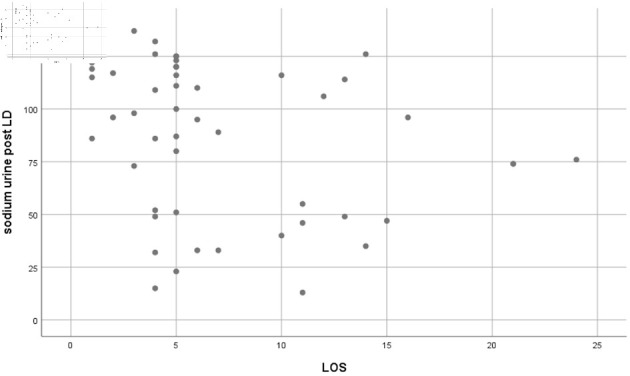
Pearson correlation result showing a statistically significant correlation between post-loop diuretic (LD) sodium urine and length of stay (LOS) (r = -0.308, P = 0.028).
Patients with lower pre-loop diuretic urinary sodium were shown to have a shorter length of stay (8.57 ± 6.161 vs. 5.30 ± 4.01, P = 0.04), while patients with lower post-loop diuretic urinary sodium showed trends of longer length of stay (8.67 ± 4.14 vs. 6.03 ± 5.39, P = 0.126) (Table 2). Beta-blocker use (P = 0.013) and pre-loop diuretic urinary sodium levels (P = 0.05) were associated with readmission 30 days after discharge; however, further multivariate analysis regarding readmission in 30 days showed no statistically significant results (Table 3). We performed multivariate linear regression analysis between select variables and length of stay. Urine sodium post-loop diuretic levels were associated with an increase in length of stay (B = -0.342, P = 0.041) (Table 4).
Table 2. Comparison of Length of Stay Between Variables.
| Length of stay (days) (mean ± SD) | P value | |
|---|---|---|
| Pre-loop diuretic urinary sodium | ||
| ≤ 50 mmol/L (n = 21) | 8.57 ± 6.161 | 0.04 |
| > 50 mmol/L (n = 30) | 5.30 ± 4.01 | |
| Post-loop diuretic urinary sodium | ||
| ≤ 50 mmol/L (n = 12) | 8.67 ± 4.14 | 0.126 |
| > 50 mmol/L (n = 39) | 6.03 ± 5.39 | |
| Gender | ||
| Male (n = 40) | 6.28 ± 4.89 | 0.417 |
| Female (n = 11) | 8.00 ± 6.34 | |
| Diuretic administration strategy | ||
| Nonaggressive (n = 21) | 6.43 ± 3.84 | 0.805 |
| Aggressive (n = 30) | 6.80 ± 6.05 |
Table 3. Factors Associated With Rehospitalization in 30 Days.
| Rehospitalization in 30 days after discharge |
P value | ||
|---|---|---|---|
| Yes (n = 9) | No (n = 42) | ||
| Male gender | 9 (100%) | 31 (73.8%) | 0.177 |
| Aggressive diuretic strategy | 4 (44.4%) | 25 (59.5%) | 1.00 |
| ACE inhibitor/angiotensin receptor blocker use during hospitalization | 5 (55.6%) | 35 (83.3%) | 0.087 |
| Beta-blocker use during hospitalization | 3 (33.3%) | 33 (78.6%) | 0.013 |
| Non-adherence to medication prescribed | 6 (66.67%) | 34 (80.9%) | 0.958 |
| Serum sodium pre-loop diuretic | 132.00 ± 4.183 | 134.64 ± 4.536 | 0.115 |
| Serum sodium post-loop diuretic | 133.89 ± 5.968 | 135.88 ± 3.902 | 0.362 |
| Pre-loop diuretic urinary sodium | 46.44 ± 30.99 | 72.27 ± 40.64 | 0.05 |
| ≤ 50 mmol/L (n = 21) | 5 (55.6) | 16 (38.1) | 0.46 |
| > 50 mmol/L (n = 30) | 4 (44.4) | 26 (61.9) | |
| Post-loop diuretic urinary sodium | 77.44 ± 30.67 | 90.00 ± 37.43 | 0.300 |
| ≤ 50 mmol/L (n = 12) | 2 (22.2) | 10 (23.8) | 1.000 |
| > 50 mmol/L (n = 39) | 7 (77.8) | 32 (76.2) | |
| Age (years), mean ± SD | 53.22 ± 13.24 | 49.89 ± 16.28 | 0.536 |
| Left ventricular ejection fraction (%), mean ± SD | 38.03 ± 18.16 | 35.22 ± 17.75 | 0.674 |
| SBP (mm Hg), mean ± SD | 127.45 ± 27.29 | 109.11 ± 14.56 | 0.057 |
| DBP (mm Hg), mean ± SD | 76.05 ± 18.30 | 68.78 ± 11.745 | 0.261 |
| Glomerular filtration rate, mean ± SD | 55.88 ± 27.62 | 63.89 ± 25.96 | 0.429 |
| Random blood glucose (mg/dL), median (IQR) | 137.24 ± 50.38 | 121.33 ± 22.215 | 0.361 |
ACE: angiotensin-converting enzyme; SD: standard deviation; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Table 4. Multivariate (Linear Regression) Analysis on Factors Associated With Length of Stay.
| Variables | Unstandardized coefficients |
Standardized coefficient |
t | P value | |
|---|---|---|---|---|---|
| B | SE | B | |||
| Glomerular filtration rate | -0.014 | 0.026 | -0.074 | -0.537 | 0.594 |
| Serum sodium pre-loop diuretic | 0.484 | 0.254 | 0.423 | 1.908 | 0.063 |
| Serum sodium post-loop diuretic | -0.387 | 0.230 | -0.322 | -1.683 | 0.099 |
| Urine sodium pre-loop diuretics | -0.026 | 0.021 | -0.202 | -1.265 | 0.212 |
| Urine sodium post-loop diuretics | -0.49 | 0.023 | -0.342 | -2.100 | 0.041 |
SE: standard error.
Discussion
In this study, the mean age of subjects was 52.63 years and dominated by males, which is similar to the Framingham cohort and European Society of Cardiology study [10-12]. The predominantly older age of male patients suffering from acute decompensated heart failure might be attributed to several factors such as: 1) long-standing poor lifestyle of choice; 2) the increased life expectancy in patients with type 2 diabetes mellitus and hypertension, which is a predisposing factor to acute decompensated heart failure; 3) increase in the prevalence of ischemic heart disease, valvular heart disease and hypertensive heart disease; 4) presence of other comorbidities such as atrial fibrillation, renal dysfunction, chronic obstructive pulmonary disease, and peripheral arterial disease. The presence of these comorbidities in patients increases the risk of heart failure and its complications [13].
Most of the patients in this study suffer from heart failure with reduced ejection fraction (HFrEF) with an ejection fraction lower than 40% (62.7%). This is similar to the Framingham cohort study, which found that HFrEF is more commonly found in male acute decompensated heart failure patients compared to females [10, 11].
Analysis showed a higher length of stay in patients with pre-loop diuretics urinary sodium levels below 50 mmol/L compared to above 50 mmol/L (8.57 ± 6.161 vs. 5.30 ± 4.01 days, P = 0.04), while analysis of post-loop diuretic urinary sodium only showed trends of longer length of stay in patients with levels < 50 mmol/L (8.67 ± 4.14 vs. 6.03 ± 5.39, P = 0.126). Pre-loop diuretic urinary sodium levels reflect the constant state of reduced sodium excretion in chronic heart failure patients, which is caused by the increased activation of the RAAS system. These indices are associated with the increased sodium avidity, which is intrinsically present in heart failure patients [14]. Pre-loop diuretic sodium levels reflect chronic RAAS activation and sodium retention in heart failure patients, which will cause congestion and hospitalizations.
Renal under-perfusion is a common phenomenon in patients with heart failure. This in turn will activate the RAAS and increase sodium retention. Chronic sodium retention, which is a hallmark of heart failure, will increase extracellular volume, and cause congestion and clinical symptoms [3, 15]. The low levels of urinary sodium levels in acute heart failure episodes are related to this phenomenon, which is more commonly known as “cardiorenal syndrome”. In this phenomenon, chronic renal underperfusion reduced systemic blood pressure, increased renal venous pressure, and right ventricular dysfunction caused renal dysfunction in patients with heart failure [16-18]. These mechanisms are the most likely cause of low urinary sodium levels after administration of loop diuretics.
In our study, rehospitalized patients show lower pre-loop diuretic urinary sodium levels compared to non-rehospitalized patients (46.44 ± 30.99 vs. 72.27 ± 40.64 mmol/L, P = 0.05). Post-loop diuretic urinary sodium was not found to be significantly associated with rehospitalization. Patients with lower pre-loop diuretic urinary sodium (< 50 mmol/L), which reflects a more severe state of RAAS activation were also observed to have longer length of stay in this study (8.57 ± 6.16 vs. 5.30 ± 4.01 days, P = 0.04).
Loop diuretics are primarily used as a decongestive agent in episodes of acute decompensated heart failure. Variability in diuresis response to loop diuretics exists and has been linked to adverse outcomes [19].
Low adherence to medication was also prescribed in both readmitted and non-readmitted patients in this study (66.7% vs. 80.9%, P = 0.958). Adherence was established during the initial encounter with the patients. Good medication adherence was defined as a patient’s ability to fulfill all the physician’s prescriptions correctly, including the dosage as prescribed by the physician. Failure to meet this criterion was defined as medication nonadherence. Several factors might be causing this finding, including poor understanding from patients, financial barriers, lack of insurance, and lack of access to medications. Further studies might be needed in this matter.
In patients with acute decompensated heart failure, diuretic-induced natriuresis reflects the ability of the kidney to excrete salt after administration of loop diuretics. It is commonly followed by spontaneous natriuresis in the post-diuretic period. Studies have shown post-diuretic spontaneous natriuresis is associated with the amount of diuretic-induced natriuresis [19]. Post-loop diuretic natriuresis reflects the rate of decongestion in patients with acute decompensated heart failure. In our study, we observe a trend of higher length of stay in patients with lower post-loop diuretic urinary sodium levels (< 50 mmol/L) (8.67 ± 4.14 vs. 6.03 ± 5.39 days, P = 0.126). This trend is further confirmed using linear regression multivariate analysis, which shows an inverse relation between post-loop diuretic urinary sodium levels and length of stay (B = -0.342; standard error (SE) = 0.023, P = 0.041) (Table 4). This finding also correlates with the findings of other studies. Biegus et al showed that in acute decompensated heart failure patients with fluid overload given intravenous loop diuretic, urinary sodium level at 48 h post-administration has prognostic value over the effectiveness of decongestion and hospitalization outcome [20, 21]. In an analysis of the ROSE-AHF trial by Hodson et al [9], sodium excretion was strongly associated with 6-month mortality. Patients excreting less than 2 g of sodium per day, showed higher levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) and a lower glomerular filtration rate. Furthermore, patients with lower sodium excretion were significantly associated with 6-month all-cause mortality in both univariate and multivariate analysis [9]. A study by Testani et al showed in patients being treated for acute decompensated heart failure, poor natriuretic response to loop diuretics is predictive of increased length of stay and can be predicted from a spot urine measurement post diuretic administration [8]. In a study by Singh et al, insufficient natriuretic response to furosemide was found to be associated with a greater likelihood of renal worsening and future adverse long-term outcomes independent of glomerular filtration rate, with adverse outcomes such as death, cardiac transplantation, and heart failure rehospitalization [6].
Low post-loop diuretic urinary sodium levels reflect ineffective decongestion, diuretic resistance, and renal tubular injury reflected by elevated kidney injury molecule I and neutrophil gelatinase-associated lipocalin [20].
Differences in outcomes of hospitalization were also analyzed from the perspective of diuretic administration strategy. Aggressive administration of loop diuretic, which is defined as administration as per the newest European Society of Cardiology guideline [7], was however not found to be associated with lower length of stay and lower risk of rehospitalization. This finding might be explained due to the side effects of aggressive decongestion in acute heart failure patients, such as worsening renal function, hypotension, and profound electrolyte imbalance, which might increase the length of hospitalization [22]. Also, upon seeing these adverse effects on a patient, treating physicians would most likely de-intensify the loop diuretic regimen, causing slower decongestion and increased length of stay.
Study limitation
This study employs consecutive sample collection without randomization which might cause a discrepancy between study groups. This study only evaluated a small sample of patients. Further studies with larger samples are needed in the future. During the time of this study, sodium-glucose cotransporter-2 inhibitors (SGLT2i) and angiotensin receptor-neprilysin inhibitors (ARNI) were not covered by our national health insurance, since our hospital is a public hospital, in which most of the patients utilized our national health insurance for treatment, SGLT2i was rarely given except in out-of-pocket cases. Furthermore, we acknowledge that even with clinical signs of decongestion achieved during hospitalization, objective tissue decongestion cannot be measured and might affect readmission rates.
Conclusions
In this study, we observed that patients with higher pre-loop diuretic urinary sodium levels had lower rates of rehospitalization. Post-loop diuretic urinary sodium level was shown to be inversely related to length of stay in acute decompensated heart failure patients.
Acknowledgments
None to declare.
Funding Statement
This paper received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
All authors declare no conflict of interest.
Informed Consent
Informed consent was was deemed inappropriate for this research due to usage of secondary data from hospital medical records.
Author Contributions
Rarsari Soerarso: conceptualization, project administration, writing - original draft. Emir Yonas: formal analysis, writing - original draft. Fikri Muhamad Yamin Tawari: formal analysis, writing - original draft. Dian Yaniarti Hasanah: writing - review and editing. Sunu Budhi Raharjo: writing - review and editing. Bambang Budi Siswanto:writing - review and editing. M.I.F.J. Oerlemans: writing - review & editing. Pim van der Harst: writing - review and editing. Maarten J. Cramer: writing - review and editing.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Siswanto BB. Accurate diagnoses, evidence-based drugs, and new devices (3 Ds) in heart failure. Medical Journal of Indonesia. 2012;21:52. doi: 10.13181/mji.v21i1.478. [DOI] [Google Scholar]
- 2.Siswanto BB, Radi B, Radi B. et al. Heart Failure in NCVC Jakarta and 5 hospitals in Indonesia. Glob Heart. 2010;5(1):35. doi: 10.1016/j.cvdpc.2010.03.005. [DOI] [Google Scholar]
- 3.Damman K, Ter Maaten JM, Coster JE, Krikken JA, van Deursen VM, Krijnen HK, Hofman M. et al. Clinical importance of urinary sodium excretion in acute heart failure. Eur J Heart Fail. 2020;22(8):1438–1447. doi: 10.1002/ejhf.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKie PM, Schirger JA, Costello-Boerrigter LC, Benike SL, Harstad LK, Bailey KR, Hodge DO. et al. Impaired natriuretic and renal endocrine response to acute volume expansion in pre-clinical systolic and diastolic dysfunction. J Am Coll Cardiol. 2011;58(20):2095–2103. doi: 10.1016/j.jacc.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verbrugge FH, Dupont M, Steels P, Grieten L, Swennen Q, Tang WH, Mullens W. The kidney in congestive heart failure: 'are natriuresis, sodium, and diuretics really the good, the bad and the ugly?'. Eur J Heart Fail. 2014;16(2):133–142. doi: 10.1002/ejhf.35. [DOI] [PubMed] [Google Scholar]
- 6.Singh D, Shrestha K, Testani JM, Verbrugge FH, Dupont M, Mullens W, Tang WH. Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long-term outcomes in acute decompensated heart failure. J Card Fail. 2014;20(6):392–399. doi: 10.1016/j.cardfail.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H. et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 8.Testani JM, Hanberg JS, Cheng S, Rao V, Onyebeke C, Laur O, Kula A. et al. Rapid and highly accurate prediction of poor loop diuretic natriuretic response in patients with heart failure. Circ Heart Fail. 2016;9(1):e002370. doi: 10.1161/CIRCHEARTFAILURE.115.002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodson DZ, Griffin M, Mahoney D, Raghavendra P, Ahmad T, Turner J, Wilson FP. et al. Natriuretic response is highly variable and associated with 6-month survival: insights from the ROSE-AHF trial. JACC Heart Fail. 2019;7(5):383–391. doi: 10.1016/j.jchf.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cremers HP, Theunissen LJHJ, Essers PPM, van de Ven ART, Spee R, Verbunt R. Gender differences in heart failure; data on outcomes and costs. European Society of Cardiology. 2020 [Google Scholar]
- 11.Bhatia V, Bajaj NS, Sanam K, Hashim T, Morgan CJ, Prabhu SD, Fonarow GC. et al. Beta-blocker use and 30-day all-cause readmission in medicare beneficiaries with systolic heart failure. Am J Med. 2015;128(7):715–721. doi: 10.1016/j.amjmed.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph SM, Cedars AM, Ewald GA, Geltman EM, Mann DL. Acute decompensated heart failure: contemporary medical management. Tex Heart Inst J. 2009;36(6):510–520. [PMC free article] [PubMed] [Google Scholar]
- 13.Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, Ky B. et al. Sex differences in heart failure. Eur Heart J. 2019;40(47):3859–3868c. doi: 10.1093/eurheartj/ehz835. [DOI] [PubMed] [Google Scholar]
- 14.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341(8):577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham JW, Sun JL, Mc Causland FR, Ly S, Anstrom KJ, Lindenfeld J, Givertz MM. et al. Lower urine sodium predicts longer length of stay in acute heart failure patients: Insights from the ROSE AHF trial. Clin Cardiol. 2020;43(1):43–49. doi: 10.1002/clc.23286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW. et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51(13):1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 17.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB. et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullens W, Abrahams Z, Skouri HN, Francis GS, Taylor DO, Starling RC, Paganini E. et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol. 2008;51(3):300–306. doi: 10.1016/j.jacc.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 19.Cox ZL, Rao VS, Ivey-Miranda JB, Moreno-Villagomez J, Mahoney D, Ponikowski P, Biegus J. et al. Compensatory post-diuretic renal sodium reabsorption is not a dominant mechanism of diuretic resistance in acute heart failure. Eur Heart J. 2021;42(43):4468–4477. doi: 10.1093/eurheartj/ehab620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biegus J, Zymlinski R, Sokolski M, Todd J, Cotter G, Metra M, Jankowska EA. et al. Serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome in patients with acute heart failure. Eur J Heart Fail. 2019;21(5):624–633. doi: 10.1002/ejhf.1428. [DOI] [PubMed] [Google Scholar]
- 21.Luk A, Groarke JD, Desai AS, Mahmood SS, Gopal DM, Joyce E, Shah SP. et al. First spot urine sodium after initial diuretic identifies patients at high risk for adverse outcome after heart failure hospitalization. Am Heart J. 2018;203:95–100. doi: 10.1016/j.ahj.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Klein L, O'Connor CM, Leimberger JD, Gattis-Stough W, Pina IL, Felker GM, Adams KF Jr. et al. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation. 2005;111(19):2454–2460. doi: 10.1161/01.CIR.0000165065.82609.3D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.


