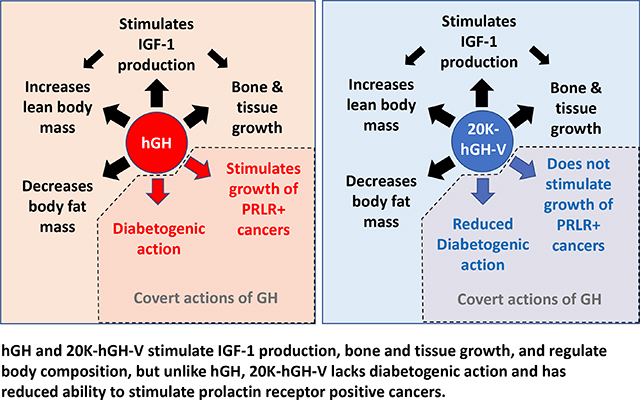
Graphical Abstract
Keywords: growth hormone, placental growth hormone, growth hormone variant, growth hormone 2, GH-V, GH-2
Introduction
Growth hormone (GH) is a 22-kDa polypeptide secreted from the anterior pituitary. While it is well known for its role in controlling longitudinal bone growth, GH also acts on multiple tissues either directly or indirectly to control many aspects of metabolism during development and throughout life. For example, GH plays an important role in nutrient partitioning as it alters the synthesis, oxidation, and distribution of carbohydrates, proteins, and lipids. To this end, one of the earliest observed actions of GH is a potent anti-insulin/diabetogenic activity. This anti-insulin activity was discovered ~90 years ago by Dr. Bernardo Houssay in a series of experiments for which he was awarded the 1947 Nobel Prize in medicine. In these experiments, Dr. Houssay demonstrated that pituitary gland removal in animals - such as dogs and toads - enhances insulin sensitivity; in contrast, animals injected with high levels of pituitary extracts develop insulin resistance and diabetes [1, 2]. In the 1960s, Rabinowitz and colleagues confirmed that GH has anti-insulin activity in humans by demonstrating that it acutely blocks insulin stimulated glucose uptake [3, 4]. Multiple studies have since reinforced that GH is a diabetogenic agent with anti-insulin activity especially when administered at high doses [5–11].
GH-V
In humans, the GH gene family (Fig.1A) is made up of five related genes that share similar amino acid sequence and protein structure likely evolving from a common ancestor [12]: 1) GH-N (also called GH1 or pituitary GH); 2) GH-V (also referred to as GH2 or placental GH); 3) placental lactogen 1 (also called chorionic somatomammotropin hormone 1); 4) placental lactogen 2 (also called chorionic somatomammotropin hormone 2); and 5) a pseudogene called chorionic somatomammotropin-like hormone. While GH-N is produced mainly in the pituitary, GH-V or placental GH as its name implies, is produced by specialized cells called syncytiotrophoblasts in the placenta. GH-V levels increase during pregnancy and replace pituitary derived GH in circulation. At the amino acid level (Fig.1B and C), GH-V differs from GH-N by 13 of 191 total residues [12, 13]. Like GH-N, the most abundant form of GH-V is the 22-kDa isoform (isoform 1 of 4 total), which has been shown to promote growth as well as maternal insulin resistance. In 1998, a rare 20-kDa isoform (isoform 4) of GH-V was detected in human placenta [14]. Similar to GH-N, the 20-kDa isoform of GH-V is the product of a 45-bp deletion resulting from the use of an alternative or cryptic precursor mRNA acceptor site within exon 3. While the existence of 20-kDa GH-V is known, it is not believed to have any natural biological relevance in humans since it is either not detected or only detected at extremely low levels – accounting for only 0.6% of GH-V mRNA isoform abundance in placenta [15].
Fig. (1).
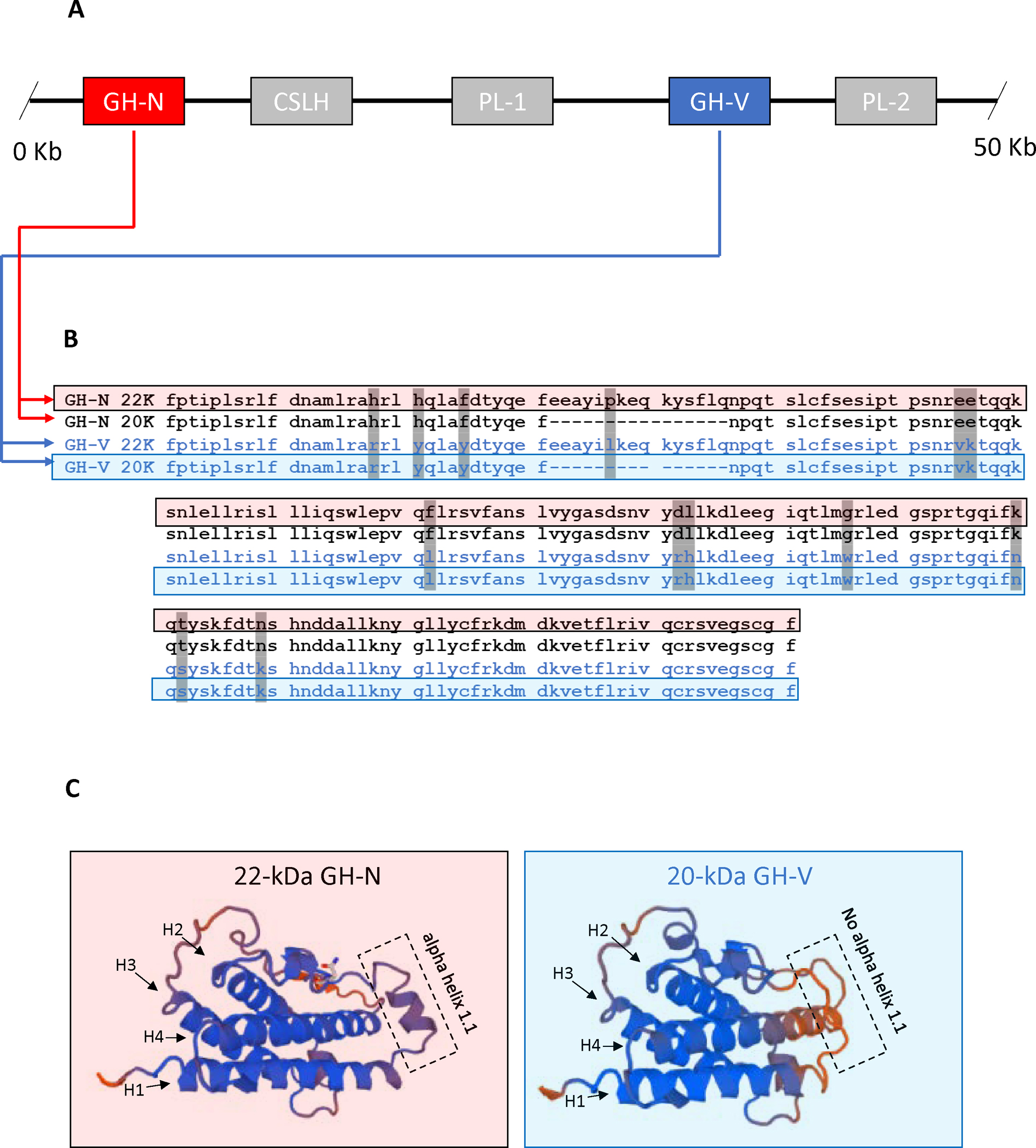
Gene family, amino acid comparison, and predicted structure of 22-kDa GH-N and 20-kDa GH-V. (A) The human GH gene family spans a 50 Kb region in chromosome 17. The two GH genes GH-N (red box) and GH-V (blue box) are shown with placental lactogens also shown (grey boxes). (B) Amino acid sequence comparison for the 20 and 22 kDa isoforms of GH-N (black lettering) and GH-V (blue lettering). Grey shading indicates where amino acids differ between GH-N and GH-V. (C) SWISS-MODEL (swissmodel.expasy.org) predicted protein structures of 22-kDa GH-N (left side) and 20-kDa GH-V (right side) reveals the absence of a small alpha-helix between alpha-helix 1 and alpha-helix 2 for 20-kDa GH-V which may explain the differences in activities reported between these two hormones.
20-kDa GH-V lacks diabetogenic activity
Despite a lack of natural utility, a study by Vickers and colleagues in 2009 indicates that 20-kDa GH-V may have potential as a therapeutic drug because it lacks the diabetogenic activity of hGH [16]. Because of this, our laboratory recently tested 20-kDa GH-V in a mouse model of GH deficiency. We showed that 20-kDa GH-V treatment given to young GH deficient mice produces significant increases to circulating IGF-1, femur length, and body length compared to saline treated controls [17]. Furthermore, these increases were similar to GH deficient mice treated with hGH (ie. 22-kDa GH-N), indicating that 20-kDa GH-V has full ability to stimulate IGF-1 and longitudinal bone growth. Since GH is known to increase lean mass and reduce fat mass, we also tested the ability of 20-kDa GH-V to alter body composition in GH deficient mice. We reported that treatment with 20-kDa GH-V, similar to hGH administration, significantly increased lean body mass while decreasing fat mass [17]. These data were in agreement with data from Vickers et al., as 20-kDa GH-V reduced body fat in high fat fed rats similar to those treated with hGH [16]. Importantly, in our mouse study, analysis of glucose homeostasis revealed that 20-kDa GH-V lacked the diabetogenic activity observed in mice treated with GH. That is, 20-kDa GH-V failed to cause hyperinsulinemia seen in hGH treated mice, and mice treated with 20-kDa GH-V were significantly more sensitive to insulin compared to hGH treated mice [17]. These findings in mice agree with the study in rats [16] as 20-kDa GH-V treatment in non-GH deficient high fat fed rats reduced insulin and C-peptide levels compared to rat receiving GH treatment. Importantly, these data suggest that 20-kDa GH-V may represent improvements to current GH therapies for individuals at risk insulin resistance/metabolic disease.
Does pituitary 20-kDa GH-N also lack diabetogenic activity?
In the 1980’s through the early 2000’s, studies in animals [18–21] and humans [22] suggested that the short form of pituitary GH, 20-kDa GH-N, may also have decreased diabetogenic activity. Unfortunately, the timing of blood sampling in the lone human study appears to be inappropriate for evaluating a diabetogenic effect (or lack thereof). More specifically, the diabetogenic activity of GH is transient and measures of insulin sensitivity need to be performed within a short window (~1–5 hours) after GH treatment to detect this diabetogenic effect [9, 10]. Because Hayakawa and colleagues [22] tested subjects 24 hours after GH injection, it is not surprising that alterations to glucose homeostasis were not detected. Furthermore, the reports in animal studies [18–21] are in disagreement with other animal studies performed in the same species (dogs and rats) which demonstrate that pituitary 20-kDa GH-N indeed has diabetogenic activity [23, 24]. Therefore, it appears that more studies are needed to help settle this debate about 20-kDa GH-N.
20-kDa GH-V lacks lactogenic activity: implication for PRLR-positive cancers
In addition to lacking diabetogenic activity, two separate laboratories have demonstrated that unlike GH-N, 20-kDa GH-V does not bind the prolactin receptor (PRLR) [13, 16]. This finding has clinical importance since PRLR signaling has been suggested to play a role in progression of certain cancer types including prostate [25, 26], breast [25, 27], ovarian [28, 29] and colon [30]. GH is also implicated in progression in the same cancers as well as others [31–37]. Since hGH binds strongly to PRLR in addition to the GHR, the binding of hGH to PRLR may be responsible for hGH’s ability to stimulate growth of these cancers [31, 36, 37]. Because 20-kDa GH-V does not bind to PRLR, we tested its ability to stimulate proliferation of PRLR-positive human cancers compared to that of hGH. Three distinct PRLR positive human cancer cell lines (two breast and one colon cancer cell line) showed a significantly reduced proliferation rate when treated with 20-kDa GH-V compared to hGH. Therefore, these results are in agreement with the theory that GH stimulation of PRLR is partially responsible for proliferation of certain PRLR-positive cancers. Importantly, these data also suggest that 20-kDa GH-V may represent improvements to current GH therapies for individuals at risk for PRLR-positive cancers.
Concluding remarks
Early studies by three separate laboratories (Solomon, Vickers, and our laboratory) indicate that 20-kDa GH-V may have potential use for GH replacement therapy especially in instances where insulin resistance and/or PRLR-positive cancers are a concern. While the clinical relevance of GH’s diabetogenic activity and the ability to stimulate PRLR-positive cancers are controversial and less of a concern in GHD children, treatment in GHD adults is a more appropriate target since metabolic syndrome/diabetes and cancer rates increase with advancing age. As we are in the early stages of evaluating 20-kDa GH-V, more research is needed to better evaluate the benefits and safety of this new therapeutic candidate. However, early studies in cultured cells and in rodents are promising.
Footnotes
Disclosure Summary: I certify that neither I nor my co-authors have a conflict of interest as described above that is relevant to the subject matter or materials included in this work.
References
- [1].Houssay B; Biasotti A, The hypothesis, carbohydrate metabolism and diabetes. Endocrinology 1931, 15, 511. [Google Scholar]
- [2].Houssay B, The hypophysis and metabolism. N Engl J Med 1936, 214, 961–985. [Google Scholar]
- [3].Rabinowitz D; Klassen GA; Zierler KL, Effect Of Human Growth Hormone On Muscle And Adipose Tissue Metabolism In The Forearm Of Man. J Clin Invest 1965, 44, 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rabinowitz D; Zierler KL, A Metabolic Regulating Device Based on the Actions of Human Growth Hormone and of Insulin, Singly and Together, on the Human Forearm. Nature 1963, 199, 913–5. [DOI] [PubMed] [Google Scholar]
- [5].Luft R; Cerasi E, Human growth hormone as a regulator of blood glucose concentration and as a diabetogenic substance. Diabetologia 1968, 4 (1), 1–9. [PubMed] [Google Scholar]
- [6].Bornstein J; Armstrong JM; Gould MK; Harcourt JA; Jones MD, Mechanism of the diabetogenic action of growth hormone. I. Effect of polypeptides derived from growth hormone on glycolysis in muscle. Biochim Biophys Acta 1969, 192 (2), 265–70. [DOI] [PubMed] [Google Scholar]
- [7].Lostroh AJ, Diabetogenic hormones (human choriosomatomammotrophin and ovine growth hormone): anti-insulin action in hypophysectomized rats. Acta Endocrinol (Copenh) 1974, 77 (1), 96–102. [DOI] [PubMed] [Google Scholar]
- [8].Cameron CM; Kostyo JL, Influence of age on responsiveness to diabetogenic action of growth hormone. Diabetes 1987, 36 (1), 88–92. [DOI] [PubMed] [Google Scholar]
- [9].Jorgensen JO; Moller N; Lauritzen T; Alberti KG; Orskov H; Christiansen JS, Evening versus morning injections of growth hormone (GH) in GH-deficient patients: effects on 24-hour patterns of circulating hormones and metabolites. J Clin Endocrinol Metab 1990, 70 (1), 207–14. [DOI] [PubMed] [Google Scholar]
- [10].Krusenstjerna-Hafstrom T; Clasen BF; Moller N; Jessen N; Pedersen SB; Christiansen JS; Jorgensen JO, Growth hormone (GH)-induced insulin resistance is rapidly reversible: an experimental study in GH-deficient adults. J Clin Endocrinol Metab 2011, 96 (8), 2548–57. [DOI] [PubMed] [Google Scholar]
- [11].Dal J; List EO; Jorgensen JO; Berryman DE, Glucose and Fat Metabolism in Acromegaly: From Mice Models to Patient Care. Neuroendocrinology 2016, 103 (1), 96–105. [DOI] [PubMed] [Google Scholar]
- [12].Miller WL; Eberhardt NL, Structure and evolution of the growth hormone gene family. Endocr Rev 1983, 4 (2), 97–130. [DOI] [PubMed] [Google Scholar]
- [13].Solomon G; Reicher S; Gussakovsky EE; Jomain JB; Gertler A, Large-scale preparation and in vitro characterization of biologically active human placental (20 and 22K) and pituitary (20K) growth hormones: placental growth hormones have no lactogenic activity in humans. Growth Horm IGF Res 2006, 16 (5–6), 297–307. [DOI] [PubMed] [Google Scholar]
- [14].Boguszewski CL; Svensson PA; Jansson T; Clark R; Carlsson LM; Carlsson B, Cloning of two novel growth hormone transcripts expressed in human placenta. J Clin Endocrinol Metab 1998, 83 (8), 2878–85. [DOI] [PubMed] [Google Scholar]
- [15].Mannik J; Vaas P; Rull K; Teesalu P; Rebane T; Laan M, Differential expression profile of growth hormone/chorionic somatomammotropin genes in placenta of small- and large-for-gestational-age newborns. J Clin Endocrinol Metab 2010, 95 (5), 2433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vickers MH; Gilmour S; Gertler A; Breier BH; Tunny K; Waters MJ; Gluckman PD, 20-kDa placental hGH-V has diminished diabetogenic and lactogenic activities compared with 22-kDa hGH-N while retaining antilipogenic activity. Am J Physiol Endocrinol Metab 2009, 297 (3), E629–37. [DOI] [PubMed] [Google Scholar]
- [17].List EO; Berryman DE; Basu R; Buchman M; Funk K; Kulkarni P; Duran-Ortiz S; Qian Y; Jensen EA; Young JA; Yildirim G; Yakar S; Kopchick JJ, The Effects of 20-kDa Human Placental GH in Male and Female GH-deficient Mice: An Improved Human GH? Endocrinology 2020, 161 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Takahashi S; Shiga Y; Satozawa N; Hayakawa M, Diabetogenic activity of 20 kDa human growth hormone (20K-hGH) and 22K-hGH in rats. Growth Horm IGF Res 2001, 11 (2), 110–6. [DOI] [PubMed] [Google Scholar]
- [19].Ishikawa M; Tachibana T; Kamioka T; Horikawa R; Katsumata N; Tanaka T, Comparison of the somatogenic action of 20 kDa- and 22 kDa-human growth hormones in spontaneous dwarf rats. Growth Horm IGF Res 2000, 10 (4), 199–206. [DOI] [PubMed] [Google Scholar]
- [20].Ishikawa M; Hiroi N; Kamioka T; Tanaka T; Tachibana T; Ishikawa H; Miyachi Y, Metabolic effects of 20 kDa and 22 kDa human growth hormones on adult male spontaneous dwarf rats. Eur J Endocrinol 2001, 145 (6), 791–7. [DOI] [PubMed] [Google Scholar]
- [21].Lewis UJ; Singh RN; Tutwiler GF, Hyperglycemic activity of the 20,000-dalton variant of human growth hormone. Endocr Res Commun 1981, 8 (3), 155–64. [DOI] [PubMed] [Google Scholar]
- [22].Hayakawa M; Shimazaki Y; Tsushima T; Kato Y; Takano K; Chihara K; Shimatsu A; Irie M, Metabolic effects of 20-kilodalton human growth hormone (20K-hGH) for adults with growth hormone deficiency: results of an exploratory uncontrolled multicenter clinical trial of 20K-hGH. J Clin Endocrinol Metab 2004, 89 (4), 1562–71. [DOI] [PubMed] [Google Scholar]
- [23].Kostyo JL; Cameron CM; Olson KC; Jones AJ; Pai RC, Biosynthetic 20-kilodalton methionyl-human growth hormone has diabetogenic and insulin-like activities. Proc Natl Acad Sci U S A 1985, 82 (12), 4250–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ader M; Agajanian T; Finegood DT; Bergman RN, Recombinant deoxyribonucleic acid-derived 22K- and 20K-human growth hormone generate equivalent diabetogenic effects during chronic infusion in dogs. Endocrinology 1987, 120 (2), 725–31. [DOI] [PubMed] [Google Scholar]
- [25].Jacobson EM; Hugo ER; Tuttle TR; Papoian R; Ben-Jonathan N, Unexploited therapies in breast and prostate cancer: blockade of the prolactin receptor. Trends Endocrinol Metab 2010, 21 (11), 691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Thomas LN; Merrimen J; Bell DG; Rendon R; Too CK, Prolactin- and testosterone-induced carboxypeptidase-D correlates with increased nitrotyrosines and Ki67 in prostate cancer. Prostate 2015, 75 (15), 1726–36. [DOI] [PubMed] [Google Scholar]
- [27].Wen Y; Zand B; Ozpolat B; Szczepanski MJ; Lu C; Yuca E; Carroll AR; Alpay N; Bartholomeusz C; Tekedereli I; Kang Y; Rupaimoole R; Pecot CV; Dalton HJ; Hernandez A; Lokshin A; Lutgendorf SK; Liu J; Hittelman WN; Chen WY; Lopez-Berestein G; Szajnik M; Ueno NT; Coleman RL; Sood AK, Antagonism of tumoral prolactin receptor promotes autophagy-related cell death. Cell Rep 2014, 7 (2), 488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Levina VV; Nolen B; Su Y; Godwin AK; Fishman D; Liu J; Mor G; Maxwell LG; Herberman RB; Szczepanski MJ; Szajnik ME; Gorelik E; Lokshin AE, Biological significance of prolactin in gynecologic cancers. Cancer Res 2009, 69 (12), 5226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tan D; Chen KE; Khoo T; Walker AM, Prolactin increases survival and migration of ovarian cancer cells: importance of prolactin receptor type and therapeutic potential of S179D and G129R receptor antagonists. Cancer Lett 2011, 310 (1), 101–8. [DOI] [PubMed] [Google Scholar]
- [30].Neradugomma NK; Subramaniam D; Tawfik OW; Goffin V; Kumar TR; Jensen RA; Anant S, Prolactin signaling enhances colon cancer stemness by modulating Notch signaling in a Jak2-STAT3/ERK manner. Carcinogenesis 2014, 35 (4), 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wennbo H; Tornell J, The role of prolactin and growth hormone in breast cancer. Oncogene 2000, 19 (8), 1072–6. [DOI] [PubMed] [Google Scholar]
- [32].Subramani R; Nandy SB; Pedroza DA; Lakshmanaswamy R, Role of Growth Hormone in Breast Cancer. Endocrinology 2017, 158 (6), 1543–1555. [DOI] [PubMed] [Google Scholar]
- [33].Chatzistamou I; Schally AV; Varga JL; Groot K; Armatis P; Busto R; Halmos G, Antagonists of growth hormone-releasing hormone and somatostatin analog RC-160 inhibit the growth of the OV-1063 human epithelial ovarian cancer cell line xenografted into nude mice. J Clin Endocrinol Metab 2001, 86 (5), 2144–52. [DOI] [PubMed] [Google Scholar]
- [34].Melmed GY; Devlin SM; Vlotides G; Dhall D; Ross S; Yu R; Melmed S, Anti-aging therapy with human growth hormone associated with metastatic colon cancer in a patient with Crohn’s colitis. Clin Gastroenterol Hepatol 2008, 6 (3), 360–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang Z; Prins GS; Coschigano KT; Kopchick JJ; Green JE; Ray VH; Hedayat S; Christov KT; Unterman TG; Swanson SM, Disruption of growth hormone signaling retards early stages of prostate carcinogenesis in the C3(1)/T antigen mouse. Endocrinology 2005, 146 (12), 5188–96. [DOI] [PubMed] [Google Scholar]
- [36].Xu J; Sun D; Jiang J; Deng L; Zhang Y; Yu H; Bahl D; Langenheim JF; Chen WY; Fuchs SY; Frank SJ, The role of prolactin receptor in GH signaling in breast cancer cells. Molecular Endocrinology 2013, 27 (2), 266–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xu J; Zhang Y; Berry PA; Jiang J; Lobie PE; Langenheim JF; Chen WY; Frank SJ, Growth hormone signaling in human T47D breast cancer cells: potential role for a growth hormone receptor-prolactin receptor complex. Molecular Endocrinology 2011, 25 (4), 597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]


