Abstract
Introduction:
As epoch-making anticancer drugs, paclitaxel and docetaxel have been extensively used in the clinic over the past three decades. Although the patents of these first-generation taxanes have expired, their clinical applications, particularly new formulations and combination therapies are under active investigations. Inspired by the notable success of Abraxane and Lipusu, new formulations have been extensively developed. In parallel, to overcome multidrug resistance (MDR) and to eradicate cancer stem cells, immense efforts have been made on the discovery and development of new-generation taxanes with improved potency and superior pharmacological properties.
Areas Covered:
Starting from three taxanes used in clinic, this review covers (a) natural sources of advanced intermediates used for semi-synthesis of taxane API, (b) new formulations, (c) the major issues of FDA approved taxanes, (d) the design and development of next-generation taxanes, particularly those already in clinical trials or advanced preclinical development, (e) new mechanisms of action, and (f) a variety of taxane-based drug delivery systems. In particular, the next-generation taxanes are not only highly potent and capable of overcoming MDR, but also critically important as the payloads of efficacious tumor-targeted drug delivery systems.
Expert Opinion:
As the highly potent next-generation taxanes can eradicate cancer stem cells and overcome MDR, the priority is to develop these superior compounds as an integral part of cancer therapy, especially for the treatment of pancreatic, colon and prostate cancer patients who hardly respond to checkpoint inhibitors. In order to mitigate undesirable side effects, the exploration of effective nanoformulations and tumor-targeted drug delivery systems are essential. Therefore, it is expected that these research programs will be the major focus in taxane-based drug development for years to come.
Keywords: paclitaxel, cancer, next-generation taxanes, nanoformulation, drug delivery system
1. Introduction
Since approved by the U.S. Food and Drug Administration (FDA), paclitaxel and docetaxel (Figure 1A) have been used extensively as front-line anti-cancer drugs for treatment of most solid tumors over the past three decades. Their manufacturing and clinical use have been continuously expanding through the generic drug market, especially in countries such as China and India. The exploration of their newer clinical applications is also actively ongoing, especially through new formulations and combination therapies.
Figure 1.
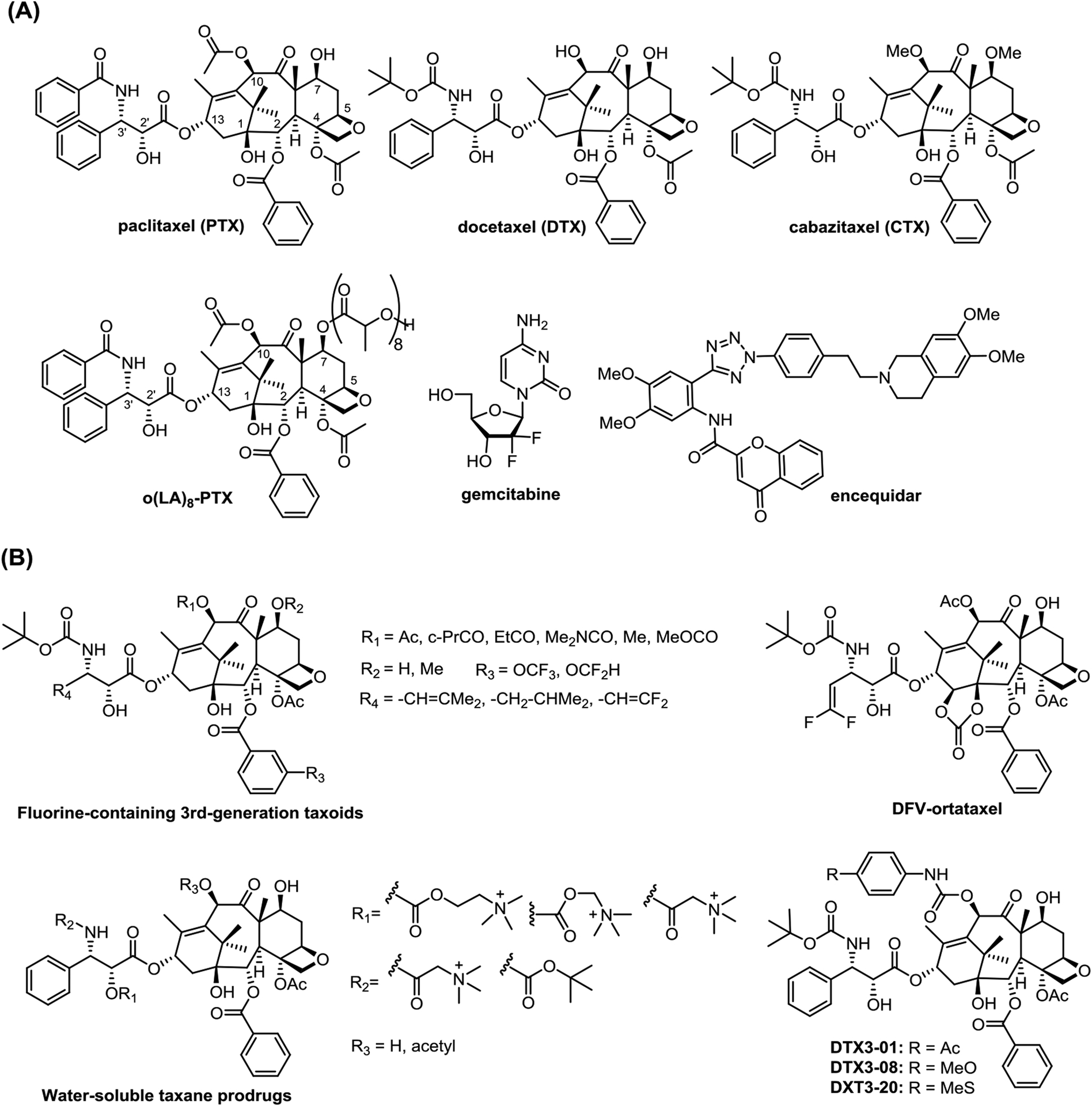
(A) Structures of 1st-generation taxanes, o(LA)8-PTX, gemcitabine and encequidar; (B) Selected next-generation taxanes disclosed in the last 3 years
Although new strategies in cancer treatment, such as cancer immunotherapy, have been explored to bring in success and hope, these applications have also shown serious and sometimes fatal adverse effects among cancer patients [1,2]. Accordingly, established chemotherapies such as taxane-based chemotherapy will continue to play a major role in the foreseen future. Noteworthy is the expansion of potential indications of new-generation taxanes to triple-negative breast, prostate, pancreatic, colon and gastric cancers and even neurodegenerative diseases. The development of new-generation taxanes with superior pharmacological properties and higher potency against multi-drug resistant cancers and cancer stem cells are expected to meet the medical needs, especially with new nanoformulations. These new taxanes will also be critically important as the payloads of tumor targeting drug conjugates [3].
2. FDA approved first-generation taxane anticancer drugs: paclitaxel, docetaxel and cabazitaxel
Taxane anticancer drugs stabilize microtubule assembly by binding β-tubulin thereby preventing depolymerization, and this results in blocking cell division at G2/M phase, followed by apoptosis [4]. Paclitaxel is a naturally occurring diterpenoid, approved by FDA in 1992. Both docetaxel and cabazitaxel (Figure 1A) are semi-synthetic taxanes developed by Rhone-Poulenc Rorer (now Sanofi). Docetaxel exhibited better efficacy than paclitaxel in stabilizing microtubule [5]. Although used for limited tumor types, the market of cabazitaxel has kept increasing since its approval in 2010, reaching $606 million in 2020 [6].
2.1. Commercial production of the active pharmaceutical ingredients (APIs) of taxane anticancer drugs
In the 1980’s the only known source of paclitaxel was from the bark of Pacific yew (taxus brevifolia) [7] and persistent over-harvesting of this slow growing tree once resulted in serious environmental issues [7]. Fortunately, this problem was resolved by the development of practical semi-synthesis of paclitaxel from 10-deacetylbaccatin III (10-DAB III), which is much more abundant in the needles of European yew (Taxus baccata), up to ~1g/kg of fresh biomass, and these yew needles are renewable. Thus, abundantly culturable European yew secured the production of paclitaxel and docetaxel [5,7], which allowed INDENA SpA, Italy to supply 10-DAB III to most of the global market [7]. Now, the “South No.1 Yew”, an elite clone from Taxus wallichiana Zucc var mairei, the fastest growing species of Taxus spp. that is endemic to China, is widely cultivated in Fujian and Yunnan provinces of China to produce 10-DAB III (0.9–1% of dry biomass) at much cheaper cost [8].
2.2. Major issues of the FDA approved taxanes
Several issues with the taxanes already in clinic have been identified. One of these is the use of excipients which is required to overcome their very poor aqueous solubility [9]. There have been some cases in which the use of these excipients have led to serious side effects. Once this happens, the patients must be removed from the treatment immediately [9]. The second problem is multidrug resistance [10,11]. There are quite a few major mechanisms for multidrug resistance (MDR): 1) Overexpression of efflux pumps, especially P-glycoprotein (Pgp) and multidrug resistance-associated proteins 1 (MRP1); 2) Decrease in the cellular uptake of drug by overexpression of plastin-3, decrease of organic anion transporting polypeptide 1B3, and changing membrane compositions mostly via increasing cholesterol percentage; 3) Changing apoptotic pathways by overexpression of Bcl-2 protein that strongly binds to paclitaxel; 4) Point mutation of tubulin, particularly the expression of βIII isoform in β-tubulin; 5) Widely existing hypoxic microenvironment in solid tumors; and 6) Metabolism-based resistance [9,11,12].
Another major issue is due to the presence of cancer stem cells (CSCs) [13,14]. Growing evidence indicates that CSCs play a key role in tumor development, metastasis, MDR and relapse. The expression of many stem cell-related genes endow CSCs with tumor initiating capacity in most cancer types, making it notoriously malignant [13,14]. The inefficiency of taxane-based treatment is partially attributed to these relatively rare, quiescent or slowly proliferating CSCs. Moreover, the current taxane-based drugs have very limited efficacy in treating melanoma, pancreatic, triple-negative breast, gastric, brain and renal cancers.
2.3. New formulations of paclitaxel and docetaxl
Inspired by the successful development of Abraxane (albumin-bound paclitaxel) and Lipusu (paclitaxel liposome injection), new paclitaxel formulations have been developed and approved in different countries. For example, 7-oligo(lactic acid)-paclitaxel, o(LA)8-PTX (Figure 1A), in poly(ethylene glycol)-block-poly(D,L-lactide) (PEG-b-PLA) micelle is a micellar formulation of paclitaxel approved for treating metastatic breast cancer and advanced lung cancer in South Korea [15]. Samyang Biopharm uses its own plant cell culture technology for API supply. Various clinical investigations for other types of cancers are ongoing in several countries [15].
Polymeric micellar paclitaxel developed by Shanghai Yizhong Pharmaceutical was recently approved by SFDA to treat patients with advanced non-small cell lung cancer (NSCLC) [16]. In comparison with the standard combination of paclitaxel and cisplatin, the Phase III clinical data of this micellar formulation indicated that progress free survival (PFS) is increased to 6.4 months from 5.3 months with reduced adverse effects. Combination therapy of polymeric micellar paclitaxel and PD-1 for the treatment of breast and gastric cancers is also under clinical evaluation [16]. Additionally, polymeric micellar docetaxel and cabazitaxel are projected to be filed for IND in 2023 [16].
Oral formulations have also been intensively studied. For example, DHP107 (Liporaxel), developed by Daehwa Pharmaceutical based on their “DH-LASED technology”, was approved in South Korea for treatment of advanced gastric cancer with similar efficacy and safety to paclitaxel (i.v.) [17,18]. The phase III study in China is close to completion and its clinical trials for metastatic breast cancer and other tumors are ongoing [19].
New formulations of paclitaxel and docetaxel approved or in clinical development in the last 5 years are summarized in Table 1, wherein Abraxaneand Lipusu are included for comparison. The tumor-targeted delivery, including nanoformulations of paclitaxel and docetaxel, will be discussed in Section 6.
Table 1.
New formulations of paclitaxel and docetaxel approved or in clinical development
| Product | Developer | Taxane | Formulation | Administration | Ref. |
|---|---|---|---|---|---|
| Abraxane | Abraxis BioScience | paclitaxel | HSA nanoparticle | i.v. | [20] |
| Lipusu | Luye Pharmaceuticals | paclitaxel | Liposome lecithin and cholesterol | i.v. | [21] |
| Genexol-PM | Samyang Corp. | paclitaxel | o(LA)8-PTX, PEG-b-PLA micelle | i.v. | [15,22] |
| Nanoxel | Samyang Corp. | docetaxel | PEG-b-PLA micelle | i.v. | [23] |
| LEP-ETU | Insys Therapeutics | paclitaxel | Liposome dioleoyl-sn-glycero-3-phosphocholine, cholesterol, and cardiolipin | i.v. | [21] |
| EndoTAG-1 | Medigene AG | paclitaxel | cationic liposome (dioleoyl-3-trimethylammonium propane/dioleoyl-sn-glycero-3-phosphocholine) | i.v. | [24] |
| PTX-LDE | Palantir Technologies | paclitaxel | Lipid core nanoparticle protein-free liposome phosphatidylcholine, cholesterol and triolein), mimicking LDL | i.v. | [25] |
| LE-DT | NeoPharm | docetaxel | Liposome dioleoyl-sn-glycero-3-phosphocholine, cholesterol, cardiolipin, alpha-tocopheryl succinate | i.v. | [26] |
| ATI-1123 | Azaya Therapeutics | docetaxel | Liposome phospholipids, cholesterol, human serum albumin, saccharose |
i.v. | [24] |
| Paclical | Oasmia Pharmaceutical | paclitaxel | Polymeric micelle N-retinoyl-L-cysteic acid methyl ester sodium salt, retinoid derivatives | i.v. | [27] |
| Paclitaxel polymer micelles | Shanghai Yizong | paclitaxel | Polymeric micelle mPEG-PDLLA (methoxy polyethylene glycol 2000-polylactide amphiphilic block copolymer) | i.v. | [16] |
| Liporaxel (DHP107) |
Daehwa Pharmaceutical | paclitaxel | lipid-based oral formulation monoolein, tricaprylin, polysorbate 80 | oral | [17,18] |
| Oraxol | Athenex | paclitaxel | lipid-based oral capsule combination with encequidar | oral | [28] |
2.4. Combination therapy
To enhance the overall efficacy and to overcome MDR of taxane-based treatment, hundreds of clinical studies, taking advantage of the synergy of taxane-including combination chemotherapies are underway [12]. Paclitaxel and carboplatin are one of the earliest combination treatments for diverse solid tumors. To date there are still numerous ongoing clinical trials of this combination therapy [24]. After approved by the FDA in 2013, the combination of Abraxane and gemcitabine (Figure 1A) is one of the most frequently used therapy for metastatic pancreatic cancer treatment [29]. Athenex’s clinical trials of “oral paclitaxel” plus encequidar (P-glycoprotein inhibitor, Figure 1A) (Oraxol) for the treatment of metastatic breast cancer [28] revealed some issues, particularly the neutropenia-related sequelae, and this was raised as a potential safety risk by the FDA. To address these issues, another round of Phase III clinical trials is planned [30].
Combinations of taxanes with immunotherapy are recently attracting considerable interest. For example, paclitaxel with ramucirumab, approved by the FDA in 2014 for the treatment of progressed advanced gastric carcinomas after the first line chemotherapy, is one of the most successful combinations [31]. Other combinations include paclitaxel-carboplatin-bevacizumab for non-small cell lung cancer (NSCLC) [32], paclitaxel-carboplatin-bevacizumab-atezolizumab for NSCLC [33], paclitaxel-carboplatin-pembrolizumab for squamous head and neck carcinoma [34], paclitaxel-cetuximab for squamous head and neck carcinoma [35], and paclitaxel-gemcitabine-pamrevlumab for pancreatic cancer [36].
4. Development of new-generation taxanes
Since other review articles have covered the new-generation taxanes derived from 10-deacetylbaccatin III, 14β-hydroxy-10-deacetylbaccatin III and C-seco-baccatins [3,10,37,38], this review focuses on the newer taxanes disclosed during the last 5 years, which are under recent clinical or advanced preclinical development.
4.1. New-generation taxanes developed from 10-deacetylbaccatin III and 14β-hydroxy-10-deacetylbaccatin III disclosed in the last 5 years
Recently, a library of new 3rd-generation taxanes by incorporating OCHF2 and OCF3 groups at the C2-benzoate position were developed and evaluated (Figure 1B) [39,40]. These fluoromethoxy groups are not only well tolerated, but also enhanced potency, especially against the MDR cancer cells. For drug-sensitive cancer cell lines MCF7 and LCC6-WT, these newer taxanes exhibited up to 2–3 orders of magnitude higher potency than that of paclitaxel against drug-resistant ovarian, breast and colon cancer cell lines with MDR-phenotype (NCI/ADR, LCC6-MDR and LDL-1), as well as pancreatic cancer cell line, CFPAC-1 [39]. The introduction of a difluorovinyl (DFV) group at the C3’ position exhibited synergistic effect with the OCF3/OCF2H group at the 3-position of C2-benzoate moiety [40]. These DFV-taxanes not only exhibited subnanomolar IC50 values against drug-sensitive human cancer cell lines, A549, HT29, Vcap and PC3, as well as CFPAC-1, but also exhibited 2–4 orders of magnitude greater potency against extremely drug-resistant cancer cell lines, LCC6-MDR and DLD-1 as compared to that of paclitaxel. Furthermore, they exhibited excellent metabolic stability [40].
Molecular modeling analysis of the 3rd-generation fluorine-containing taxoids indicated that both 3-CF3O/3-CHF2O group of the C2-benzoate moiety and the 3’-DFV moiety are well accommodated in the deep hydrophobic binding pocket of β-tubulin [40]. Compared to the previously reported taxoids, there is an extra hydrogen bond between a fluorine of the OCF3 group and the NH of His229 (C-F---H-N), or between the hydrogen in the OCHF2 group and the nitrogen of His229 (C-H---N). These unique features may be contributing to the 10–100 pM IC50 values displayed by some of these newer fluorine-containing taxoids.
As a DFV-analog of ortataxel, which has advanced to Phase II clinical trials for the treatment of breast, NSCL cancers and glioblastoma, DFV-ortataxel (Figure 1B) was developed and its potency and efficacy examined [41]. DFV-ortataxel exhibited higher potency and better efficacy than ortataxel in taxane-resistant MCF-7R and MDA-MB-231R tumor xenografts in mice, wherein MDA-MB-231R cells overexpress ABCB1/ABCG2 efflux pumps [41]. A recent study on newer DFV-taxanes against anaplastic thyroid cancer also exhibited excellent potency in vitro and promising efficacy in vivo [42].
Two recent Chinese patent applications disclosed different libraries of newer taxanes. One library was made by incorporating water-soluble modules such as a trimethylammonium group to produce paclitaxel and docetaxel prodrugs (Figure 1B) [43]. These modules not only increase aqueous solubility, but also possess targeted antitumor activity. Once metabolized, free paclitaxel or docetaxel is released in tumor tissues. Thus, the potency is comparable to paclitaxel against drug-sensitive tumors, but much more potent against MDR tumors with reduced toxicity [43]. The other library was created by modifying the C7 and/or C10-hydroxyl groups with various arylcarbamoyl groups, e.g., DTX3-01, DTX3-08 and DTX3-20 (Figure 1B), which exhibit higher potency and better pharmacological properties than docetaxel [44].
4.2. New-generation taxanes in clinical trials and advanced preclinical development
Tesetaxel (Figure 2), derived from 9-dihydro-10-DAB III (9-DHB), was originally developed by Daiichi-Sankyo until 2006. However, the death of a patient due to severe neutropenia led to the suspension of its clinical trials [45,46]. After tesetaxel failed to exhibit superior efficacy over existing therapies in its Phase II clinical studies for the treatment of metastatic colorectal cancer and advanced gastric cancer, combination therapy became the hope for its further development [45,46]. Odonate Therapeutics continued its development and expected a 3.5 month extension benefit in its Phase III “Contessa” study against metastatic breast cancer. However, the combination therapy of oral tesetaxel and capecitabine only achieved a modest 2.9 month improvement as compared to capecitabine alone [47]. Furthermore, neutropenia was observed in 71.2% of patients, while the control group treated with capecitabine only exhibited 8.3% [47]. Consequently, after development for over 10 years, Odonate Therapeutics failed to get its FDA approval in March, 2021 [45–48].
Figure 2.
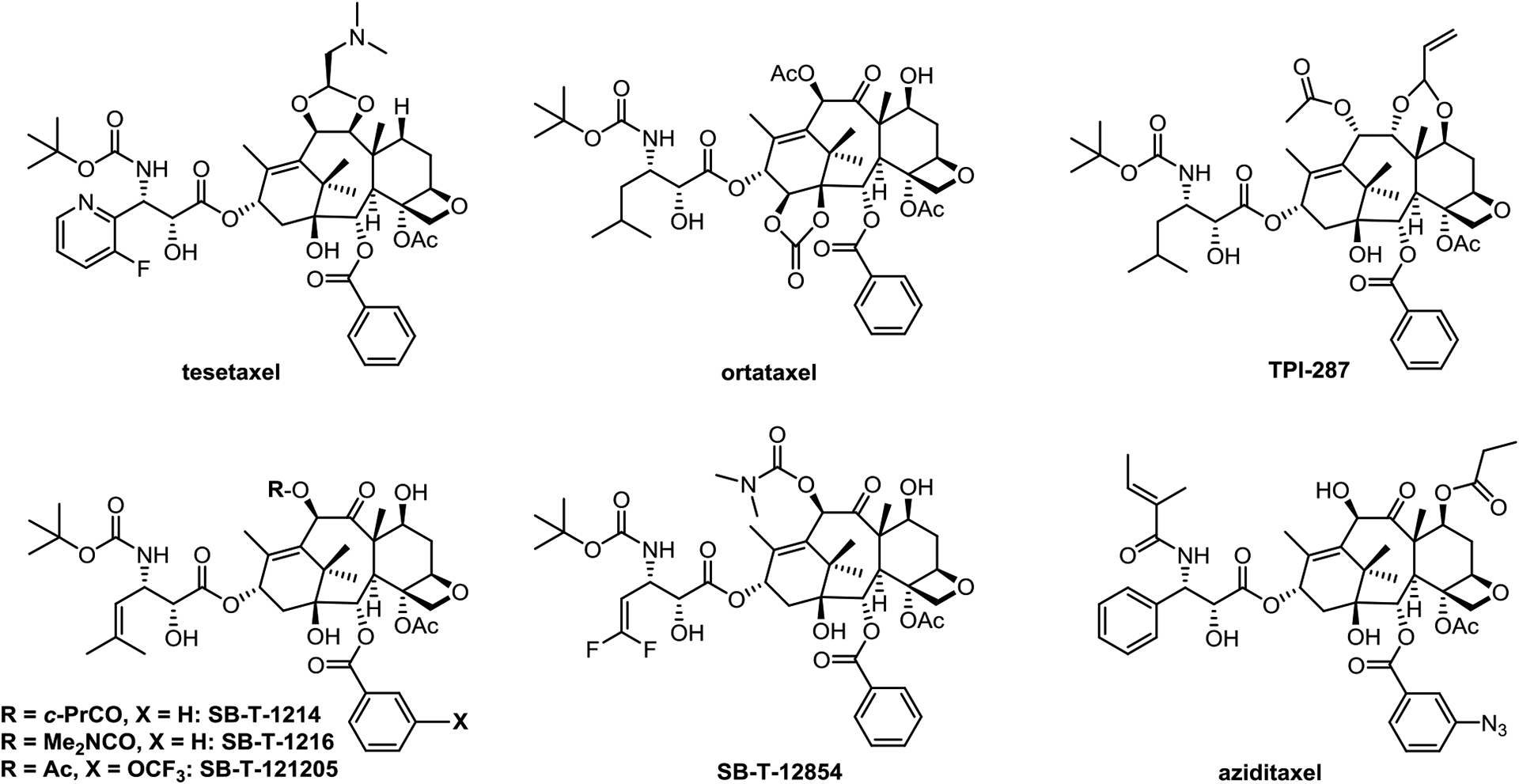
New-generation taxanes in clinical trials or advanced preclinical development
Ortataxel (SB-T-101131, IDN5109) (Figure 2) is an early new-generation taxane that was synthesized from 14-OH-DAB III. Ortataxel is an effective modulator of ABC transporters, and is also orally active [38]. To date, ortataxel completed three Phase II clinical trials for the treatment of taxane-resistant breast cancer, NSCLC [38] and recurrent glioblastoma [49]. Similar to paclitaxel in toxicity profile, ortataxel exhibited encouraging, but still rather limited efficacy against paclitaxel/docetaxel-resistant advanced breast cancer and NSCLC [38]. It failed to meet the expected progression-free survival (PFS) in the treatment of glioblastoma [49].
Abeotaxane, TPI287 (Figure 2), is the first taxane advanced to human clinical trials for treating diseases other than cancer [50]. Although it is in phase I and II clinical trials for a range of cancers, including glio-, neuro- and medulloblastoma [51,52], those for breast cancer and metastatic melanoma [53,54] were suspended. However, its clinical investigation against tauopathies deserves attention [50]. Similar to ortataxel, TPI287 can cross the the blood brain barrier (BBB) [55] and thus, TPI-287 was primarily evaluated for treating brain cancer and a variety of tumors metastasized to the brain [55]. Since microtubule stabilization of cytoskeletal components favors the physiological function of protein tau, whose dysfunction could lead to neuronal death. Cortice Biosciences assessed the safety, tolerability and pharmacodynamics of TPI287 in Alzheimer’s disease, corticobasal degeneration and progressive supranuclear palsy in humans and further clinical trials are in planning [55].
Among the new-generation taxanes developed by Ojima et al., SB-T-1214 (Figure 2) is the most extensively investigated in preclinical studies. It exhibited remarkable efficacy in various tumor xenografts in mice [56] and impressive activity against CSCs [57]. SB-T-12854 (Figure 2) is a 2nd-generation 3ʹ-DFV-taxane, exhibiting excellent metabolic stability, particularly against CYP3A4 enzyme [58,59] and showed superior efficacy to paclitaxel in suppressing rat lymphoma [58,59]. It was found that treatment of tubulins with DFV-taxanes led to the formation of uniquely thinner, shorter and straight microtubules as compared to paclitaxel [59]. These differences of taxanes-tubulin/microtubules interactions is likely a critical contribution factor for the enhanced potency and efficacy of 2nd-generation DFV-taxanes [10].
Derived from cephalomannine, aziditaxel (Figure 2) has a better microtubule-binding affinity than paclitaxel enabling it to overcome drug resistance [60,61], and results in its much better efficacy than that of paclitaxel in BGC-823 human gastric carcinoma and A549 human non-small cell lung carcinoma xenografts in mice models [62]. After being formulated with human serum albumin, the aziditaxel nanoparticles efficiently inhibited the proliferation, migration and invasion of triple negative breast cancer cells in vitro [63]. Aziditaxel is currently under advanced preclinical development.
5. Novel mechanism of actions
Many studies have shown that taxanes mainly function by binding to β-tubulin, triggering mitotic arrest at G2/M phase through suppressing the dynamics of microtubule and this leads to apoptosis [4,10]. However, more studies, particularly with newer generation taxanes are uncovering other mechanisms of action (MOA).
5.1. Induction of multipolar division to cause chromosomal instability
Substantial amount of work has been focusing on determining and understanding the taxane binding site [10]. However, a study using a breast cancer model argues the intratumoral paclitaxel concentration produced by standard paclitaxel treatment regimen is not sufficient to initiate mitotic arrest of breast cancers [64]. It was suggested that it is likely the clustering of multipolar spindles into bipolar spindles that is responsible for paclitaxel resistance, rather than the lower intratumoral paclitaxel concentration. It was shown that longer duration of multipolarity increased instability of chromosome and conversely decreasing its duration increased cell survival and decreased sensitivity to paclitaxel treatment. Thus, the level of chromosomal stability of breast cancer patients was shown as a biomarker to determine whether they are sensitive to multipolar divisions triggered by paclitaxel treatment [64].
5.2. Suppression of CSCs genes, blockage of Hedgehog signaling and PI3K/Akt pathway
It has been demonstrated that new-generation taxanes are capable of enacting other MOA such as suppressing the cancer stem cell genes [13,14], repressing Hedgehog signaling pathway [65] and blocking the PI3K/Akt pathway [66].
As CSCs are critically important for maintenance, resistance to treatment, metastasis, and recurrence of tumor, the suppression of their stemness genes is critical to eradicate these relatively rare, highly drug-resistant, quiescent or slowly proliferating tumor-initiating cells [67]. A new generation taxane, SB-T-1214 exhibited remarkable efficacy in the treatment of CSC-enriched tumors not only by inhibiting the expression of stemness genes, but also by waking up gene expressions to reverse drug-resistance [13].
Significant enhancement of Hedgehog (HH) signaling pathway is critical for the development and metastasis of pancreatic ductal adenocarcinoma (PDAC) [68]. SB-T-1216 was shown to (Figure 2) suppress the HH pathway by down-regulating most of the tested downstream genes in the Paca-44 pancreatic cancer cells, as well as in tumor xenografts in mice [65]. This discovery provides another possibility to treat aggressive pancreatic cancer with new-generation taxanes such as SB-T-1216 [65].
Another new-generation taxane, SB-T-121205 (Figure 2) was found to block the PI3K/Akt pathway by downregulating the expression of transgelin 2, p-Akt and p-GSK-3β, as well as upregulating the expression of tumor-suppressor PTEN in highly taxane-resistant MCF-7/PTX cells [66]. SB-T-121205 not only inhibited proliferation, migration and invasion of paclitaxel resistant cells by suppressing epithelial–mesenchymal transition, but exhibited less toxicity towards BEAS-2B nontumorigenic human bronchial epithelial cells, as compared to paclitaxel [66].
6. Tumor-targeted drug delivery of taxanes
To mitigate undesirable side effects, especially for the rapidly proliferating noncancer cells in certain tissues like bone marrow, extensive efforts have been made on taxane-based tumor-targeting chemotherapy [69–78]. Along this line, the development of “tumor-targeting prodrugs” has been a promising approach [72,73,77,78]. Methods such as new liposomal nanoformulations [79,80], peptide-drug conjugates (PDC) [81–86], “TumorSelect” technology [87], omega-3 polyunsaturated fatty acids (PUFAs) [71,88], vitamin Bs [72,73,77], poly(2-oxazoline)s micelles [9], monoclonal antibodies (mAbs) [78], hyaluronic acid (HA) [89,90], single-walled carbon nanotubes [76], and PAMAM dendrimers [77] have been used as tumor-specific targeting modules for the development of taxane-based tumor-targeting prodrugs and each of these will be briefly presented here.
6.1. Liposomal nanoformulations
Liposomal formulations have been extensively explored for taxane development. Constructed with dioleoyl-3-trimethylammonium propane/dioleoyl-sn-glycero-3-phosphocholine, a cationic liposomal formulation, “EndoTAG®−1”, has exhibited good efficacy against pancreatic cancer and advanced breast cancer by targeting the negatively charged surface of newly formed epithelial cells of tumor vasculature [79]. Phase III clinical trials in pancreatic cancer, as a first-line therapy, is currently underway in China and as a second-line therapy in a number of other countries [91].
Another very promising formulation is ginposomal paclitaxel, prepared by replacing liposomal cholesterol and PEG with a ginsenoside Rh2 [80]. Ginsenoside is the major bioactive substance obtained from ginseng and is responsible for a variety of pharmacological functions, including anticancer activity [92]. Ginsenoside can promote the cytotoxic and apoptotic effects of paclitaxel through inhibiting the NF-κB signaling pathway and regulating the Bax/Bcl-2 expression in breast cancer [93]. Relative to cholesterol, Rh2 can stabilize the liposome, prolong its blood circulation, and enhance its accumulation in tumors by targeting the glucose transporters of tumor cells. Moreover, ginposome works well in tumor microenvironment where the clinical therapeutic efficacy of liposome is limited [80]. Ginposomal paclitaxel exhibited much better efficacy than Abraxane in preclinical studies [94], and is currently in Phase I clinical trial. The ginposomal formulation of docetaxel and cabazitaxel are also under preclinical development.
6.2. Peptide-taxane conjugates (PDCs) and FK506-taxane conjugate
Two taxane-based peptide drug candidates, ANG-1005 and TH1902 (Figure 3) have advanced to human clinical trials [85,86]. ANG-1005 was designed and developed by linking three molecules of paclitaxel to Angiopep-2 (AP-2), a flexible peptide-based drug delivery platform for transporting drugs across the BBB. It efficaciously decreased ovarian cancer originated metastatic tumors within the brain. Although it failed to meet expectations in a phase II breast cancer metastasis study, clear improvement and prolonged overall survival were observed in patients with leptomeningeal carcinomatosis [85]. For further evaluation of the patients with poor prognosis, a randomized Phase III clinical trial is underway [85].
Figure 3.
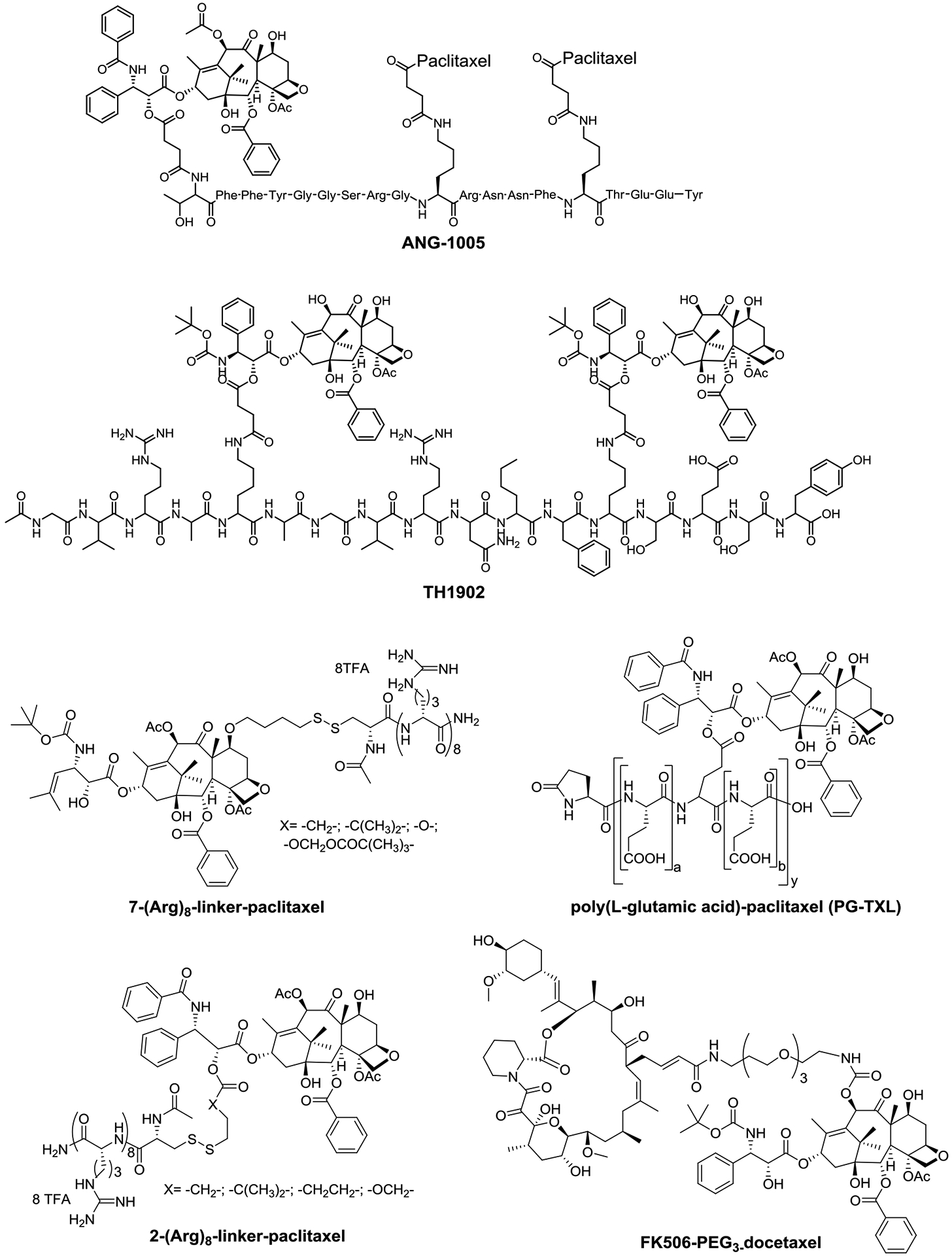
ANG-1005, TH1902, C2’-/C7-polyarginine-paclitaxel, poly(glutamic acid)-paclitaxel (PG-TXL) and FK506-PEG3-docetaxel
Based on its own “SORT1+ Technology”, TH1902 was designed and developed by Theratechnologies to treat patients with recurrent sortilin tumors that are refractory to normal therapy. It is a docetaxel linked to a sortilin receptor targeting peptide, and is currently under Phase I evaluation as a single agent treatment for all sortilin receptor positive advanced refractory solid tumors [86].
To increase aqueous solubility and for tumor targeting, oligopeptides are used to develop taxane-based prodrugs [82,95] (Figure 3). Poly(L-glutamic acid)-paclitaxel (PG-TXL) was advanced to Phase III clinical trial for the treatment of non-small cell lung cancer (NSCLC), ovarian cancer and glioblastoma [37]. Similarly, paclitaxel has also been conjugated to oligoarginine with a disulfide linker at the C2’ or C7 position to overcome the efflux-based MDR in human ovarian carcinoma [83]. Another example, to reduce taxane neurotoxicity, an immunosuppressive and neuroprotective agent FK506 was conjugated to docetaxel via a PEG3 linker (Figure 3). This conjugate exhibited high potency in SKOV3 ovarian, PC3 prostate and MCF7 breast cancer cell lines [84].
6.3. ART-207 using TumorSelect® technology
Taking advantage of an emerging anticancer technology, “TumorSelect”, ART-207 (Figure 4) was recently reported as a promising chemotherapy [87]. As tumor cells are voracious for cholesterol and very lipophilic molecules, ART-207 incorporates an acid-labile lipophilic chain on the C2’-OH of paclitaxel for recognition by tumor cell LDL receptors. This allows ART-207 to selectively accumulate in tumor tissues, get internalized and hydrolyzed in acidic environment of the cancer cell to release free active paclitaxel in a controlled manner. Consequently, this prodrug shows long-lasting effect with greatly decreased collateral damage to normal tissue. In a xenograft model of ovarian cancer in SCID mice, ART-207 exhibited an obviously better efficacy relative to Abraxane with minor weight loss [87].
Figure 4.
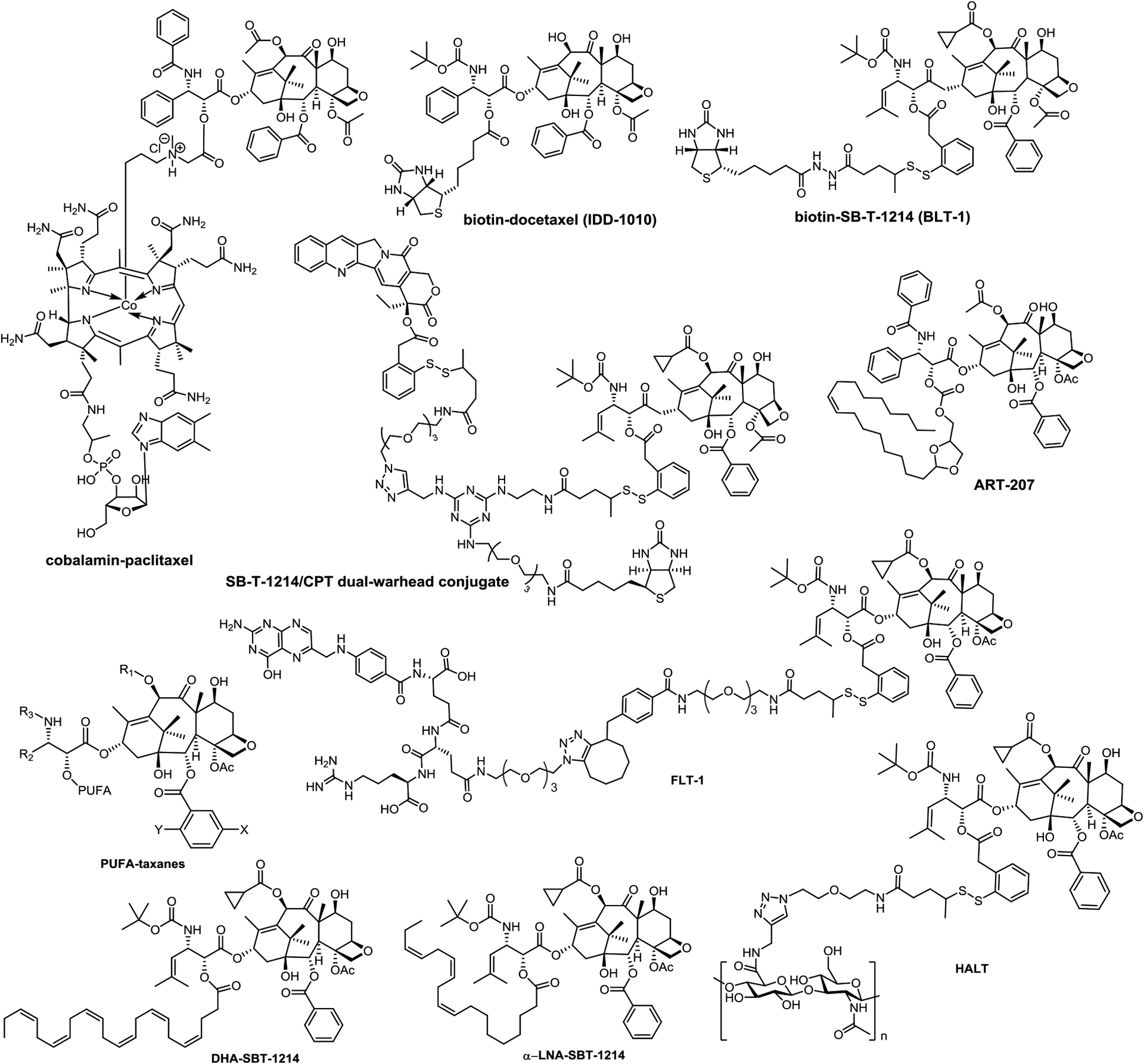
Structures of FLT-1, HALT, PUFA-taxane conjugates, DHA-SBT-1214, α-LNA-SBT-1214 and ART-207
6.4. Taxane conjugates with omega-3 polyunsaturated fatty acids (PUFAs) and their nanoemulsion formulation
Omega-3 polyunsaturated fatty acids (PUFAs) often exhibit synergistic effects with cytotoxic drugs and of these, DHA-paclitaxel, was advanced to Phase III clinical study against metastatic melanoma and pancreatic cancer [96]. Following up on the promising feature of DHA-paclitaxel, PUFA conjugates of much more potent new-generation taxanes have been extensively explored (Figure 4) [56]. As anticipated, the new-generation derived PUFA, DHA-SBT-1214, exhibited a remarkable antitumor effect [38]. It achieved complete eradication of pancreatic (PANC-1) and colon (DLD-1) tumor xenografts in mouse models [88]. Comparably, α-LNA-SBT-1214 (Figure 4) also demonstrated similar efficacy to that of DHA-SBT-1214 [97].
To enhance therapeutic efficacy and bioavailability, DHA-SBT-1214 was formulated as a nanoemulsion. Taking advantage of the “enhanced permeability and retention (EPR) effect”, taxane nanoemulsion, NE-DHA-SBT-1214, exhibited substantially improved pharmacokinetic and pharmacodynamic properties as well as reduced systemic toxicity [98]. In mice implanted with patient-derived prostate cancer stem cells PPT2, NE-DHA-SBT-1214 exhibited much better efficacy than Abraxane without causing significant weight loss [98]. Moreover, no CSCs were detected or found viable in the NE-DHA-SBT-1214 treated tumors. In contrast, the viable cells that survived Abraxane treatment still induced a substantial amount of floating spheroids and holoclones [98]. NE-DHA-SBT-1214 is well positioned for development as a comprehensive targeted therapeutic modality for both de-bulking tumor and for eradicating cancer stem cell (CSC) populations in multidrug-resistant cancers [98].
6.5. Vitamin B-taxane conjugates
Tumor cells are particularly voracious for certain vitamins, especially vitamin Bs such as folate (vitamin B9), biotin (vitamin B7) and cobalamin (vitamin B12) [75]. Receptors of these vitamins are often highly overexpressed on the surface of cancer cells [99]. These receptors are widely used for tumor-targeting taxane delivery [100]. The anti-angiogenic efficacy of a paclitaxel-cobalamin conjugate (Figure 4) was shown to be effective in the treatment of eye diseases [101]. A biotin-docetaxel conjugate, IDD-1010, exhibited remarkable efficacy in the PC3 prostate tumor xenograft in mice [102]. A folate-linker-taxane conjugate, FLT-1 (Figure 4), displayed excellent selectivity for ID8 ovarian and MX-1 breast cancer cells in comparison with normal lung fibroblast cells [103].
In addition to folate-linker-taxane conjugates (FLTs) and biotin-linker-taxane conjugates (BLTs) such as IDD-1010, FLT-1 and BLT-1 (Figure 4), a dual-warhead biotin-conjugate bearing camptothecin (CPT) and SB-T-1214 (1:1) was also developed [73] (Figure 4). This unique conjugate as a combination therapy displayed over 100 times higher selectivity for cancer cells over human normal cells [73].
6.6. Taxane conjugates with hyaluronic acid
Hyaluronic acid or hyaluronan (HA), a water-soluble polysaccharide, is used to target the CD44 marker of cancer stem cells (CSCs) [89,90]. HA is very common in the extracellular tissue matrix and is important for cell proliferation [104]. Additionally, HA-based nanoparticles have been shown to accumulate in tumor tissue and exhibit prolonged circulation by taking advantage of the enhanced permeability and retention (EPR) effect [105]. Accordingly, HA can be an excellent vehicle for drug delivery through the attachment of drugs to its hydroxyl or carboxyl groups. Thus, a hyaluronic acid-linker-taxane conjugate, HALT (Figure 4), was constructed by attaching SB-T-1214 via a self-immolative disulfide linker to ensure the release of the drug only after internalization [106]. The biological evaluations of HALT showed promising activity against CSC spheroids [106].
6.7. EPR-based tumor-targeted taxane delivery using poly(2-oxazoline)-based micelles
A series of new-generation taxanes were successfully encapsulated in micelles of doubly amphiphilic poly(2-oxazoline)s (POx) with high loading capacity for EPR-based tumor-targeted drug delivery [9]. Among these POx-taxanes, POx-SB-T-1214 exhibited up to 100 times higher activity than the corresponding POx-paclitaxel against LCC6/MDR human breast cancer cell line in vitro [9]. Furthermore, POx-SB-T-1214 also exhibited impressive suppression of the growth of LCC6/MDR and T11 orthotopic tumors in mouse models [9].
6.8. Monoclonal antibody-taxane conjugates
The first antibody drug conjugate (ADC) using paclitaxel as a payload only exhibited limited cytotoxic activity in vivo, even though it was better than paclitaxel in vitro [107]. In pioneering work of Chari and Ojima, highly efficacious EGFR-targeting ADCs using 2nd-generation taxanes as warheads were developed (Figure 5). These early ADCs were not only highly selective to cancer cells in vitro, but also exhibited excellent efficacy in the treatment of A431 human squamous tumor xenografts in mice without showing systemic toxicity [78]. Considering the loss of potency from C10 modification of the original taxane structure, highly efficient self-immolative disulfide smart linkers were developed to release the original taxane without compromising potency [75].
Figure 5.
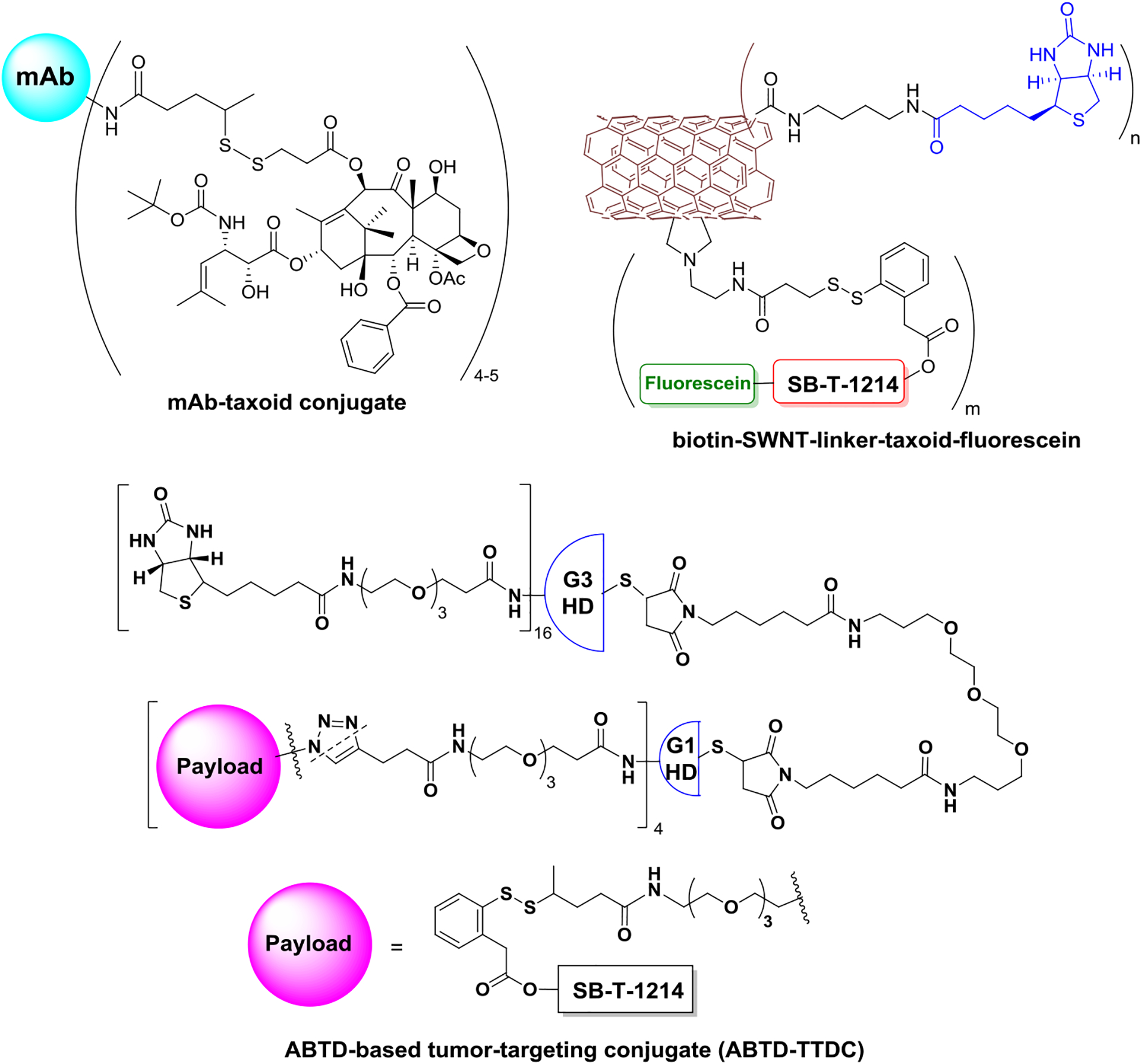
EGFR-mAb-taxoid conjugate, vitamin-SWNT-taxoid conjugate, and ABTD-TTDC-taxoid.
6.9. Taxane conjugates with surface-modified single-walled carbon nanotubes
Chen et. al. successfully attached 178 molecules of biotin and 71 molecules of SB-T-1214-fluorescein to single-wall carbon nanotubes (SWNTs) of 250 nm in length and average diameter of 1 nm to produce a novel SWNT-taxane conjugate, SWNT-SB-T-1214 (Figure 5) [76]. Subsequent confocal fluorescence microscopy (CFM) analysis indicated this huge tumor targeting drug carrier “Trojan horse” was completely internalized via receptor-mediated endocytosis (RME). SWNT-SB-T-1214 exhibited remarkable cytotoxicity and selectivity (>150) against biotin-receptor expressing (BR+) cancer cells compared to normal human cells (BR−) [76].
6.10. Asymmetric bow-tie poly(amidoamine) (PAMAM) dendrimer-taxane conjugates
The well-defined structure and functionalization feature of dendrimers make it a promising drug delivery system [77,108]. Starting from poly(amidoamine) dendrimers with cystamine cores, Wang et. al. successfully constructed an asymmetric bowtie dendrimer (ABTD)-tumor-targeting conjugate (TTC) (Figure 5). ABTD-TTC, for biotin receptor recognition, bears 16 biotins in the G3 half-dendron moiety and 4 taxanes connected to self-immolative linkers in the G1 half-dendron moiety [77]. This dendrimer exhibited an extraordinary cancer cell specificity of 1,000–5,000 times more selective for ID-8 ovarian (BR+) and MX-1 breast (BR+) cancer cells than to WI38 human lung fibroblast cells (BR−) [77].
7. Summary
This review has covered the exploration and development of new formulations of FDA approved taxanes, and the new-generation taxanes that possess superior pharmacological properties and higher potencies than the parent taxanes. The new-generation taxanes, other than initiating tubulin polymerization much faster than the 1st-generation taxanes, also revealed new mechanisms of action [109]. Favorably, the new-generation taxanes are able to eradicate CSCs in vivo by suppressing “stemness genes” and promoting cell differentiation [13]. Furthermore, the taxane SB-T-121205 can overcome MDR by blocking the invasion and metastasis, as well as the epithelial-mesenchymal transition of cancer cells by suppressing the PI3K/Akt pathway[66]. These are previously unknown MOA of taxanes.
Starting from three 1st-generation taxanes used in the clinic, this review also covered the supply of API intermediates, new formulations, the major issues of FDA-approved taxanes, the design and development of new-generation taxanes, particularly those already in clinical trials or advanced preclinical development, and a variety of taxane-based drug delivery systems. Considering that efficacious tumor-targeted drug delivery systems are the key to advance the new-generation taxanes to become clinical drugs, this account covered taxane-based delivery systems, especially nanoparticle formulations.
8. Expert opinion
It is expected that the three FDA-approved taxane drugs, paclitaxel, docetaxel and cabazitaxel will continue to be used as first line chemotherapies in the foreseen future. The heavily invested cultivation of yew trees in China will keep supplying substantial amounts of API intermediates for the global market. The extraordinary efficacy of Abraxane, specifically its combination therapy with gemcitabine for pancreatic cancer patients indicates that one future for taxane drugs will be in combination therapies. Additionally, there is continuous exploration and development of tumor targeting drug delivery systems, especially highly efficacious formulations that spare normal cells and are superior to Abraxane. These show promise to produce single agent taxane drugs with less toxic side effects.
To overcome multi-drug resistance, eradicate cancer stem cells, solve aqueous solubility issues, development of new-generation taxanes with superior pharmacological properties and higher potencies are extremely important, especially for pancreatic and colon cancer patients who do not respond to checkpoint inhibitors well. New-generation taxanes are also expected to expand their applications to treating prostate cancer, triple-negative breast, gastric, melanoma, brain and renal cancer patients on whom currently used taxanes have very limited efficacy. Highly potent new-generation taxanes will be critical as the payloads/warheads of efficacious tumor-targeted drug delivery systems and are expected to be developed as an integral part of cancer therapy.
Usually, improved clinical efficacy of taxane-based treatments are achieved by increasing the dosage through formulation, however, the raised systemic exposure also increases incidence of undesired toxic side effects. Ortataxel, for example, failed to realize its optimal efficacy partially because of dose-limiting toxicity resulting from the conventional Tween 80/ethanol/saline formulation. If maximum tolerated dose (MTD) can be raised by using a new drug delivery formulation and higher doses can be achieved, with minimal side effects, its efficacy would be substantially improved, like in the case of Abraxane. Similarly, to obtain the FDA approval, a new drug delivery formulation is also the most practical and efficient strategy for tesetaxel and other new-generation taxanes already in clinical trials.
The progression of new-generation taxanes heavily relies on the exploration of ideal formulations and drug delivery systems. It is one of the major fields of taxane-based drug development. Certain vitamins like folate and biotin, PUFAs especially DHA, hyaluronic acid, peptides, antibody as well as various nanoscale vehicles are all promising as tumor-targeting modules. Particularly, taxane-based liposomal formulations are expected to be a hot field in drug development.
To date, no taxane-ADCs have been advanced to clinical studies, simply because the cytotoxicity level was not high enough. For the first-generation taxane-ADCs, the payload needed to be in the 10–100 pM IC50 range. However, in more recently developed second-generation taxane-ADCs a 1 nM level IC50 is acceptable, due to the “bystander effects” in which delivered free drug diffuses from antigen positive to neighboring antigen negative cancer cells and so contributes to the efficacy.
Alternatively, small molecule drug conjugates (SMDCs) are also highly specific and smaller in size, as well as have shorter retention time, better penetration, and are capable of delivering cytotoxic agents selectively to cancer cells, thereby reducing the off-target side effects observed with traditional chemotherapy [110]. Owing to their low molecular weight, SMDCs are excreted with significantly faster kinetics than the large ADC counterparts [110]. By minimizing the time spent in circulation, the probability of premature drug release in healthy tissues is reduced, and thereby the likelihood of mitigating off-target toxicities is improved [110].
Results from recent studies indicate that investigations into the new mechanisms of action of taxanes are important for taxane-based drug development, specifically for combination therapies. The disclosed new mechanisms of action of new-generation taxanes include overcoming MDR by suppressing CSC genes, as well as blocking Hedgehog and PI3K/Akt signaling pathways. Paclitaxel was also reported to destabilize chromosomes by inducing multipolar division. These new MOA may help to expand utility to other cancers and disease indications in which taxanes are currently not used.
Further exploration of broader applications of taxanes beyond cancer is an interesting and promising field of research. As indicated by TPI-287, an abeotaxane in clinical trials for the treatment of Alzheimer’s disease, corticobasal degeneration and progressive supranuclear palsy, tubulin related diseases like neural system disorders would be a field for further investigation with taxanes.
Lastly, it is very important to emphasize that the library of fluorine-containing 3rd-generation taxanes is a very valuable asset for drug development, particularly those bearing the DFV moiety in the isoserine side chain. Endued with excellent pharmacological properties, these extremely potent taxanes are highly promising as payloads/warheads of ADCs and SMDCs or for various emerging new tumor-targeted drug delivery systems and nanoformulations.
Article Highlights:
The FDA approved taxanes will continue to play significant roles in a foreseen future as the first-line drugs for cancer chemotherapy.
Efficacious drug delivery systems, especially nanoformulations, are the promising directions of taxane-based drug development.
Next-generation taxanes are critically important as the payloads of efficacious tumor-targeted drug delivery systems.
Next-generation taxanes overcome MDR, suppress CSC genes, block Hedgehog signaling and PI3K/Akt pathway.
Highly potent fluorine-containing 3rd-generation taxanes exhibit excellent pharmacological properties for development.
TPI-287, an abeotaxane in clinical trials for brain cancers, also exhibited efficacy in Alzheimer’s disease, corticobasal degeneration and progressive supranuclear palsy.
Acknowledgments
The authors thank Mr. Huaxing Zhan of Shanghai Ginposome Pharmatech and Mr. Changming Li of Fujian Yew Park Biological Co. Ltd have for providing useful information. Generous support from the Institute of Chemical Biology & Drug Discovery, Stony Brook University, USA is gratefully acknowledged.
Funding
This work was funded, in part, by the International Collaboration Foundation of Guangdong Province, China, Grant 2016A050502039 and the National Institutes of Health, USA, Grant CA103314.
Abbreviations:
- 10-DAB III
10-deacetylbaccatin III
- 9-DHB
9-dihydro-10-DAB III
- 14-OH-DAB III
14 β-hydroxy-10-deacetylbaccatin III
- ABTD-TTC
asymmetric bowtie dendrimer tumor-targeting conjugate
- ADCs
antibody-taxane conjugates
- AP-2
Angiopep-2
- APIs
Active Pharmaceutical ingredients
- BBB
Blood Brain Barrier
- BLTs
biotin-linker-taxane conjugates
- BR+
biotin-receptor expressing
- CFM
confocal fluorescence microscopy
- CSC
cancer stem cell
- CPT
camptothecin
- DFV
difluorovinyl
- DHA
docosahexaenoic acid
- EGFR
epidermal growth factor receptor
- EPR effect
enhanced permeability and retention effect
- FDA
Food and Drug Administration, U.S.A.
- FLTs
folate-linker-taxane conjugates
- G2/M phase
growth 2/mitotic phase
- HA
hyaluronic acid or hyaluronan
- HALT
hyaluronic acid-linker-taxane conjugate
- mAbs
monoclonal antibodies
- MDR
multidrug resistance
- MRP1
multidrug resistance-associated proteins
- MTD
maximum tolerated dose
- NSCLC
non-small cell lung cancer
- o(LA)8-PTX
7-Oligo(lactic acid)-paclitaxel
- PAMAM
asymmetric bow-tie poly(amidoamine)
- PDAC
pancreatic ductal adenocarcinoma
- PDC
peptide-drug conjugates
- PEG-b-PLA
poly(ethylene glycol)-block-poly(D, L-lactide)
- PFS
progress free survival
- Pgp
P-glycoprotein
- PG-TXL
Poly(L-glutamic acid)-paclitaxel
- PI3K/Akt pathway
phosphoinositide 3-kinases/protein kinase B pathway
- POx
poly(2-oxazoline)s
- PUFAs
omega-3 polyunsaturated fatty acids
- RME
receptor-mediated endocytosis
- SFDA
State Food and Drug Administration, China
- SMDCs
small molecule drug conjugates
- SWNTs
single-wall carbon nanotubes
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Moslehi JJ, Salem JE, Sosman JA, et al. Reporting of immune checkpoint inhibitor-associated myocarditis Reply. Lancet. 2018. Aug 4;392(10145):384–385. [DOI] [PubMed] [Google Scholar]
- 2.Moslehi JJ, Salem JE, Sosman JA, et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018. Mar 10;391(10124):933–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ojima I, Lichtenthal B, Lee S, et al. Taxane anticancer agents: a patent perspective. Expert Opin Ther Pat. 2016;26(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiff PB, Fant J, Horwitz SB. Promotion of Microtubule Assembly Invitro by Taxol. Nature. 1979;277(5698):665–667. [DOI] [PubMed] [Google Scholar]
- 5.Suffness M Taxol: Science and Applications. CRC Press: New York, 1995.: CRC Press: New York; 1995. [Google Scholar]
- 6.Sanofi - Annual Reports, 2020. Available from: https://www.annualreports.com/HostedData/AnnualReports/PDF/NYSE_SNY_2020.pdf
- 7.Jordan Goodman VW. The Story of Taxol: Nature and Politics in the Pursuit of an Anti-Cancer Drug. Cambridge University Press; 2001. [Google Scholar]
- 8.Zhang H, Tan J, Xie J, et al. , inventors; Diqing Shunyuan Biotechnology Company Ltd., assignee. A method of extrract, seperate and purify 10-DAB, CN202110448063.3 patent CN 113149935 A. 2021.; *This patent describes the cultivation of an elite clone of the fastest growing Mairei yew, ensuring the sustainable supply of API for global need.
- 9.He Z, Schulz A, Wan X, et al. Poly(2-oxazoline) based micelles with high capacity for 3rd generation taxoids: Preparation, in vitro and in vivo evaluation. J Control Release. 2015;208:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ojima I, Wang X, Jing YR, et al. Quest for Efficacious Next-Generation Taxoid Anticancer Agents and Their Tumor-Targeted Delivery. J Nat Prod. 2018. Mar;81(3):703–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das T, Anand U, Pandey SK, et al. Therapeutic strategies to overcome taxane resistance in cancer. Drug Resist Updat. 2021. Mar;55:100754. [DOI] [PubMed] [Google Scholar]; *This manuscript summarizes the drug resistance issue and mechanism of FDA approved taxanes.
- 12.Skubnik J, Pavlickova V, Ruml T, et al. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants (Basel). 2021. Mar 17;10(3):569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Botchkina GI, Zuniga ES, Das M, et al. New-generation taxoid SB-T-1214 inhibits stem cell-related gene expression in 3D cancer spheroids induced by purified colon tumor-initiating cells [journal article]. Mol Cancer. 2010. July 14;9(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Botchkina GI, Zuniga ES, Rowehl RH, et al. Prostate Cancer Stem Cell-Targeted Efficacy of a New-Generation Taxoid, SBT-1214 and Novel Polyenolic Zinc-Binding Curcuminoid, CMC2.24. PLoS ONE. 2013;8(9):e69884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tam YT, Shin DH, Chen KE, et al. Poly(ethylene glycol)-block-poly(d,l-lactic acid) micelles containing oligo(lactic acid)8-paclitaxel prodrug: In Vivo conversion and antitumor efficacy. J Control Release. 2019. Mar 28;298:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi M, Gu A, Tu H, et al. Comparing nanoparticle polymeric micellar paclitaxel and solvent-based paclitaxel as first-line treatment of advanced non-small-cell lung cancer: an open-label, randomized, multicenter, phase III trial. Ann Oncol. 2021. Jan;32(1):85–96. [DOI] [PubMed] [Google Scholar]; **This article describes the advantage of nanoparticle polymeric micellar paclitaxel that recently approved by SFDA.
- 17.Daehwa Pharma earns approval for world’s first oral anti-cancer drug 2016. Available from: http://www.koreaherald.com/view.php?ud=20160909000885
- 18.Ryu MH, Ryoo BY, Kim TW, et al. A Phase I/IIa Study of DHP107, a Novel Oral Paclitaxel Formulation, in Patients with Advanced Solid Tumors or Gastric Cancer. Oncologist. 2017;22(2):129-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rugo HS, Pluard TJ, Sharma P, et al. Pharmacokinetic evaluation of an oral paclitaxel DHP107 (Liporaxel (R)) in patients with recurrent or metastatic breast cancer (MBC): Phase II study (OPERA, NCT03326102). Cancer Res. 2021. Feb;81(4):615. [Google Scholar]
- 20.Abraxane 2022. Available from: https://www.abraxanepro.com/
- 21.ClinicalTrials.gov.: Paclitaxel liposome 2021. [cited 2021]. Available from: https://www.clinicaltrials.gov/ct2/results?cond=&term=lipusu&cntry=&state=&city=&dist=&Search=Search
- 22.ClinicalTrials.gov. Genexol-PM 2021. Available from: https://www.clinicaltrials.gov/ct2/results?cond=&term=genexol-PM&cntry=&state=&city=&dist=&Search=Search
- 23.ClinicalTrials.gov. Nanoxel and Nanoxel M 2021. Available from: https://www.clinicaltrials.gov/ct2/results?cond=&term=NAnoxel&cntry=&state=&city=&dist=&Search=Search
- 24.ClinicalTrials.gov. EndoTAG1 2021. Available from: https://www.clinicaltrials.gov/ct2/results?cond=&term=endotag&cntry=&state=&city=&dist=&Search=Search
- 25.Graziani SR, Vital CG, Morikawa AT, et al. Phase II study of paclitaxel associated with lipid core nanoparticles (LDE) as third-line treatment of patients with epithelial ovarian carcinoma. Med Oncol. 2017;34(9):151. [DOI] [PubMed] [Google Scholar]
- 26.ClinicalTrials.gov. Liposome Entrapped Docetaxel (LE-DT) 2021. Available from: https://www.clinicaltrials.gov/ct2/results?cond=&term=LE-DT&cntry=&state=&city=&dist=&Search=Search
- 27.ClinicalTrials.gov. Paclical® 2021. Available from: https://www.clinicaltrials.gov/ct2/results?cond=&term=paclical&cntry=&state=&city=&dist=&Search=Search
- 28.Athenex Receives FDA Complete Response Letter for Oral Paclitaxel Plus Encequidar for the Treatment of Metastatic Breast Cancer 2021. Available from: https://www.globenewswire.com/en/news-release/2021/03/01/2184118/0/en/Athenex-Receives-FDA-Complete-Response-Letter-for-Oral-Paclitaxel-Plus-Encequidar-for-the-Treatment-of-Metastatic-Breast-Cancer.html
- 29.Wainberg ZA, Hochster HS, Kim EJ, et al. Open-label, Phase I Study of Nivolumab Combined with nab-Paclitaxel Plus Gemcitabine in Advanced Pancreatic Cancer. Clin Cancer Res. 2020. Sep 15;26(18):4814–4822. [DOI] [PubMed] [Google Scholar]
- 30.Athenex Provides Update from FDA Type A Meeting Regarding Oral Paclitaxel + Encequidar in Metastatic Breast Cancer 2021. Available from: https://finance.yahoo.com/news/athenex-provides-fda-type-meeting-120000761.html
- 31.Casak SJ, Fashoyin-Aje I, Lemery SJ, et al. FDA Approval Summary: Ramucirumab for Gastric Cancer. Clin Cancer Res. 2015. Aug 1;21(15):3372–6. [DOI] [PubMed] [Google Scholar]
- 32.Clinicaltrials.gov. NCT04325698. BEVACIZUMAB PLUS PACLITAXEL-CARBOPLATIN IN NSCLC 2021. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04325698?term=NCT04325698&draw=2&rank=1 [Google Scholar]
- 33.ClinicalTrials.gov. NCT03991403, Study of Atezolizumab Combination Carboplatin + Paclitaxel + Bevacizumab in EGRF Mutation or ALK Translocation NSCLC 2021. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03991403?term=NCT03991403&draw=2&rank=1
- 34.ClinicalTrials.gov. NCT04489888. A Study of Pembrolizumab (MK-3475) Plus Carboplatin and Paclitaxel as First-line Treatment of Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma 2021. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04489888?term=NCT04489888&draw=2&rank=1 [Google Scholar]
- 35.ClinicalTrials.gov. NCT04278092. Paclitaxel Plus Cetuximab After First-line Checkpoint Inhibitor Failure 2021. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04278092?term=NCT04278092&draw=2&rank=1 [Google Scholar]
- 36.ClinicalTrials.gov. NCT03941093. Evaluation of Efficacy and Safety of Neoadjuvant Treatment With Pamrevlumab in Combination With Chemotherapy in Participants With Locally Advanced Pancreatic Cancer 2021. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03941093?term=NCT03941093&draw=2&rank=1 [Google Scholar]
- 37.Yared JA, Tkaczuk KH. Update on taxane development: new analogs and new formulations. Drug Des Devel Ther. 2012;6:371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ojima IKA, Seitz JD. Taxol, taxoids and related taxanes. In: Hanessian S, editor Natural products in medicinal chemistry Weinheim (Germany): Wiley- VCH. 2013:127–180. [Google Scholar]
- 39.Wang C, Wang X, Sun Y, et al. Design, synthesis and SAR study of 3rd-generation taxoids bearing 3-CH3, 3-CF3O and 3-CHF2O groups at the C2-benzoate position. Bioorg Chem. 2020. Jan;95:103523. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This article describes highly potent 3rd-generation taxanes
- 40.Wang C, Chen L, Sun Y, et al. Design, synthesis and SAR study of Fluorine-containing 3rd-generation taxoids. Bioorg Chem. 2022;119:103523. [DOI] [PubMed] [Google Scholar]; *This article describes extremely potent 3rd-generation fluorine-containing taxanes.
- 41.Rong D, Wang C, Zhang X, et al. A novel taxane, difluorovinyl-ortataxel, effectively overcomes paclitaxel-resistance in breast cancer cells. Cancer Lett. 2020. Oct 28;491:36–49. [DOI] [PubMed] [Google Scholar]
- 42.Wang MC, Wang CW, Feng C, et al. Potent antitumor activity of novel taxoids in anaplastic thyroid cancer. Endocrine. 2021. Sep 30;75:465–477. [DOI] [PubMed] [Google Scholar]
- 43.He JA, Zaipaer; Li Yan; Zhang Jin; Du Qianqian; Sun Chenglong; Zhang Ruiping; Jin Hongtao; Song Xiaowei; Luo Zhigang, inventor; Institute of Materia Medica, Chinese Academy of Medical Sciences, assignee. Quaternization modified taxane derivatives, pharmaceutical compositions thereof, synthetic routes and uses thereof. Peop. Rep. China patent WO 2020177748 A1. 2019.
- 44.Geng ZW, Xiugen; Li Zhaoguang; Wei Furong, inventor; Jiangsu Jibeier Pharmaceutical Co., Ltd., assignee. Taxane derivatives for pharmaceutical compositions. Peop. Rep. China patent CN 111196790 A. 2020.
- 45.Moore M, Jones C, Harker G, et al. Phase II trial of DJ-927, an oral tubulin depolymerization inhibitor, in the treatment of metastatic colorectal cancer. J Clin Oncol. 2006;24:3591. [Google Scholar]
- 46.Roche M, Kyriakou H, Seiden M. Drug evaluation: tesetaxel--an oral semisynthetic taxane derivative. Curr Opin Investig Drugs. 2006. Dec;7(12):1092–9. [PubMed] [Google Scholar]
- 47.Karlovitch S FDA Feedback Leads to Discontinuation of Tesetaxel Development in Solid Tumors: MJH Life Sciences And Targeted Oncology; 2021. Available from: https://www.targetedonc.com/view/fda-feedback-leads-to-discontinuation-of-tesetaxel-development-in-solid-tumors
- 48.Beeram M, Papadopoulos K, Pantnaik A, et al. Phase I dose-ranging, pharmacokinetic (PK) study of tesetaxel, a novel orally active tubulin-binding agent. J Clin Oncol. 2010;28:13075. [Google Scholar]
- 49.Silvani A, De Simone I, Fregoni V, et al. Multicenter, single arm, phase II trial on the efficacy of ortataxel in recurrent glioblastoma. J Neurooncol. 2019. May;142(3):455–462. [DOI] [PubMed] [Google Scholar]
- 50.THERAPEUTICS TPI-287: Cortice Biosciences; 2017. Available from: http://www.alzforum.org/therapeutics/tpi-287
- 51.Mitchell D, Bergendahl G, Ferguson W, et al. A Phase 1 Trial of TPI-287 as a Single Agent and in Combination With Temozolomide in Patients with Refractory or Recurrent Neuroblastoma or Medulloblastoma. Pediatr Blood Cancer. 2016. Jan;63(1):39–46. [DOI] [PubMed] [Google Scholar]
- 52.Zumbar CT, Usubalieva A, King PD, et al. The CNS penetrating taxane TPI-287 and the AURKA inhibitor alisertib induce synergistic apoptosis in glioblastoma cells. J Neurooncol. 2018. May;137(3):481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McQuade JL, Posada LP, Lecagoonporn S, et al. A phase I study of TPI-287 in combination with temozolomide for patients with metastatic melanoma. Melanoma Res. 2016. Dec;26(6):604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitzgerald DP, Emerson DL, Qian Y, et al. TPI-287, a new taxane family member, reduces the brain metastatic colonization of breast cancer cells. Mol Cancer Ther. 2012. Sep;11(9):1959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsai RM, Miller Z, Koestler M, et al. Reactions to Multiple Ascending Doses of the Microtubule Stabilizer TPI-287 in Patients With Alzheimer Disease, Progressive Supranuclear Palsy, and Corticobasal Syndrome: A Randomized Clinical Trial. JAMA Neurol. 2020. Feb 1;77(2):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This article describes the first taxane advanced to clinical trial other than the treatment of cancer.
- 56.Ojima I, inventorTaxoid-Fatty Acid Conjugates and Pharmaceutical Compositions Thereof, WO2005041881, patent US7820839B2. 2010.
- 57.Botchkina GI, Zuniga ES, Das M, Wang Y, Wang H, Zhu S, Savitt AG, Rowehl RA, Leyfman Y, Ju J, Shroyer K, Ojima I. New-generation taxoid SB-T-1214 inhibits stem cell-related gene expression in 3D cancer spheroids induced by purified colon tumor-initiating cells. Mol Cancer. 2010;9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ojima I, Das M. Recent advances in the chemistry and biology of new generation taxoids. J Nat Prod. 2009. Mar 27;72(3):554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuznetsova L, Sun L, Chen J, et al. Synthesis and biological evaluation of novel 3 ‘-difluorovinyl taxoids. J Fluorine Chem. 2012. Nov;143:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao X, Wu J, Trigili C, et al. Effects of C7 substitutions in a high affinity microtubule-binding taxane on antitumor activity and drug transport. Bioorg Med Chem Lett. 2011. Aug 15;21(16):4852–6. [DOI] [PubMed] [Google Scholar]
- 61.Zhou Q, Li Y, Jin J, et al. Lx2-32c, a novel taxane derivative, exerts anti-resistance activity by initiating intrinsic apoptosis pathway in vitro and inhibits the growth of resistant tumor in vivo. Biol Pharm Bull. 2012;35(12):2170–9. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Li H, Zuo M, et al. Lx2-32c, a novel taxane and its antitumor activities in vitro and in vivo. Cancer Lett. 2008. Sep 8;268(1):89–97. [DOI] [PubMed] [Google Scholar]
- 63.Chen LQ, Zhang ZM, Huang W, et al. Inhibiting the proliferation, migration and invasion of triple negative breast cancer cells through anti-tumor human serum albumin nanoparticles loading aziditaxel as a novel taxane derivative. Pharmazie. 2017. Mar 1;72(3):152–160. [DOI] [PubMed] [Google Scholar]
- 64.Scribano CM, Wan J, Esbona K, et al. Chromosomal instability sensitizes patient breast tumors to multipolar divisions induced by paclitaxel. Sci Transl Med. 2021. Sep 8;13(610):eabd4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohelnikova-Duchonova B, Kocik M, Duchonova B, et al. Hedgehog pathway overexpression in pancreatic cancer is abrogated by new-generation taxoid SB-T-1216. Pharmacogenomics J. 2017;17(5):452–460. [DOI] [PubMed] [Google Scholar]
- 66.Zheng XW, Wang CW, Xing YM, et al. SB-T-121205, a next-generation taxane, enhances apoptosis and inhibits migration/invasion in MCF-7/PTX cells. Int J Oncol. 2017. Mar;50(3):893–902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells [10.1038/35102167]. Nature. 2001. 11/01/print;414(6859):105–111. [DOI] [PubMed] [Google Scholar]
- 68.Witkiewicz AK, McMillan EA, Balaji U, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaracz S, Chen J, Kuznetsova LV, et al. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem. 2005;13:5043–5054. [DOI] [PubMed] [Google Scholar]
- 70.Koshkaryev A, Sawant R, Deshpande M, et al. Immunoconjugates and long circulating systems: Origins, current state of the art and future directions. Adv Drug Deliv Rev. 2013. Jan;65(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen S, Zhao X, Chen J, et al. Mechanism-Based Tumor-Targeting Drug Delivery System. Validation of Efficient Vitamin Receptor-Mediated Endocytosis and Drug Release. Bioconjugate Chem. 2010;21(5):979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vineberg JG, Wang T, Zuniga ES, et al. Design, Synthesis, and Biological Evaluation of Theranostic Vitamin–Linker–Taxoid Conjugates. J Med Chem. 2015. 2015/03/12;58(5):2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vineberg JG, Zuniga ES, Kamath A, et al. Design, Synthesis, and Biological Evaluations of Tumor-Targeting Dual-Warhead Conjugates for a Taxoid–Camptothecin Combination Chemotherapy. J Med Chem. 2014. 06/05 04/22/received;57(13):5777–5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Warnecke A Site-Specific Prodrug Activation and the Concept of Self-Immolation. In: Kratz F, Peter Senter P, Steinhagen H, editors. Drug Delivery in Oncology: From Basic Research to Cancer Therapy. Vol. 2. Weinheim: Wiley-VCH; 2011. p. 553–589. [Google Scholar]
- 75.Ojima I Guided molecular missiles for tumor-targeting chemotherapy-case studies using the second-generation taxolds as warheads. Acc Chem Res. 2008. Jan;41(1):108–119. [DOI] [PubMed] [Google Scholar]
- 76.Chen J, Chen S, Zhao X, et al. Functionalized single-walled carbon nanotubes as rationally designed vehicles for tumor-targeted drug delivery. J Am Chem Soc. 2008. Dec 10;130(49):16778–16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang T, Zhang YZ, Wei LF, et al. Design, Synthesis, and Biological Evaluations of Asymmetric Bow-Tie PAMAM Dendrimer-Based Conjugates for Tumor-Targeted Drug Delivery. ACS Omega. 2018. Apr;3(4):3717–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ojima I, Geng XD, Wu XY, et al. Tumor-specific novel taxoid-monoclonal antibody conjugates. J Med Chem. 2002. Dec 19;45(26):5620–5623. [DOI] [PubMed] [Google Scholar]
- 79.Fasol U, Frost A, Buchert M, et al. Vascular and pharmacokinetic effects of EndoTAG-1 in patients with advanced cancer and liver metastasis. Ann Oncol. 2012. Apr;23(4):1030–6. [DOI] [PubMed] [Google Scholar]
- 80.Hong C, Liang J, Xia J, et al. One Stone Four Birds: A Novel Liposomal Delivery System Multi-functionalized with Ginsenoside Rh2 for Tumor Targeting Therapy. Nanomicro Lett. 2020. Jun 16;12(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This article describes the ginposomal nanoparticle for delivering taxane.
- 81.Kozlowski A, Riley TA, McManus SP, inventorsMulti-arm polymeric prodrug conjugates of taxane-based compounds as a drug delivery systems, WO2012088422A12012.
- 82.Paz-Ares L, Ross H, O’Brien M, et al. Phase III trial comparing paclitaxel poliglumex vs docetaxel in the second-line treatment of non-small-cell lung cancer. Br J Cancer. 2008. May 20;98(10):1608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wender PAG WC; Bhat NM; Pillow TH; Bieber MM; Teng NN Taxol-oligoarginine conjugates overcome drug resistance in-vitro in human ovarian carcinoma. Gynecol Oncol. 2012;126:118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mutz MW, Webb R, Gestwicki JE, inventorsTherapeutic agents having reduced toxicity, WO2011130317A22011.
- 85.Kumthekar P, Tang SC, Brenner AJ, et al. ANG1005, a Brain-Penetrating Peptide-Drug Conjugate, Shows Activity in Patients with Breast Cancer with Leptomeningeal Carcinomatosis and Recurrent Brain Metastases. Clin Cancer Res. 2020. Jun 15;26(12):2789–2799. [DOI] [PubMed] [Google Scholar]
- 86.Demeule M, Charfi C, Currie JC, et al. TH1902, a new docetaxel-peptide conjugate for the treatment of sortilin-positive triple-negative breast cancer. Cancer Sci. 2021. Oct;112(10):4317–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haider S, Penfornis P, Claudio PP, et al. Balancing the efficacy vs. the toxicity of promiscuous natural products: Paclitaxel-based acid-labile lipophilic prodrugs as promising chemotherapeutics. Eur J Med Chem. 2022. Jan 5;227:doi: 10.1016/j.ejmech.2021.113891. [DOI] [PubMed] [Google Scholar]; ** This article describes the TumorSelect technology for tumor-targeting drug delivery of taxane.
- 88.Kuznetsova L, Chen J, Sun L, et al. Syntheses and evaluation of novel fatty acid-second-generation taxoid conjugates as promising anticancer agents. Bioorg Med Chem Lett. 2006. Feb 15;16(4):974–977. [DOI] [PubMed] [Google Scholar]
- 89.Maneval DC, Shepard HM, Thompson CB, inventorsProtein sequences of human hyaluronidase and combination therapy with an anti-hyaluronan agent and a tumor-targeted taxane, WO2013151774A12013.
- 90.Klostergaard J, Farquhar D, Ghosh SC, et al. , inventorsAnticancer agent-hyaluronic acid conjugate compositions and methods of use patent WO2008134528A1. 2008.
- 91.SB05PC (EndoTAG®-1) Phase III been Approved in China by NMPA 2019. Available from: https://www.syncorebio.com/en/sb05pc-endotag-1-phase-iii-been-approved-in-china-by-nmpa/
- 92.Kim H, Lee JH, Kim JE, et al. Micro-/nano-sized delivery systems of ginsenosides for improved systemic bioavailability. Journal of Ginseng Research. 2018. Jul;42(3):361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yuan ZG, Jiang H, Zhu XH, et al. Ginsenoside Rg3 promotes cytotoxicity of Paclitaxel through inhibiting NF-kappa B signaling and regulating Bax/Bcl-2 expression on triple-negative breast cancer. Biomed Pharmacother. 2017. May;89:227–232. [DOI] [PubMed] [Google Scholar]
- 94.Wang X, Zheng W, Shen Q, et al. Identification and construction of a novel biomimetic delivery system of paclitaxel and its targeting therapy for cancer. Signal Transduct Target Ther. 2021. Jan 27;6(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bai H, Tsang KY, Jin Y, et al. , inventorsLarge scale process for preparing poly(glutamyl-glutamate) conjugates for anticancer drug delivery, WO2014175898A12014.
- 96.Tuller ERB AL; Yu H; Lou JR; Benbrook DM; Ding W-Q, . PPARα signaling mediates the synergistic cytotoxicity of clioquinol and docosahexaenoic acid in human cancer cells. Biochem. Pharmacol 2009, 77, 1480–1486. Biochem Pharmacol 2009;77:1480–1486. [DOI] [PubMed] [Google Scholar]
- 97.Seitz JD. The design, synthesis and biological evaluation of novel taxoid anticancer agents and their tumor-targeted drug conjugates: Stony Brook University; 2013. [Google Scholar]
- 98.Ahmad G, El Sadda R, Botchkina G, et al. Nanoemulsion formulation of a novel taxoid DHA-SBT-1214 inhibits prostate cancer stem cell-induced tumor growth. Cancer Lett. 2017. Oct 10;406:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This article describes a highly efficacious nanoemersion formulation of a DHA-taxane conjugate.
- 99.Russell-Jones G, McTavish K, McEwan J, et al. Vitamin-mediated targeting as a potential mechanism to increase drug uptake by tumours. J Inorg Biochem. 2004. Oct;98(10):1625–1633. [DOI] [PubMed] [Google Scholar]
- 100.Leamon CP. Folate-targeted drug strategies for the treatment of cancer. Curr Opin Invest Drugs. 2008. Dec;9(12):1277–1286. [PubMed] [Google Scholar]
- 101.Gebhard JR, Patel D, inventorsCobalamin taxane bioconjugates for treating eye disease, WO2010088287A12010.
- 102.Rayan A, inventorConjugate of a taxane and biotin and uses thereof patent WO2014191989A1. 2014.
- 103.Seitz JD, Vineberg JG, Herlihy E, et al. Design, synthesis and biological evaluation of a highly-potent and cancer cell selective folate–taxoid conjugate. Bioorg Med Chem. 2015;23:2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fraser JRE, Laurent TC, Laurent UBG. Hyaluronan: Its nature, distribution, functions and turnover. J Intern Med. 1997. Jul;242(1):27–33. [DOI] [PubMed] [Google Scholar]
- 105.Yoon HY, Koo H, Choi KY, et al. Photo-crosslinked hyaluronic acid nanoparticles with improved stability for in vivo tumor-targeted drug delivery. Biomaterials. 2013. Jul;34(21):5273–5280. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y Development of nanotechnology-based drug delivery platforms for tumor-targeted chemotherapy: Stony Brook University; 2018. [Google Scholar]
- 107.Guillemard V, Saragovi HU. Taxane-antibody conjugates afford potent cytotoxicity, enhanced solubility, and tumor target selectivity. Cancer Res. 2001. Jan 15;61(2):694–9. [PubMed] [Google Scholar]
- 108.Ojima I, Wang T, Teng Y-HG, inventors; Research Foundation of the State University of New York, assignee. Asymmetric Bow-Tie Dendrimers as Versatile Platform for Drug Delivery and Diagnosis patent WO 2015038493 A1. 2015.
- 109.Ojima I, Chen J, Sun L, et al. Design, synthesis, and biological evaluation of new-generation taxoids. J Med Chem. 2008. Jun 12;51(11):3203–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Srinivasarao MGC, Low PS. . Principles in the design of ligandtargeted cancer therapeutics and imaging agents. Nat Rev Drug Discov. 2015;14:203–219. [DOI] [PubMed] [Google Scholar]


