Abstract
Schizophrenia is a severe neuropsychiatric disorder with a polygenic mode of inheritance which is also governed by non-genetic factors. Candidate genes identified on the basis of biochemical and pharmacological evidence are being tested for linkage and association studies. Neurotransmitters, especially dopamine and serotonin have been widely implicated in its etiology. Genome scan of all human chromosomes with closely spaced polymorphic markers is being used for linkage studies. The completion and availability of the first draft of Human Genome Sequence has provided a treasure-trove that can be utilized to gain insight into the so far inaccessible regions of the human genome. Significant technological advances for identification of single nucleotide polymorphisms (SNPs) and use of microarrays have further strengthened research methodologies for genetic analysis of complex traits. In this review, we summarize the evolution of schizophrenia genetics from the past to the present, current trends and future direction of research.
Keywords: Association strategy, candidate genes/sites, linkage analysis, neurotransmitters, schizophrenia
1. Introduction
Schizophrenia is a common disorder with a proven genetic basis but complex mode of inheritance. Onset is generally during adolescence with a lifetime morbid risk being approximately 1% in the general population (American Psychiatric Association 1987). A constellation of features including hallucinations and delusions characterize schizophrenia. Notwithstanding several studies on segregation analyses, a consistent Mendelian inheritance pattern has not been observed. This failure can be attributed to both, the genetic and phenotypic heterogeneity underlying the disease. Twin and adoption studies as well as familial clustering have supported a genetic etiology of this illness. However, absence of full concordance among monozygotic twins suggests a role for non-genetic factors in addition to the genetic component.
Till now, no susceptibility gene for schizophrenia has been detected consistently, though a large number of genes have been speculated to be associated with the disorder. A number of parametric and non-parametric linkage studies done so far have failed to pinpoint a single major gene responsible for the causation of the disease. Multiple genes conferring moderate effects have been proposed to provide susceptibility to schizophrenia (McGuffin et al 1995) and this is consistent with the pattern of risk to the family members and epistatic model of multiple contributing loci given by Risch (1990). However, as yet there is neither a clue to the number of genes involved nor to the degree of interaction between the genes and the contribution of each gene to the overall susceptibility to this multifactorial disorder.
Lack of a consistent phenotypic, cytogenetic, or biochemical marker or a neurophysiological test or any specific features (like neurofibrillary tangles in case of Alzheimer’s disease) have been a major drawback in both schizophrenia diagnosis and research. One of the most consistent phenotypic marker that has been observed is the eye movement dysfunction. Though eye movement dysfunction was observed and reported by Diefendorf and Dodge in 1908, further advances did not occur till Holzman et al (1973) investigated it further. Since then there have been several reports with elevated rates of eye tracking dysfunction in patients suffering from schizophrenia (reviewed by Levy et al 1993), and also non-psychotic first-degree relatives. Recently, Arolt et al (1996) defined eye tracking dysfunction as a putative phenotypic marker which mapped to a locus on chromo-some 6, a region already implicated for schizophrenia, but it would be premature to draw firm conclusions unless additional replicative studies are undertaken.
2. History
The approach to find the putative biochemical basis in schizophrenia has been a little different from most other disorders. The fortuitous discovery of chlorpromazine, a drug to treat schizophrenia in the 1950’s paved the way for such research. Chlorpromazine was found to have beneficial effect on patients with schizophrenia and its extraordinary success led to the hope of understanding biochemistry of the illness. In vitro studies on the brain tissues of animals conducted revealed that drugs such as chlorpromazine, haloperiodol and other antipsychotic drugs effective in treatment of schizophrenia, were dopamine receptor antagonists. The clinical efficacy of these drugs was correlated with affinity for dopamine D2 receptors. This led to the development of “dopamine theory” of schizophrenia which postulated brain dopaminergic hyperactivity (Thomson 1996). However, dopamine levels or receptors have not shown to be elevated consistently in affected individuals. This aroused the interest in various other neurotransmitters and their receptors present in the brain. The dopamine theory dominated schizophrenia research for the next two decades and still continues to do so.
A large number of early studies based on segregation analyses supported a genetic etiology underlying this disease. However, it was the development of restriction fragment length polymorphism (RFLP) as a mapping tool by Botstein et al (1980) which provided markers that could be used to track the chromosomal region(s) of interest. A cytogenetic marker for the probable location of a schizophrenia gene came much later in 1980’s when a balanced translocation was reported in a family with two males affected with schizophrenia, an uncle and a nephew. Both the males were found to be partially trisomic for chromo-some 5q11⋅2-q13⋅3 and carried an extra copy of this region translocated onto chromosome 1. This initial excitement also occurred because chromosome 5q harbours the gene for glucocorticoid receptor, and disturbances in its metabolism could induce psychotic symptoms (though subsequent studies showed the locus for this receptor to be located outside the 5q11-q13 region). Using RFLP markers, Sherrington et al (1988) found linkage in two British and five Icelandic families with 39 cases of schizophrenia and Kennedy et al (1988) provided evidence against linkage in this region in Swedish samples. Linkage to this region was also ruled out by McGuffin (1990). Subsequently, all the putative biochemical and cytogenetic markers were evaluated with fervour using linkage and association studies but none have provided consistent results.
The completion of a comprehensive genetic map of human genome using the microsatellite markers in middle of 1990’s enabled genome-wide linkage analysis. With a large collection of well-documented families and densely spaced microsatellite markers, all human chromosomes were screened worldwide by independent groups or in collaborations. Many promising candidate regions have since been found and work is in progress to establish linkage or association. The late 1990’s has given an additional tool in the form of SNP’s, which are present abundantly in the genome and can be used in conjunction with the microsatellite markers.
3. Strategies for finding genes for schizophrenia
The genetic analysis of multifactorial disorders has remained a challenge due to the possible risk conferrred by multiple genes of small effect, incomplete penetrance, complex and uncertain mode of inheritance along with the involvement of non-genetic factors. Currently, both association strategies with robust statistical methods as well as the linkage analyses are being used to unravel the genes for schizophrenia.
The methods to discover the genes for schizophrenia can be broadly dichotomized into parametric and non-parametric methods. (i) Parametric methods need the specification of the Mendelian mode of inheritance, number of genes involved, frequency of each susceptibility gene and their penetrance. These methods are useful for finding the genes for rare disorders or genes of major effect. Conventional linkage analysis includes the study of segregation of marker along with the disease condition within a family and requires generally a large multigenerational and multi-member affected pedigree. However, the main disadvantage of this approach is that it cannot detect genes of small effect, a hallmark of complex traits. Moreover, the mode of inheritance, which is unclear for schizophrenia, has to be specified. (ii) Non-parametric methods appropriately termed “model free analysis” do not require any model specification. A non-parametric method has also been developed for linkage analysis where affected sib-pairs are used but the major limitation of this method is the large number of sib-pairs required for finding a gene of modest effect.
3.1. Association studies
Unlike linkage methods, association studies are population based and provide an alternative to linkage studies for detecting the genes of small effect. Both case-control approach and family based designs are commonly used to detect association. In contrast to the conventional case-control approach, often marred by the population stratification bias, family based designs like haplotype relative risk (HRR) and transmission disequilibrium test (TDT) are methods of choice (Ewens and Spielman 1995). TDT however, requires at least one heterozygous parent and uses information only from the informative matings and hence a large number of families are discarded from the analysis resulting in loss of power to detect putative association.
4. Current direction of research in schizophrenia
Current research in schizophrenia includes four directions: (i) Detection of structural abnormalities in brain, (ii) evaluation of the effect of drugs on the target receptors, neurotransmitters and the physiology of the brain, (iii) association and linkage studies with putative genes identified based on the biochemical or pharmacological evidence or candidate regions identified by the genome scan studies and (iv) role of epigenetic factors in disease causation.
Several sophisticated techniques like computed tomography (CT), magnetic resonance imaging (MRI), functional imaging and positron emission tomography (PET) along with the conventional post-mortem studies have been used to study the anatomy of the brain of affected individuals and controls. However, this aspect does not fall in the purview of this review and therefore, will not be discussed.
4.1. Role of neurotransmitters and drugs in the etiology of schizophrenia
Most of the therapeutic drugs used for the treatment of schizophrenia interfere with the function of neurotransmitter(s) or its receptor(s). Several key neurotransmitters especially dopamine and serotonin have thus become the focal point of schizophrenia research.
Neurotransmitters have been implicated in the etiology of schizophrenia since the time dopamine hypothesis was conceived. A large number of investigations were carried out to explain the role of dopamine, its various receptors and the genes involved in the dopamine neurotransmission pathway but none of the genes so far have been convincingly implicated in the etiology of schizophrenia. Further, it was observed that the drugs blocking dopamine receptors primarily were not effective in the treatment of all patients. Recently several drugs like clozapine (trade name Clozaril) have been marketed. These drugs appear to be as efficacious as conventional medications, with fewer side effects.
The monoaminergic group of neurotransmitters, their receptors and the genes involved in their uptake, synthesis, transport, reuptake and degradation may all play an important role in the pathogenesis of schizophrenia. Though most studies have focused on the dopaminergic and serotonergic group, role of other neurotransmitters and the genes involved in their metabolism like γ-amino-butyric acid (GABA) type A has also been evaluated. Briefly, the role of some of the candidate genes would be discussed.
4.1a. Dopaminergic system:
Five dopamine receptors have been identified (Grandy et al 1989; Dearry 1990; Giros et al 1990; Sunhara et al 1991; Van Tol et al 1991) so far and these can be broadly classified into 2 families: D1 and D2 (Kebabian and Calne 1979). Each of these receptors comprise of about 400 amino acids and are transmembrane protein with seven hydrophobic domains spanning the neural membrane. The structure of some of these receptors is similar to that of other G-protein coupled receptors.
• Dopaminergic receptors
D1 family:
This family consists of two receptors: Dopamine receptor D1 (DRD1) and DRD5 (table 1).
Table 1.
Genes involved in dopaminergic pathway.
| Receptor | Location | Function and significance | Linkage and association studies | Result |
|---|---|---|---|---|
| D1 family | ||||
| DRD1 | 5q35⋅1 | Involved in memory, emotion and cognition, the functions that are highly disturbed in schizophrenics | Grandy et al 1990; Litt et al 1991; Nothen et al 1993a; Cichon et al 1994; Liu et al 1995; Kojima et al 1999 | No significant association with any of the polymorphisms |
| Several polymorphisms in 5′ and 3′ UTR of the gene (Grandy et al 1990; Litt et al 1991; Dollfus et al 1996; Kojima et al 1999) | ||||
| DRD5 | 4p 15⋅3 | DRD5 is neuron specific and is localized within the limbic regions of the brain | Asherson et al 1998 | No significant association |
| Displays high affinity for dopamine | ||||
| D2 family | ||||
| DRD2 | 11q 22-q23 | Second most abundant dopamine receptor | Gejman et al 1994; Nanko et al 1994; Asherson et al 1994; Nothen et al 1994; Sobell et al 1994 | No significant association |
| Higher density of DRD2 in schizophrenic brains (Sedvall and Farde 1995) | ||||
| Ser311/Cys311 polymorphism reported (Arinami et al 1994). Cys311 variant more in schizophrenics | Shaikh et al 1994 | Positive association | ||
| DRD3 | 3q13⋅3 | Expression of DRD3 is restricted to the limbic areas of the brain, site for emotion and cognition that is highly disturbed inschizophrenics | Crocq et al 1992; Nimgaonkar et al 1993; Mant et al 1994 | Significant association |
| Selective loss of DRD3 mRNA in parietal and motor regions of postmortem schizophrenic brains (Schmauss et al 1993) | Nothen et al 1993b; Jonsson et al 1993; Nanko et al 1993; Chen et al 1997a; Prasad et al 1999 | No association | ||
| A to G substitution polymorphism leading to creation of MscI site in exon 1 of DRD3 gene and increased homozygosity at either allele is associated with schizophrenia (Crocq et al 1992) | Please see Jonsson et al (1999) for exhaustive reference | |||
| DRD4 | 11p15⋅5 | Higher affinity than other dopamine receptors for atypical antipsychotic clozapine | Sommer et al 1993 | No significant association |
| Polymorphic 48 base pair repeat in putative third cytoplasmicloop | ||||
D2 family:
This family consists of three receptors: DRD2, DRD3 and DRD4 (table 1).
• Dopamine transporter
Beside the dopaminergic receptors, dopamine transporter (DAT) gene which is located on chromosome 5p15⋅3 (Vandenbergh et al 1992a) and involved in the reuptake of dopamine back in the presynaptic terminal has also been considered as a possible candidate. A 40 bp VNTR polymorphism was identified in the 3′ untranslated region of this DAT gene. Alleles with 9 and 10 repeats have been observed to be predominant in most of the populations (Vandenbergh et al 1992b; Perisco et al 1993; Nakatome et al 1996). But no significant association (Daniels et al 1995; Pean et al 1995; Perisco and Macciardi 1997) or linkage (Byerley et al 1993; Perisco et al 1995) has been detected with any of the DAT alleles. Association studies with a RFLP polymorphism due to Taq1 restriction site in the DAT gene have also not yielded any significant results (Perisco et al 1995; Pean et al 1995).
• Tyrosine hydroxylase
Tyrosine hydroxylase (TH) is a rate-limiting enzyme in the dopaminergic pathway. A rare allele of a tetranucleotide repeat HUMTHO1, located in the first intron of TH is suggested to be associated with schizophrenia (Meloni et al 1995) but there are also several contrary reports (Burgert et al 1998; Jonsson et al 1998).
4.1b. Serotonergic system:
Serotonin is another important neurotransmitter that governs some of the behavioural aspects like sleep, moods, hallucinations and depression. Apart from the brain, serotonin is present in certain body tissues like smooth muscles and in blood platelets. Till now seven serotonin receptors have been identified: 5HT-1, 5HT-2, 5HT-3, 5HT-4, 5HT-5, 5HT-6, and 5HT-7. There is evidence that there are 5 distinct subtypes of 5HT-2 and 3 distinct subtypes of 5HT-3 (Barnes and Sharp 1999; Matsumoto and Yoshioka 2000).
Structural similarities between LSD, a potent hallucinogen and serotonin have been observed. In addition, drugs like Reserpine have been shown to reduce brain serotonin concentrations and can also be beneficial in treatment (Stahl and Wets 1987). These observations have been some of the major lines of evidence to suggest that serotonin may have an important etiological role. Arora and Meltzer (1991) reported a significant decrease in the number of 5HT2a receptors in patients with schizophrenia as compared to the controls and negated the role of neuroleptics for this difference. Hallmeyer et al (1992) did not find any linkage between 5HTR2a gene and schizophrenia. Joyce et al (1993) found that serotonin receptors and its uptake sites are altered in the limbic systems of people suffering from schizophrenia.
Polymorphism has been described in several genes in the serotonergic pathway and most of these genes have been studied to detect the association with schizophrenia. These include serotonin transporter (SERT or 5-HTT), serotonin receptors: 5-HT1A (Erdmann et al 1995), 5HT1d beta (Nothen et al 1994; Sidenberg et al 1993); 5-HT2A (Warren et al 1993), 5-HT2C, 5-HT6 (Kohen et al 1996; Shinkai et al 1999), 5-HT7 (Erdmann et al 1996). A meta analysis study by Arranz et al (1998) showed association of the 102-T/C polymorphism of 5-HT2A with clozapine response in extreme responders (table 2).
Table 2.
Genes invovled in serotonergic pathway.
| Receptor | Location | Function and significance | Linkage and association studies | Result |
|---|---|---|---|---|
| 5HT2A gene | 13q14 | Binds clozapine (antipsychotic) more effectively than dopamine | Williams et al 1996, 1997; Spurlock et al 1998 | Significant association |
| Both gene and promoter have been investigated. | ||||
| Gene polymorphism: T to C polymorphism viz. T102C (Warren et al 1993; Inayama et al 1994) | Nimgaonkar et al 1996; Chen et al 1997b; Hawi et al 1997; Verga et al 1997; He et al 1999; Lin et al 1999 | No significant association | ||
| 5HT2A promoter | Chromosome 13 | Regulatory region of 5HT2A gene and thus mutations in this region can influence the 5HT2a gene expression and its density | Spurlock et al 1998; Kouzmenko et al 1999 | No significant association |
| A to G polymorphism at position – 1438 (Spurlock et al 1998) | ||||
| SERT or 5HTT | 17q11⋅2-q12 | VNTR in intron 2 | Heils et al 1996; Hranilovic et al 2000 | |
| 44 base pair deletion/insertion in the 5HTT promoter region resulting in long and short variant | Brilhault et al 1997 | Significant Association | ||
| Short variant leads to reduced 5HTT expression (Heils et al 1996) | Naylor et al 1998; Oliveira et al 1998; Rao et al 1998 | No significant association | ||
| TPH gene | Chromosome 11 | Rate limiting enzyme in the synthesis of serotonin | Serretti et al 1999 | No significant association |
| Polymorphic site resulting in U and L allele in the intron | ||||
| TPH promoter | Chromosome 11 | A to G transition at position 6526 | Rotando et al 1999 | No significant association |
4.1c. Genes involved in the degradation of monoaminergic neurotransmitters:
Monoamine oxidase (MAO) and catechol o-methyltransferase (COMT) are two important enzymes that are involved in the catabolism of the neurotransmitters.
MAO is present at both pre-synaptic terminal and postsynaptic cell and breaks down excess dopamine and serotonin. COMT is another inactivating enzyme present in postsynaptic cells of dopamine or NE synapse but not serotonin. Floderus et al (1981) reported a higher COMT activity in schizophrenics which has not been replicated in many subsequent studies. COMT gene has been mapped to chromosome 22q11⋅2 and microdeletions in this region leading to a number of diseases including the velocardiofacial syndrome (VCFS) encompass the COMT locus (Dunham et al 1992). Further, Pulver et al (1994) have reported VCFS patients to have a high risk for developing schizophrenia.
4.2. Association and linkage studies with putative genes and candidate regions
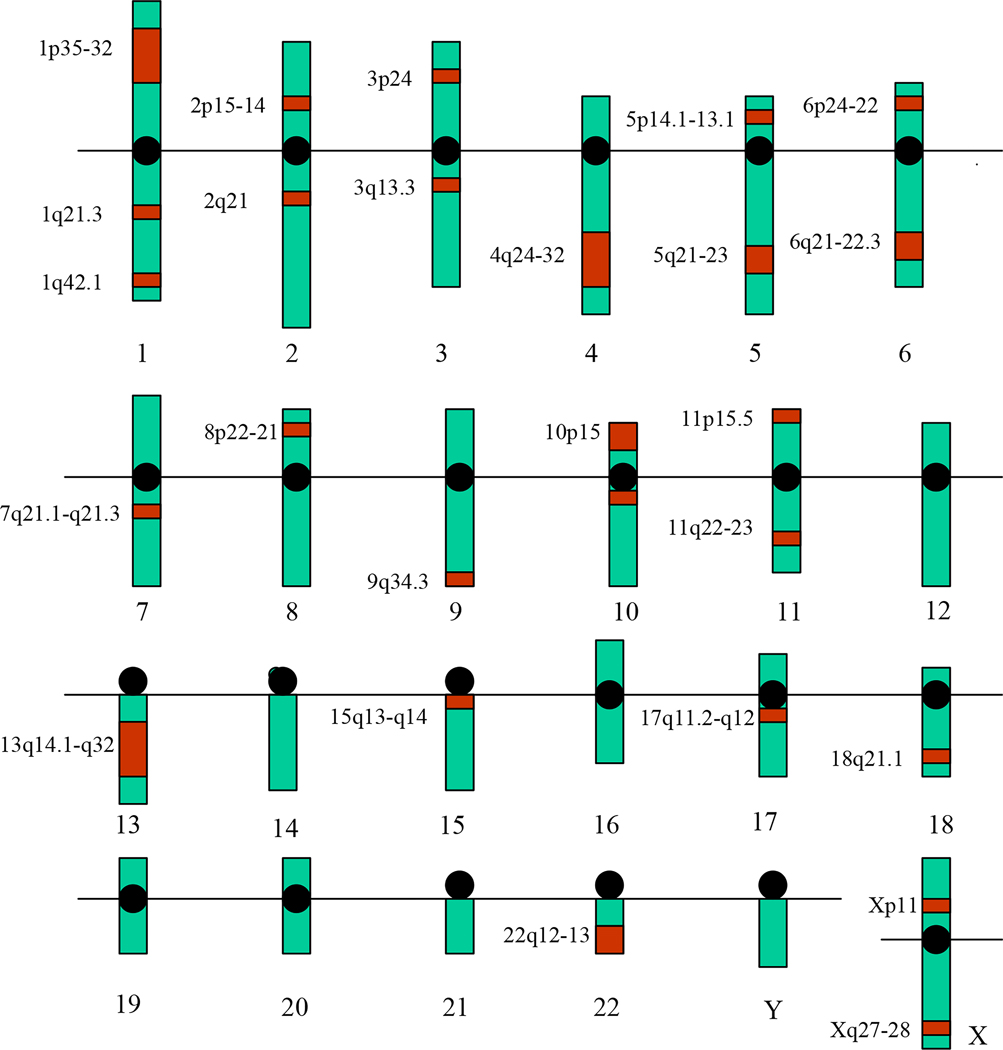
A large number of putative genes chosen on the basis of biochemical and pharmacological evidence and candidate sites identified using genome scans have been tested for association or linkage with schizophrenia. Table 3 lists the location of some important putative genes and chromo-some sites based on some of the important linkage/ association studies undertaken for schizophrenia. Figure 1 is a graphical presentation of their distribution on different chromosomes.
Table 3.
Notable putative genes and chromosomal regions studied for schizophrenia gene using linkage and association strategies.
Indicates candidate regions identified by genome scan studies.
Figure 1.
A glimpse of genes/chromosomal sites implicated for schizophrenia based on linkage and association studies. Black circle denotes centromere. Red colour denotes candidate sites/location of candidate genes.
4.3. Role of epigenetic factors in disease causation
Epigenetics is a branch of developmental biology that investigates the mechanism by which heritable changes in gene expression occur without the change in the genetic material. Epigenetic phenomenon has been observed in many organisms and it includes position effect variegation, paramutation, age dependent DNA modification etc. (Bestor et al 1994; Petronis et al 1999). The epigenetic factors may show only partial stability (metastability) when transmitted from one generation to the next generation (Petronis et al 1999). New lines of investigations suggest that epigenetic mechanisms might play an important role in the etiology of complex traits like schizophrenia. Various epigenetic factors like DNA methylation and interaction(s) among gene products might complement the genetic component (Holliday 1989). However, the role of epigenetics in disease causation has not been investigated intensively at present.
Environmental factors can also cause epigenetic changes in the DNA that can result in altered gene expression leading to disease manifestation. Recent studies are suggestive of the role of viruses in the development of schizophrenia. Neurodevelopmental defects have been seen frequently in children of women encountering viral exposure during pregnancy. This theory is also supported by the fact that viral infections like influenza, measles etc. are more frequent during winter months and several studies indicate that children born during winter months develop schizophrenia more often than others (Battle et al 1999). It has been speculated that neurodevelopmental defects occur when a mother contracts a viral infection early during pregnancy. Various viruses like influenza (Crow 1997), borna (Iwahashi et al 1998), herpes (DeLisi et al 1986) etc. have thus been investigated in schizophrenics.
Retroposons have also been advocated for causation of schizophrenia (O’Reily and Singh 1996; Yolken et al 2000). Various endogenous retroposon like sequences are present in the genome which remain latent until some trigger such as an infection from other viruses, hormones or chemical messengers released by immune system trigger them into action resulting in the development of the disease (Yolken et al 2000).
Four important theories of schizophrenia have been discussed from an epigenetic point of view in a review by Petronis et al (1999) with an intent to answer some very basic aspects of schizophrenia genetics. These hypotheses encompass ‘neurodevelopmental hypothesis’, ‘dopamine dysregulation hypothesis’, ‘viral hypothesis’ and ‘genetic anticipation and the unstable DNA hypothesis’. DNA methylation occurring during meiosis, gametogenesis etc. has been suggested to have the potential to cause subtle changes in brain development, resulting in neurodevelopmental defects (Petronis et al 1999). Dopamine dysregulation has been correlated to control of DRD2 expression, which is believed to be due to differential methylation patterns. In addition, integration of viruses at specific positions and their activity is advocated to be under control of epigenetic mechanisms, leading to disruption of neurodevelopmental processes by viral toxic substances, resulting in brain abnormalities associated with schizophrenia. Epigenetic mechanisms have also been suggested to be the molecular equivalents or a cause of anticipation by Petronis et al (1999). Subsequently, Petronis (2000) has affirmed the role of genomic imprinting and differential methylation of regulatory regions as epigenetic factors in regulation of Serotonin 2A receptor gene (HTR2A) as well.
Besides neurotransmitter disturbances, immune dysfunction has also been observed in a subset of schizophrenics. Various markers from immune system have thus been studied. A large number of reports indicate that schizophrenia like most other neurological disorders shows phenomenon of anticipation. These two aspects are briefly discussed below.
5. Immune response in schizophrenics
A large number of inherited biochemical markers have been studied for association with schizophrenia including the immunoglobulins, haptoglobin types, blood groups, substance P, enzymes like porphobilinogen deaminase, adenosine deaminase, tyrosine hydroxylase etc. (summarized in Nimgaonkar et al 1992). None of the markers have shown a consistent association. However, an increasing number of studies have suggested occurrence of immune dysfunction in a subset of schizophrenics.
Altered levels and numbers of various components of immune system like immunoglobulins, complements, interleukins (IL2), CD3+ and CD4+ have been reported (Solomon 1981; DeLisi et al 1982; Ganguli and Rabin 1989; Muller et al 1991; Zamani et al 1994). Since the HLA system governs the immune responses, HLA genes were implicated in the etiology of schizophrenia. Past association studies with various HLA class I alleles yielded inconsistent results (summarized in Nimgaonkar et al 1992) except HLA-A9. The reason suggested for inconsistencies include population stratification, case-control mismatch, different diagnostic criteria and use of serological techniques which are error prone (Nimgaonkar et al 1992). It has been further observed that HLA class II alleles might be more important than HLA class I alleles in view of the autoimmune pathology suggested for schizophrenia.
Autoimmune pathology has been advocated (Knight 1982; Ganguli and Rabin 1987) because of increased levels of antibrain antibodies (Heath and Krupp 1967; Shima et al 1991). Also, an inverse relationship between schizophrenia and autoimmune diseases like rheumatoid arthritis (RA) (Eaton et al 1992; Wright et al 1996) and insulin dependent diabetes mellitus (IDDM) (Finney 1989) has been reported.
Human leucocyte antigen (HLA) class II alleles play an important role in autoimmune responses (Trucco 1992; Lechler 1994) and many autoimmune diseases seem to be HLA DR and DQ associated (Mignot et al 1995). Several studies have reported linkage with schizophrenia around the HLA locus (Moises et al 1995; Schwab et al 1995). Since RA and IDDM are inversely related to schizophrenia, HLADRB1*04 (DR4) and HLADQB1 region positively associated with RA and IDDM respectively, were studied (Finney 1989; Knight et al 1992). A negative association of schizophrenia with DR4 (Wright et al 1996) and HLADQB1*0602 (Nimgaonkar et al 1992, 1995, 1997) was found. However, Gibson et al (1999) in their population did not find any association of schizophrenia with A9, DR4 or DQB1*0602.
Recently Wei and Hemmings (2000) reported that the HSMHC3A5 locus present near the major histocompatibility complex (MHC) class I and class II junction is significantly associated with schizophrenia. This locus harbours the Notch 4 gene, which consists of 30 exons and spans 36⋅8 kb region inclusive of a putative promoter. Haplotype combination of markers especially SNP2(CTG)n from Notch 4 locus showed strong association with schizophrenia. The function of Notch 4 is not known in humans but animal studies have shown that it is involved in neurodevelopmental process. Thus, nonimmune related genes localized to the HLA region may have an etiological role in schizophrenia.
6. Anticipation in schizophrenia
Dynamic mutations characterized by expansion of triplet repeats are known to cause at least 13 different neuro-degenerative disorders. Genetic anticipation, a phenomenon characterized by increase in disease severity combined with an early onset of disease with succeeding generations, has been observed in several neuropsychiatric disorders with trinucleotide expansions (Ross 1993; Johnson et al 1997) and suggested in schizophrenia as well (Bassett and Honer 1994). Various trinucleotide repeats have been studied using candidate gene as well as the repeat expansion detection (RED) technique (Rubinsztein et al 1994; Jain et al 1996; McInnis et al 1996; Gaitonde et al 1997; Saleem et al 1998). Large CAG repeats have been reported to be more frequent in patients than in controls (Morris et al 1995; O’Donovan et al 1995). Therefore, genes with unstable trinucleotide repeats are being considered as suitable candidates for underlying pathology of schizophrenia and are being analysed.
Chandy et al (1998) described one such candidate gene which is a human calcium-activated potassium channel gene (hsKCa3), and has been mapped to 1q21⋅3. This gene at the N-terminal region contains two contiguous CAG repeats with the second repeat being polymorphic (repeat size varying from 12 to 28 repeats). The long allelic variant of the polymorphic repeat is shown to be overrepresented in the schizophrenics in comparison to controls (McInnis et al 1998). However, most of the reports have been controversial (Bowen et al 1998; Austin et al 1999; Guy et al 1999. Antonarkis et al 1999; Joober et al 1999. Saleem et al 2000a).
Several other ion-channels like g-aminobutyric acid (GABA), N-methyl-D-aspartate (NMDA) receptor and its ligand (e.g. AMPA) are also being investigated since antagonists to these receptors produce schizophrenic symptoms (Tsai et al 1998; Gargus et al 1998; Aghajanian and Marek 2000).
7. Schizophrenia research in India
A large number of studies for schizophrenia have been conducted mainly in the Caucasoid population. However, it is desirable to perform similar studies in different ethnic groups to reaffirm the status of the association or linkage identified. Indian population is very well suited for such studies as it satisfies many criteria required for the investigation of complex traits. Besides, Indian population is an attractive large reservoir of samples both due to its population size as well as the high rate of marital stability, the latter essential for family based analysis.
Work on schizophrenia genetics has been initiated since the last 5–6 years by two major groups, one of the authors and the other one based at NIMHANS in Bangalore. The Hindi version of the Diagnostic Interview for Genetic Studies (DIGS) and Family Interview for Genetic studies (FIGS) (Deshpande et al 1998) has been validated and is being used by the authors group. A total of 260 families including sib-pairs and trios have been collected and used for genetic analysis. Genes in the dopaminergic and serotonergic pathways in the brain have been studied in predominantly North Indian samples by the authors. Using family based association test, DRD3 locus did not show any association (Prasad et al 1999). In another study, four genes (5HT2A, TPH, COMT and DAT) involved in monoaminergic pathway evaluated using TDT, have also failed to show any significant association (Semwal et al 2001). An association between cytosolic phospholipase A2 (cPLA2) locus and schizophrenia has also been evaluated in the family based samples among Indians but no significant association has been detected (Chowdari et al 2001). A large difference in CAG repeat allele sizes in case of patients suffering from schizophrenia compared to the controls has been reported at the Machado-Joseph Disease (MJD) locus. Though longer CAG repeat at MJD locus is not associated with schizophrenia, the role of smaller unstable repeats in disease manifestation cannot be excluded (Saleem et al 1998). Saleem et al (2000a) observed no association with serotonin transporter (5-HT) in patients suffering from bipolar disorder based on case-control approach. Though no association has been observed with the hSKCa3 polymorphism by the same group (Saleem et al 2000b) they have shown that a large difference in allele sizes greater than or equal to five occurs in patients compared to controls. Association studies with candidate genes that may play a role in susceptibility to Tardive Dyskinesia among schizophrenia patients is being investigated by the author’s group. This is expected to serve as a model for pharmacogenetic studies especially of complex traits. Presently, the author’s group is carrying out high throughput SNP genotyping of several candidate genes in schizophrenia.
8. Future strategies
Considering the complex nature of the common disorders, with no clue to the number and nature of genes involved or their location, it is difficult to search for the genes using the conventional techniques that help unravel the genes for single gene disorders. Various new experimental and statistical methodologies have been developed and are in current use to hunt for the genes underlying schizophrenia.
Non-parametric method of linkage analysis using large collection of affected sib-pairs along with the genome scan studies using affected sib-pairs would be a more effective and favoured strategy to track genes of minor effect. Considering schizophrenia is a polygenic disorder, gene-gene interaction among putative markers is expected to yield important clues to the underlying pathology. Haplotype analysis could be informative where genotypic combination might be crucial in providing the susceptibility. Along with the exons of candidate genes, intron sequences should also be searched for mutations. SNPs coupled with the powerful technique of DNA microarrays can add a new dimension to the existing research methodologies. Strategies employed in parallel studies on other complex traits such as diabetes and hypertension would also be useful in navigating the search for genes. Type I diabetes exemplifies the role of non-parametric linkage and association mapping in identifying the susceptibility genes and loci. Initially, case-control association studies revealed association of type I diabetes to HLA at 6p21 and the insulin gene on 11p15 chromosomal region (reviewed by Thomson et al 1989). Using affected sib–pairs, linkage was subsequently demonstrated to HLA (IDDM1) (Cudworth and Woodrow 1975) and non-HLA genes like IDDM4 (11q13), IDDM5 (6q25), and IDDM12 (2q33) while other non-HLA genes like IDDM2 were identified using association studies (reviewed by Thomson 2001). In hypertension, on the other hand, SNP identification in candidate genes is already underway in search for common variants that might provide susceptibility. Around 874 SNPs in 75 candidate genes for hypertension have been identified by Halushka et al 1999 (see review by Gray et al 2000).
Association studies, but with more powerful analytical tools such as SNPs, would remain instrumental in searching the genes for common disorders because of their significant power to identify the pathogenic allele(s) in contrast to the linkage strategy. Limitation of small sample size in such studies could be overcome by meta-analysis approach. The meta-analysis method synthesizes information by pooling relatively weak signals from independent genetic association studies into consolidated stronger evidence for genetic effects. Thus, evidence from multiple studies are pooled and variations among studies are accounted for, to arrive at a pooled measure of genetic linkage (Gu et al 2001). However, the limitation in performing association studies would still be the requirement of prior knowledge about the candidate genes/candidate chromosomal sites. Therefore, availability of extensive data on polymorphism in the whole genome could provide a large number of markers for effective association studies. SNPs coupled with microsatellite markers seem to be the ideal tools for such studies and use of microarrays could provide a high-throughput genotyping method in the near future.
Abbreviations used:
- COMT
Catechol o-methyltransferase
- DAT
dopamine transporter
- DR
dopamine receptor
- HLA
human leucocyte antigen
- IDDM
insulin dependent diabetes mellitus
- MAO
monoamine oxidase
- MHC
major histocompatibility complex
- RA
rheumatoid arthritis
- RFLP
restriction fragment length polymorphism
- SNPs
single nucleotide polymorphisms
- TDT
transmission disequilibrium test
- TH
tyrosine hydroxylase
- VCFS
velocardiofacial syndrome
References
- Aghajanian GK and Marek GJ 2000. Serotonin model of schizophrenia: emerging role of glutamate mechanisms; Brain Res. Brain Res. Rev 31 302–312 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association 1987. Diagnostic and Statistical Manual Revised 3rd edition (Washington DC: American Psychiatric Association; ) [Google Scholar]
- Antonarakis SE, Blouin JL, Lasseter VK, Gehrig C, Radhakrishna U, Nestadt G, Housman DE, Kazazian HH, Kalman K, Gutman GA, Fantino E, Chandy KG, Gargus JJ and Pulver AE 1999. Lack of linkage or association between schizophrenia and the polymorphic trinucleotide repeat within the KCNN3 gene on chromosome 1q21; Am. J. Med. Genet 88 348–351 [PubMed] [Google Scholar]
- Arolt V, Lencer R, Achim N, Muller-Myhsok B, Purmann S, Schurmann M, Leutelt J, Pinnow M and Schwinger E 1996. Eye tracking dysfunction is a putative phenotypic susceptibility marker of schizophrenia and maps to a locus on chromosome 6p in families with multiple occurrence of the disease; Am. J. Med. Genet. Neuropsychiatric Genet 67 560–563 [DOI] [PubMed] [Google Scholar]
- Arora RC and Meltzer HY 1991. Serotonin2 (5HT2) receptor binding in the frontal cortex of schizophrenic patients; J. Neural. Trans 85 19–29 [DOI] [PubMed] [Google Scholar]
- Arranz MJ, Munro J, Sham P, Kirov G, Murray RM, Collier DA and Kerwin RW 1998. Meta-analysis of studies on genetic variation in 5-HT2A receptors and clozapine response; Schizophr. Res 32 93–99 [DOI] [PubMed] [Google Scholar]
- Asherson P, Mant R, Williams N, Cardno A, Jones L, Murphy K, Collier DA, Nanko S, Craddock N, Morris S, Muir W, Blackwood McGuffin P and Owen MJ 1998. A study of chromosome 4p markers and dopamine D5 receptor gene in schizophrenia and bipolar disorder; Mol. Psychiatry 3 310–320 [DOI] [PubMed] [Google Scholar]
- Asherson P, Williams N, Roberts E, McGuffin M and Owen M 1994. DRD2 Ser311/Cys311 polymorphism in schizophrenia; Lancet 343 1045. [PubMed] [Google Scholar]
- Austin CP, Holder DJ, Ma L, Mixson LA, Caskey CT 1999. Mapping of hKCa3 to chromosome 1q21 and investigation of linkage of CAG repeat polymorphism to schizophrenia; Mol. Psychiatry 4 261–266 [DOI] [PubMed] [Google Scholar]
- Barden N and Morissette J 1999. Chromosome 13 workshop report; Am. J. Med. Genet 88 260–262 [DOI] [PubMed] [Google Scholar]
- Barnes NM and Sharp T 1999. A review of central 5-HT receptors and their function; Neuropharmacology 38 1083–1152 [DOI] [PubMed] [Google Scholar]
- Basset AS and Chow EW 1999. 22q11 deletion syndrome: a genetic subtype of schizophrenia; Biol. Psychiatry 46 882–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset AS, Hodgkinson K, Chow EW, Correia S, Scutt LE and Weksberg R 1998. 22q11 deletion syndrome in adults with schizophrenia; Am. J. Med. Genet 81 328–337 [PMC free article] [PubMed] [Google Scholar]
- Basset AS and Honer WG 1994. Evidence for anticipation in schizophrenia; Am. J. Hum. Genet 54 864–870 [PMC free article] [PubMed] [Google Scholar]
- Battle YL, Martin BC, Dorfman JH and Miller LS 1999. Seasonality and infectious disease in schizophrenia: the birth hypothesis revisited; J. Psychiatr. Res 33 501–509 [DOI] [PubMed] [Google Scholar]
- Bestor TH, Chandler VL and Feinberg AP 1994. Epigenetic effects in eukaryotic gene expression; Dev. Genet 15 458–462 [DOI] [PubMed] [Google Scholar]
- Blouin JL, Dombroski BA, Nath S, Lasseter VK, Wolyniec PS, Nestadt G, Thornquist M, Ulrich G, McGrath JA, Kasch L, Lamacz M, Thomas MG, Gehrig C, Radhakrishna U, Snyder SE, Balk KG, Neufeld K, Swartz KL, DeMarchi N, Papdimitriou GN, Dikeos DG, Stefanis CN, Chakravarti A, Childs B, Housman DE, Kazazian H, Antonarakis SE and Pulver AE 1998. Schizophrenia susceptibility loci on chromosomes 13q32 and 8p21; Nature Genet. 10 70–73 [DOI] [PubMed] [Google Scholar]
- Botstein D, White RL, Skolnick MH and Davis RW 1980. Construction of a genetic linkage map in man using restriction fragment length polymorphisms (RFLPs); Am. J. Hum. Genet 32 314–331 [PMC free article] [PubMed] [Google Scholar]
- Bowen T, Guy CA, Craddock N, Cardno A, Williams N, Spurlock G, Murphy KC, Jones L, Gray M, 1998. Replication of association between a polymorphic CAG repeat in the hSKCa3 genes and schizophrenia; Mol. Psychiatry 3 32–37 [DOI] [PubMed] [Google Scholar]
- Brilhault BF, Laurent C, Thibaut F, Campion D, Chavand O, Samolyk D, Martinez M, Petit M and Mallet J 1997. Serotonin transporter gene polymorphism and schizophrenia: an association study; Biol. Psychiatry 42 634–636 [DOI] [PubMed] [Google Scholar]
- Burgert E, Crocq MA, Bausch E, Macher JP and Morris-Rosendahl DJ 1998. No association between the tyrosine hydroxylase microsatellite marker HUMTHO1 and schizophrenia or bipolar I disorder; Psychiatr. Genet 8 45–48 [DOI] [PubMed] [Google Scholar]
- Byerley WF, Coon H, Hoff M, Holik J, Waldo M, Freedman R, Caron MG and Giros B 1993. Human dopamine transporter gene not linked to schizophrenia in multigenerational pedigrees; Hum. Hered 43 319–322 [DOI] [PubMed] [Google Scholar]
- Byerley W, Hoff M, Holik J, Myles-Worsley M, Waldo M, Freedman R and Coon H 1995. Linkage analysis between schizophrenia and index simple-sequence repeat loci for chromosome 21; Hum. Hered 45 49–52 [DOI] [PubMed] [Google Scholar]
- Calzolari E, Aiello V, Palazzi P, Sensi A, Calzolari S, Orrico D, Calliari L, Holler H, Marzi C, Belli S, Bernardi F and Patracchini P 1996. Psychiatric disorder in a familial 15;18 translocation and sublocalization of myelin basic protein of 18q22⋅3; Am. J. Med. Genet 67 154–161 [DOI] [PubMed] [Google Scholar]
- Campbell DA, Sundaramurthy D, Markham AF and Pieri LF 1997. Fine mapping of the human 5-HTR2a gene to chromo-some 13q14 and identification of two highly polymorphic linked markers suitable for association studies in psychiatric disorders; Genet. Test 1 297–299 [DOI] [PubMed] [Google Scholar]
- Cao Q, Martinez M, Zhang J, Sanders AR, Badner JA, Cravchik A, Markey CJ, Beshah E, Guroff JJ, Maxwell ME, Kazuba DM, Whiten R, Goldin LR, Gershon ES and Gejman PV 1997. Suggestive evidence for a schizophrenia susceptibility locus on chromosome 6q and a confirmation in an independent series of pedigrees; Genomics 43 1–8 [DOI] [PubMed] [Google Scholar]
- Chandy KG, Fantino E, Kalman K, Tong LL, Ho TH, Gutman GA, Crocq MA, Ganguli R, Nimgaonkar VL, Morris-Rosendahl and Gargus JJ 1998. Isolation of a novel potassium channel gene hSKCa3 containing a polymorphic CAG repeat: a candidate for schizophrenia and bipolar disorder?; Mol. Psychiatry 3 32–37 [DOI] [PubMed] [Google Scholar]
- Chen C-H, Liu M-Y, Wei F-C, Koong F-J, Hwu H-G and Hsiao K-J 1997a. Further evidence of no association between Ser9Gly polymorphism of dopamine D3 receptor gene; Am. J. Med. Genet 74 40–43 [DOI] [PubMed] [Google Scholar]
- Chen CH, Lee YR, Wei FC, Koong FJ, Hwu HG and Hsiao KJ 1997b. Lack of association between 102T/C polymorphism of serotonin receptor type 2A gene and schizophrenia in Chinese; Psychiatr. Genet 7 35–38 [DOI] [PubMed] [Google Scholar]
- Chen CH, Lee YR, Chung MY, Wei FC, Koong FJ, Shaw CK, Yeh JI and Hsiao KJ 1999. Systematic mutation analysis of the catechol-O-methyltransferase gene as a candidate gene for schizophrenia; Am. J. Psychiatry 156 1273–1275 [DOI] [PubMed] [Google Scholar]
- Chowdari KV, Brandstaetter B, Semwal P, Bhatia T, Deshpande S, Reddy R, Wood J, Weinberg CR, Thelma BK and Nimgaonkar VL 2001. Association studies of cytosolic phospholipase A2 polymorphisms and schizophrenia among two independent family based samples; Psychiatr Genet. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon S, Nothen MM, Erdmann J and Propping P 1994. Detection of four polymorphic sites in the human D1receptor gene (DRD1); Hum. Mol. Genet 3 209. [DOI] [PubMed] [Google Scholar]
- Craddock N and Lendon C 1999. Chromosme Workshop: chromosomes 11, 14 and 15; Am. J. Med. Genet 88 244–254 [PubMed] [Google Scholar]
- Crocq M-A, Mant R, Asherson P, Williams J, Hode Y, Mayerova A, Collier D, Lannfelt L, Sokoloff P, Schwartz JC, Gill M, Macher JP, McGuffin P and Owen MJ 1992. Association between schizophrenia and homozygosity at the dopamine D3 receptor gene; J. Med. Genet 29 858–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ 1992. X-Y linkage and schizophrenia; Br. Med. J 305 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ 1997. Influenza and schizophrenia; Br. J. Psychiatry 171 91. [DOI] [PubMed] [Google Scholar]
- Cudworth AG and Woodrow JC 1975. Evidence for HLA-linked genes in “juvenile” diabetes mellitus; Br. Med. J 3 133–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis L, Blouin JL, Radhakrishna U, Gehrig C, Lasseter VK, Wolyniec P, Nestadt G, Dombroski B, Kazazian HH, Pulver AE, Housman D, Bertrand D and Antonarakis SE 1999. No evidence for linkage between schizophrenia and markers at chromosome 15q13–14; Am. J. Med. Genet 88 109–112 [DOI] [PubMed] [Google Scholar]
- d’Amato T, Waksman G, Martinez M, Laurent C, Gorwood P, Campion D, Jay M, Petit C, Savoye C, Bastard C, Babron MC, Clerget-Darpoux F and Mallet J 1994. Pseudoautosomal region in schizophrenia: linkage analysis of seven loci by sib-pair and lob score methods; Psychiatry Res. 52 135–147 [DOI] [PubMed] [Google Scholar]
- Daniels J, Williams J, Asherson P, McGuffin P and Owen M 1995. No association between schizophrenia and polymorphisms within the genes for Debrisoquine 4-hydroxylase (CYP2D6) and the dopamine transporter; Am. J. Med. Genet 60 85–87 [DOI] [PubMed] [Google Scholar]
- Dann J, DeLisi LE, Devoto M, Laval S, Shields G, Smith A, Loftus J, Peterson P, Vita A, Comazzi M, Morganti C, Levinson DF, Wildenauer D, Nancarrow DJ, Mowry BJ, Collier D, Aranz MJ, Okoro C, Owen M, Crowe RR, Andreasen NC, Silverman JM, Mohs RC, Walters MK, Lennon DF, Hayward NK, Maier W, Albus M, Lerer B and Crow TJ 1997. A linkage study of schizophrenia to markers within Xp11 near the MAOB gene; Psychiatry Res. 70 131–143 [DOI] [PubMed] [Google Scholar]
- Dawson E, Powell JF, Sham P, Shaikh S, Taylor C, Clements A, Asherson P, Sargeant M, Collier D, Nanko S, Wahtley S, Murray RM, McGuffin P and Gill M 1995. Systematic search for major genes in schizophrenia: methodological issues and results from chromosome 12; Am. J. Med. Genet. Neuropsychiatric Genet 60 424–433 [DOI] [PubMed] [Google Scholar]
- Dearry A, Gingrich JA, Falardeau P, Fremeau RT, Bates MD and Caron MG 1990. Molecular cloning and expression of the gene for a human D1 dopamine receptor; Nature (London) 347 72–75 [DOI] [PubMed] [Google Scholar]
- de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK and Simpson GM 1995. Schizophrenia and smoking: an epidemiological survey in a state hospital; Am. J. Psychiatry 152 453–455 [DOI] [PubMed] [Google Scholar]
- DeLisi LE 1997. The genetics of schizophrenia: past, present and future concepts; Schizophr. Res 28 163–175 [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Goodman S, Neckers LM and Wyatt RJ 1982. An analysis of lymphocyte subpopulation in schizophrenic patients; Biol. Psychiatry 17 1003–1009 [PubMed] [Google Scholar]
- DeLisi LE, Smith SB, Hamovit JR, Maxwell ME, Goldin LR, Dingman CW and Gershon ES 1986. Herpes simplex virus, cytomegalovirus and Epstein-Barr antibody titres in sera from schizophrenic patients; Psychol. Med 16 757–763 [DOI] [PubMed] [Google Scholar]
- Deshpande SN, Mathur MN, Das SK, Bhatia T, Sharma S and Nimgaonkar VL 1998. A hindi version of the diagnostic interview for genetic studies; Sch. Bull 24 489–493 [DOI] [PubMed] [Google Scholar]
- Detera-Wadleigh SD 1999. Chromosome 12 and 16 workshop; Am. J. Med. Genet 88 256–259 [DOI] [PubMed] [Google Scholar]
- Diefendorf AR and Dodge R 1908. An experimental study of the ocular reactions of the photographic records; Brain 31 451–489 [Google Scholar]
- Dollfus S, Campion D, Vasse T, Preterre P, Laurent C, d’Amato T, Thibaut F, Mallet J and Petit M 1996. Association study between dopamine D1, D2, D3, and D4 receptor genes and schizophrenia defined by several diagnostic systems; Biol. Psychiatry 40 419–421 [DOI] [PubMed] [Google Scholar]
- Dunham I, Collins J, Wadey R and Scambler P 1992. Possible role for COMT in psychosis associated with velo-cardiofacial syndrome; Lancet 340 1361–1362 [DOI] [PubMed] [Google Scholar]
- Eaton WW, Hayward C and Ram R 1992. Schizophrenia and rheumatoid arthritis: A review; Schiz. Res 6182–192 [DOI] [PubMed] [Google Scholar]
- Erdmann J, Shimron-Abarbanell D, Cichon S, Albus M, Maier W, Litchtermann D, Minges J, Franzek E, Ertl MA, Heberbrand J, Remschmidt H, Lehmkuhl G, Poustka F, Schmidt M, Fimmers R, Körner J, Rietschel M, Propping P and Nöthen MM 1995. Systematic screening for mutations in the promoter and the coding region of the 5HT 1A gene; Am. J. Med. Genet. Neuropsychiatric Genet 60 393–399 [DOI] [PubMed] [Google Scholar]
- Erdmann J, Shimron-Abarbanell D, Rietschel M, Albus M, Maier W, Korner J, Bondy B, Chen K, Shih JC, Knapp M, Propping P and Nothen MM 1996. Systematic screening for mutations in the human serotonin-2A (5HT2A) receptor gene: identification of two naturally occurring receptor variants and association analysis in schizophrenia; Hum. Genet 97 614–619 [DOI] [PubMed] [Google Scholar]
- Ewens WJ and Spielman S 1995. The transmission/diseqilibrium test: history, subdivision, and admixture; Am. J. Hum. Genet 57 455–464 [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Matise T, Svrakie D, Pepple J, Malaspina D, Suarez B, Hampe C, Zambuto CT, Schmitt K, Meyer J, Markel P, Lee H, Harkavy Friedman J, Kaufmann C, Cloninger CR and Tsuang MT 1998. Genome scan of European–American schizophrenia pedigrees: results of the NIMH genetics initiative and millennium constortium; Am. J. Med. Genet 81 200–295 [PubMed] [Google Scholar]
- Finney GH 1989. Juvenile onset diabetes and schizophrenia?; Lancet 2 1214–1215 [DOI] [PubMed] [Google Scholar]
- Floderus Y, Book JA and Wetterberg L 1981. Erythrocyte catechol-o-methyltransferase activity in related families with schizophrenia; Clin. Genet 19 386–389 [DOI] [PubMed] [Google Scholar]
- Foroud T, Castelluccio PF, Koller DL, Edenberg HJ, Miller M, Bowman E, Rau NL, Smiley C, Rice JP, Goate A, Armstrong C, Bierut LJ, Reich T, Detera-Wadleigh SD, Goldin LR, Badner JA, Guroff JJ, Gershon ES, McMahon FJ, Simpson S, MacKinnon D, McInnis M, Stine OC, DePaulo JR, Blehar MC and Nurnberger JI Jr 2000. Suggestive evidence of a locus on chromosome 10p using the NIMH genetics initiative bipolar affective disorder pedigrees; Am. J. Med. Genet 96 18–23 [PubMed] [Google Scholar]
- Gaitonde EJ, Sivagnanasundaram S, Morris AG, McKenna PJ, Mollon JD and Hunt DM 1997. The number of triplet repeats in five brain-expressed loci with CAG repeats is not associated with schizophrenia; Schiz. Res 25 111–116 [DOI] [PubMed] [Google Scholar]
- Ganguli R and Rabin S 1989. Increased serum interleukin-2-receptor concentration in schizophrenic and brain damaged subjects; Arch. Gen. Psychiatry 46 292. [DOI] [PubMed] [Google Scholar]
- Ganguli R, Rabin BS, Kelly RH, Lyte M and Raghu U 1987. Clinical and laboratory evidence of autoimmunity in acute schizophrenia; Ann. NY Acad. Sci. USA 496 676–685 [DOI] [PubMed] [Google Scholar]
- Gargus JJ, Fantino E and Gutman GA 1998. A piece in the puzzle: an ion channel candidate gene for schizophrenia; Mol. Med. Today 4 518–524 [DOI] [PubMed] [Google Scholar]
- Garver DL, Barnes R, Holcombe J et al. 1998. Genome wide scan and schizophrenia in African Americans; Am. J. Med. Genet 81 454–455 [Google Scholar]
- Gejman PV 1999. Chromosomes 19 and 20 report; Am. J. Med. Genet 88 271. [PubMed] [Google Scholar]
- Gejman PV, Ham A, Gelernter J, Friedman E, Cao Q, Pickar D, Blum K, Noble EP, Kranzler HR, O’Malley S, Hamer DH, Whitsitt F, Rao P, DeLisi LE, Virkkunen M, Linnoila M, Goldman D and Gershon ES 1994. No structural mutation in the dopamine D2 receptor gene in alcoholism or schizophrenia; J. Am. Med. Assoc 271 204–208 [PubMed] [Google Scholar]
- Gibson S, Hawi Z, Straub RE, Walsh D, Kendler KS and Gill M 1999. HLA and schizophrenia; refutation of reported associations with A9 (A23/A24), DR4 and DQB1*0602; Am. J. Med. Genet 88 416–421 [DOI] [PubMed] [Google Scholar]
- Gill M, McGuffin P, Parffit E, Mant R, Asherson P, Collier D, Vallada H, Powell J, Shaikh S, Taylor C et al. 1993. A linkage study of schizophrenia with DNA markers from the long arm of chromosome 11; Psychol. Med 23 27–44 [DOI] [PubMed] [Google Scholar]
- Giros B, Martese M-P, Sokoloff P and Schwartz J-C 1990. Clonage du gene recepteur dopaminergique D3 humain et identification de son chromosome; C. R. Acad. Sci. Paris 311 501–508 [PubMed] [Google Scholar]
- Grandy DK, Marchionni MA, Makam H, Stofko RE, Alfano M, Frothingham L, Fischer JB, Burke-Howie KJ, Bunzow JR, Server AC and Civelli O 1989. Cloning of the cDNA and gene for a human D2 dopamine receptor; Proc. Natl. Acad. Sci. USA 86 9762–9766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandy DK, Zhou QY, Allen L, Litt R, Magenis RE, Civelli O and Litt M 1990. A human D1 dopamine receptor gene is located on chromosome 5 at q35⋅1 and identifies an EcoRI RFLP; Am. J. Hum. Genet 47 828–834 [PMC free article] [PubMed] [Google Scholar]
- Gray IC, Campbell DA and Spurr NK 2000. Single nucleotide polymorphisms as tools in human genetics; Hum. Mol. Genet 9 2403–2408 [DOI] [PubMed] [Google Scholar]
- Gu C, Province MA and Rao DC 2001. Meta-analysis for model-free methods; Adv. Genet 42 255–272 [DOI] [PubMed] [Google Scholar]
- Gurling H 1998. Chromosome 21 workshop; Psychiatr. Genet 8 19–113 [DOI] [PubMed] [Google Scholar]
- Guy CA, Bowen T, Williams N, Jones IR, McCandless F, McGuffin P, Owen MJ, Craddock N and O’Donovan MC 1999. No association between a polymorphic CAG repeat in the human potassium channel gene hKCa3 and bipolar disorder; Am. J. Med. Genet 88 57–60 [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Kennedy JL, Wetterberg L, Sjogren B, Kidd KK and Cavalli Sforza L L 1992. Exclusion of linkage between the serotonin 2 receptor and schizophrenia in a large Swedish kindred; Arch. Gen. Psychiatry 49 216–219 [DOI] [PubMed] [Google Scholar]
- Halushka MK, Fan JB, Bentley K, Hsie L, Shen N, Weder A, Cooper R, Lipshutz R and Chakravarti A 1999. Patterns of single-nucleotide polymorphisms in candidate genes for blood-pressure homeostasis; Nat. Genet 2 239–247 [DOI] [PubMed] [Google Scholar]
- Hawi Z, Mayakishev M, Straub RE, O’Neill A, Kendler KS, Walsh D and Gill M 1997. No association or linkage between the 5-HT2a/T102C polymorphism and schizophreniain Irish families; Am. J. Med. Genet 74 370–373 [PubMed] [Google Scholar]
- He L, Li Tao, Melville C, Liu Sheng, Feng GY, Gu NF, Fox g, Shaw D, Breen G, Liu X, Sham P, Brown J, Collier D and St. Clair D 1999. 102T/C polymorphism of serotonin receptor type 2A gene is not associated with schizophrenia in either Chinese or British populations; Am. J. Med. Genet 88 95–98 [PubMed] [Google Scholar]
- Heath RG and Krupp IM 1967. Schizophrenia as an immunologic disorder. I. Demonstration of antibrain globulins by fluorescent antibody technique; Arch. Gen. Psychiatry 16 1–9 [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D and Lesch KP 1996. Allelic variation of human serotonin transporter gene expression; J. Neurochem 66 2621–2624 [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silpo G and Lichtenstein M 1999. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia; Arch. Gen. Psychiatry 56 29–36 [DOI] [PubMed] [Google Scholar]
- Holliday R 1989. DNA methylation and epigenetic mechanisms; Cell Biophys. 15 15–20 [DOI] [PubMed] [Google Scholar]
- Holzman PS, Proctor LR and Hughes DW 1973. Eye-tracking patterns in schizophrenia; Science 181 179–181 [DOI] [PubMed] [Google Scholar]
- Hovatta I, Lichtermann D, Juvonen H, Suvisaari J, Terwilliger JD, Arajarvi R, Kokko-Sahin ML, Ekelund J, Lonnqvist J and Peltonen L 1998. Linkage analysis of putative schizophrenia gene candidate regions on chromosomes 3p, 5q, 6p, 8p 20p and 22q in a population-based sampled Finnish family set; Mol. Psychiatry 3 452–457 [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Schwab SG, Jernej B, Knapp M, Lerer B, Albus M, Rietschel M, Kanyas K, Borrmann M, Lichtermann D, Maier W and Wildenauer DB 2000. Serotonin transporter gene and schizophrenia: evidence for association/linkage disequilibrium in families with affected siblings; Mol. Psychiatry 5 91–95 [DOI] [PubMed] [Google Scholar]
- Inayama Y, Yoneda H, Ishada T, Nonomura Y, Kono Y, Koh J, Kuroda K, Higashi H, Asaba H and Sakai T 1994. An association between schizophrenia and a serotonin receptor DNA marker (5HTR2); Neuropsychopharmacology (Suppl.) 10 56 [Google Scholar]
- Iwahashi K, Watanabe M, Nakamura K, Suwaki H, Nakaya T, Nakamura Y, Takahashi H and Ikuta K 1998. Borna disease virus infection and schizophrenia: seroprevalence in schizophrenia patients; Can. J. Paychiatry 43 197. [PubMed] [Google Scholar]
- Jain S, Leggo J, DeLisi LE, Crow TJ, Margolis RL, Li SH, Gooburn S, Walsh C, Paykel ES, Ferguson-Smith MA, Ross CA and Rubinsztein DC 1996. Analysis of thirteen trinucleotide repeat loci as candidate genes for schizophrenia and bipolar affective disorder; Am. J. Med. Genet 67 139–146 [DOI] [PubMed] [Google Scholar]
- Jonsson E, Lannfelt L, Sokoloff P, Schwartz J-C and Sedvall G 1993. Lack of association between schizophrenia and alleles in the dopamine D3 receptor gene; Acta Psychiatr. Scand 87 345–349 [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Geijer T, Gyllander A, Terenius L and Sedvall GC 1998. Failure to replicate an association between a rare allele of a tyrosine hydroxylase gene microsatellite and schizophrenia; Eur. Arch. Psychiatry Clin. Neurosci 248 61–63 [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Nimgaonkar VL, Zhang XR, Shaw SH, Burgert E, Crocq MA, Chakravarti A and Sedvall GC 1999. Trend for an association between schizophrenia and D3S310, a marker in proximity to the dopamine D3 receptor gene; Am. J. Med. Genet 88 352–357 [PubMed] [Google Scholar]
- Johnson JE, Cleary J, Ahsan H, Harkavy Friedman J, Malaspina D, Cloninger CR, Faraone SV, Tsuang MT and Kaufmann CA 1997. Anticipation in schizophrenia: biology or bias?; Am. J. Med. Genet 74 275–280 [DOI] [PubMed] [Google Scholar]
- Joober R, Benkelfat C, Brisebois K, Toulouse A, Lafreniere RG, Turecki G, Lal S, Bloom D, Labelle A, Lalonde P, Fortin D, Alda M, Palmour R and Rouleau GA 1999. Lack of association between the hSKCa3 channel gene CAG polymorphism and schizophrenia; Am. J. Med. Genet 88 154–157 [PubMed] [Google Scholar]
- Joyce JN, Shane A, Lexow N, Winokur A, Cassanova MF and Kleinman JE 1993. Serotonin uptake sites and serotonin receptors are altered in the limbic system of schizophrenics; Neuropsychopharmacology 8 315–336 [DOI] [PubMed] [Google Scholar]
- Kaufmann CA, Suarez B, Malaspina D, Pepple J, Svrakic D, Markel PD, Meyer J, Zambuto CT, Schmitt K, Matise TC, Harkavy Friedman J M, Hampe C, Lee H, Shore D, Wynne D, Faraone SV, Tsuang MT and Cloninger CR 1998. NIMH genetics initiative millennium schizophrenia consortium: linkage analysis of African-American pedigrees; Am. J. Med. Genet 81 282–289 [PubMed] [Google Scholar]
- Kebabian JK and Calne DB 1979. Multiple receptors for dopamine; Nature (London) 277 93–96 [DOI] [PubMed] [Google Scholar]
- Kendler KS, MacLean CJ, O’Neill FA, Burke J, Murphy B, Duke F, Shinkwin R, Easter SM, Webb BT, Zhang J, Walsh D and Straub RE 1996. Evidence for a schizophrenia vulnerability locus on chromosome 8p in the Irish study of high density schizophrenia families; Am. J. Psychiatry 153 1534–1540 [DOI] [PubMed] [Google Scholar]
- Kennedy JL, Giuffra LA, Moises HW, Cavalli-Sforza LL, Pakstis AJ, Kidd JR, Castiglione CM, Sjogren B, Wetterberg L and Kidd KK 1988. Evidence against linkage of schizophrenia to markers on chromosome 5 in a northern Swedish pedigree; Nature (London) 336 167–170 [DOI] [PubMed] [Google Scholar]
- Kennedy JL and Macciardi FM 1998. Chromosome 4 workshop; Psychiatric Genet. 8 67–71 [DOI] [PubMed] [Google Scholar]
- Knight J, Knight A and Ungarvi G 1992. Can autoimmune mechanisms account for the genetic predisposition for schizophrenia?; Br. J. Psychiatry 160 533–540 [DOI] [PubMed] [Google Scholar]
- Knight JG 1982. Dopamine receptor stimulating autoantibody: a possible cause of schizophrenia; Lancet 2 1073–1075 [DOI] [PubMed] [Google Scholar]
- Kohen R, Metcalf MA, Khan N, Druck T, Huebner K, Lachowicz JE, Meltzer HY, Sibley DR, Roth BL and Hamblin MW 1996. Cloning, characterization and chromosomal localization of a human 5-HT6 serotonin receptor; J. Neurochem 66 47–56 [DOI] [PubMed] [Google Scholar]
- Kojima H, Ohmori O, Shinkai T, Terao T, Suzuki T and Abe K 1999. Dopamine D1 receptor gene polymorphism and schizo-phrenia in Japan; Am. J. Med. Genet 88 116–119 [DOI] [PubMed] [Google Scholar]
- Kouzmenko AP, Scaffidi A, Pereira AM, Hayes WL, Copolov DL and Dean B 1999. No correlation between A(−1438)G polymorphism in 5HT2A receptor gene promoter and the density of frontal cortical 5HT2A receptors in schizophrenia; Hum. Hered 49 103–105 [DOI] [PubMed] [Google Scholar]
- Lechler R 1994. HLA and diseases (London: Academic Press; ) [Google Scholar]
- Levy DL, Holzman PS, Matthysse S, Mendell NR 1993. Eye tracking dysfunction and schizophrenia: a critical perspective; Schizophr. Bull 19 461–536 [DOI] [PubMed] [Google Scholar]
- Lin CH, Tsai SJ, Yu YW, Song HL, Tu PC, Sim CB, Hsu CP, Yang KH and Hong CJ 1999. No evidence for association of serotonin-2A receptor variant (102T/C) with schizophrenia or clozapine response in a Chinese population; Neuroreport 10 57–60 [DOI] [PubMed] [Google Scholar]
- Litt M, Al-Dhalimy M, Zhou Q, Grandy D and Civelli O 1991. A TaqI RFLP at the DRD1 locus; Nucleic Acids Res. 19 3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Janet LS, Leonard LH, Steve SS 1995. Screening the dopamine D1 receptor gene in 131 schizophrenics and eight alcoholics; Am. J. Med. Genet 60 165–171 [DOI] [PubMed] [Google Scholar]
- Maier W, Schmidt F, Schwab SG, Hallmeyer J, Minges J, Ackenheil M, Lichtermann D and Wildenauer DB 1995. Lack of linkage between schizophrenia and markers at the telomeric end of the pseudoautosomal region of the sex chromosome; Biol. Psychiatry 37 344–347 [DOI] [PubMed] [Google Scholar]
- Mant R, Williams J, Asherson P, Parfitt E, McGuffin P and Owen MJ 1994. Relationship between homozygosity at the dopamine D3 receptor gene and schizophrenia; Am. J. Med. Genet 54 21–26 [DOI] [PubMed] [Google Scholar]
- Matsumoto M and Yoshioka M 2000. Possible involvement of serotonin receptors in anxiety disorders; Nippon Yakurigaku Zasshi 115 39–44 [DOI] [PubMed] [Google Scholar]
- Maziade M, Debraekeleer M, Genest P, Cliché D, Fournier JP, Garneau Y, Shriqui C, Roy MA, Nicole L, Raymond V et al. 1993. A balanced 2:18 translocation and familial schizophrenia: falling short of an association; Arch. Gen. Psychiatry 50 73–75 [DOI] [PubMed] [Google Scholar]
- McGuffin P, Owen MJ and Farmer AE 1995. The genetic basis of schizophrenia; Lancet 346 678–682 [DOI] [PubMed] [Google Scholar]
- McGuffin P, Sargeant M, Hetti G, Tidmarsh S, Whatley S and Marchbanks RM 1990. Exclusion of a schizophrenia susceptibility gene from the chromosome 5q11–13 region: new data and a reanalysis of previous reports; Am. J. Hum. Genet 47 524–535 [PMC free article] [PubMed] [Google Scholar]
- McInnis M, Lee P, Ginnis EI, Lenane M, Kumra S, Jacobsen L, Rapport JL and Schalling M 1998. Large CAG/CTG repeats are associated with childhood-onset schizophrenia; Mol. Psychiatry 3 321–327 [DOI] [PubMed] [Google Scholar]
- McInnis MG 1996. Anticipation: an old idea in new genes; Am. J. Hum. Genet 59 973–979 [PMC free article] [PubMed] [Google Scholar]
- Meloni R, Leboyer M, Bellivier F, Barbe B, Samolyk D, Allilaire JF and Mallet J 1995. Association of manic depressive illness with the TH locus using a microsatellite marker localized in the tyrosine hydroxylase gene; Lancet 345 932. [DOI] [PubMed] [Google Scholar]
- Meszaros K, Lenzinger E, Fureder T, Hornik K, Willinger U, Stompe T, Heiden AM, Resinger E, Fathi N, Gerhard E, Fuchs K, Miller-Reiter E, Pfersmann V, Sieghart W, Aschauer HN and Kasper S 1996. Schizophrenia and the dopamine-beta hydroxylase gene: results of a linkage and association study; Psychiatric Genet. 6 17–22 [DOI] [PubMed] [Google Scholar]
- Mignot E, Kimura A, Abbal M, Thorsby E, Lin X, Voros A, Macaubas C, Bouissou F, Sollid LM, Thomsen AC, Yasunaga S and Grumet FC 1995. DQCAR microsatellite polymorphisms in three selected HLA class II-associated diseases; Tissue Antigens 46 299–304 [DOI] [PubMed] [Google Scholar]
- Moises HW, Yang L, Kristbjarmarson H, Weise C, Byerley W, Macciardi F, Arolt V, Blackwood D, Liu X, Sjogren B, Aschauer HN, Hwu HG, Jang K, Livesley WJ, Kennedy JL, Zoega T, Ivarsson O, Bui MT, Yu MH, Havesteen B, Commenges D, Weissenbach J, Schwinger E, Gottesman II, Pakstis AJ, Wetterberg L, Kidd KK and Helgason T 1995. An international two-stage genome wide search for schizophrenia susceptibility genes; Nature Genet. 11 233–234 [DOI] [PubMed] [Google Scholar]
- Morris AG, Gaitonde EJ, McKenna PJ, Mollon JD and Hunt DM 1995. CAG repeat expansions and schizophrenia: association with disease in females and with early age-at-onset; Hum. Mol. Genet 4 1957–1961 [DOI] [PubMed] [Google Scholar]
- Muller N, Ackenheil M, Hofschuster E, Mempel W and Eckstein R 1991. Cellular immunity in schizophrenic patients before and during neuroleptic treatment; Psychiatry Res. 37 147–160 [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones RG, Griffiths E, Thompson PW and Owen MJ 1998. Chromosome 22q11 deletions. An under-recognized cause of idiopathic learning disability; Br. J. Psychiatry 17 180–183 [DOI] [PubMed] [Google Scholar]
- Nakatome M, Honda K, Tun Z, Kato Y, Harihara S, Omoto K, Misawa S, Gerelsaikhan T, Nyamkhishig S, Dashnyam B, Batsuuri J and Wakasugi C 1996. Genetic polymorphism of the 3′ VNTR region of the human dopaminergic function gene DAT1 (Human Dopamine Transporter Gene) in the Mongolian population; Hum. Biol 68 509–515 [PubMed] [Google Scholar]
- Nanko S, Hattori M, Dai XY, Fukuda R and Kazamatsuri H 1994. DRD2 Ser311/Cys311 polymorphism in schizophrenia; Lancet 343 1044–1045 [PubMed] [Google Scholar]
- Nanko S, Sasaki T, Fukuda R, Hattori M, Dai XY, Kazamatsuri H, Kuwata S, Juji T and Gill M 1993. A study of association between schizophrenia and dopamine D3 receptor gene; Hum. Genet 92 336–338 [DOI] [PubMed] [Google Scholar]
- Naylor L, Dean B, Pereira A, Mackinnon A, Kouzmenko A and Copolov D 1998. No association between the serotonin transporter-linked promoter region polymorphism and either schizophrenia or density of the serotonin transporter in human hippocampus; Mol. Med 4 671–674 [PMC free article] [PubMed] [Google Scholar]
- Nimgaonkar VL, Ganguli R, Rudert WA, Vavassori C, Rabin BS and Trucco M 1992. A negative association of schizophrenia with an allele of the HLA DQB1 gene among African-Americans; Am. J. Med. Genet 8199–209 [DOI] [PubMed] [Google Scholar]
- Nimgaonkar VL, Rudert WA, Zhang XR, Trucco M and Ganguli R 1997. Negative association of schizophrenia with HLADQB1*0602: evidence from a second African-American cohort; Schizophr. Res 23 81–86 [DOI] [PubMed] [Google Scholar]
- Nimgaonkar VL, Rudert WA, Zhang XR, Tsoi WF, Trucco M and Saha N 1995. Further evidence for an association between schizophrenia and the HLADQB1 gene locus; Schizophr. Res 18 43–49 [DOI] [PubMed] [Google Scholar]
- Nimgaonkar VL, Zhang XR, Caldwell JG, Ganguli R and Chakravarti A 1993. Association study of schizophrenia with dopamine D3 receptor gene polymorphisms: Probable effects of family history; Am. J. Med. Genet 48 214–217 [DOI] [PubMed] [Google Scholar]
- Nimgaonkar VL, Zhang XR, Brar JS, DeLeo M and Ganguli R 1996. 5-HT2 receptor gene locus: association with schizophrenia or treatment response not detected; Psychiatr. Genet 6 23–27 [PubMed] [Google Scholar]
- Nöthen MM, Erdmann J, Shimron-Abarbanell D and Propping P 1994. Identification of genetic variation in the human serotonin1Dβ receptor gene; Biochem. Biophys. Commun 205 1194–1200 [DOI] [PubMed] [Google Scholar]
- Nöthen MM, Korner J, Lannfelt L, Sokoloff P, Schwartz J-C, Lanczik M, Rietschel M, Cichon S, Kramer R, Fimmers R, Möller H-J, Beckmann H, Propping P, Grandy DK, Civelli O and O’Dowd BF 1993a. Lack of association between schizophrenia and alleles of the dopamine D1, D2, D3 and D4 receptor loci; Psychiatr. Genet 3 89–94 [Google Scholar]
- Nöthen MM, Cichon S, Propping P, Fimmers R, Schwab SG and Wildenauer DB 1993b. Excess of homozygosity at the dopamine D3 receptor gene in schizophrenia not confirmed; J. Med. Genet 30 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan MC, Guy C, Craddock N, Murphy KC, Cardno AG, Jones LA, Owen MJ and McGuffin P 1995. Expanded CAG repeats in schizophrenia and bipolar disorder; Nature Genet. 6 14–18 [DOI] [PubMed] [Google Scholar]
- Oliveira MJR, Otto PA, Vallada H, Lauriano V, Elkis H, Lafer B, Vasquez L, Gentil V, Passos-Bueno MR and Zatz M 1998. Analysis of a novel functional polymorphism within the promoter region of the serotonin transporter gene (5-HTT) in Brazilian patients affected by bipolar disorder and schizophrenia; Am. J. Med. Genet 81 225–227 [DOI] [PubMed] [Google Scholar]
- O’Reilly RL and Singh SM 1996. Retroviruses and schizophrenia revisited; Am. J. Med. Genet 67 19–24 [DOI] [PubMed] [Google Scholar]
- Parfitt E, Asherson P, Roberts E, Mant R, Nanko S, Gill M, McGuffin P and Owen M 1996. No evidence for linkage between schizophrenia and eight microsatellite markers on chromosome 19; Hum. Hered 46 191–196 [DOI] [PubMed] [Google Scholar]
- Pean BS, Laurent C, Campion D, Jay M, Thibaut F, Dollfus S, Petit M, Samolyk D, d’Amato T, Martinez M and Mallet J 1995. No evidence for linkage or association between the dopamine transporter gene and schizophrenia in a French population; Psychiatr. Res 59 1–6 [DOI] [PubMed] [Google Scholar]
- Perisco AM and Macciardi F 1997. Genotypic association between dopamine transporter gene polymorphisms and schizophrenia; Am. J. Med. Genet 74 53–57 [DOI] [PubMed] [Google Scholar]
- Perisco AM, Vandenberg DJ, Smith SS and Uhl GR 1993. Dopamine transporter gene markers are not associated with polysubstance abuse; Biol. Psychiatry 34 265–267 [DOI] [PubMed] [Google Scholar]
- Perisco AM, Wang ZW, Black DW, Andreasen NC, Uhl GR and Crowe RR 1995. Exclusion of close linkage of the dopamine transpoter gene with schizophrenia spectrum disorders; Am. J. Psychiatr 152 134–136 [DOI] [PubMed] [Google Scholar]
- Petronis A 2000. The genes for major psychosis: aberrant sequence or regulation?; Neuropsychopharmacology 23 1–11 [DOI] [PubMed] [Google Scholar]
- Petronis A, Paterson AD and Kennedy JL 1999. Schizophrenia: An Epigenetic Puzzle; Schizophr. Bull 25 639–655 [DOI] [PubMed] [Google Scholar]
- Prasad S, Deshpande S, Bhatia T, Wood J, Nimgaonkar VL and Thelma BK 1999. An association study of schizophrenia among Indian families; Am. J. Med. Genet 88 298–300 [DOI] [PubMed] [Google Scholar]
- Pulver AE, Lasseter VK, Kasch L, Wolyniec PS, Nestadt G, Blouin JL, Kimberland M, Babb R, Vourlis S, Chen H, Lalioti M, Morris MA, Karayiorgou M, Ott J, Meyers D, Antonarakis S, Housman D and Kazaazian HH 1995. Schizophrenia: a genome scan targets chromosome 3p and 8p as potential sites of susceptibility genes; Am. J. Med. Genet 60 252–260 [DOI] [PubMed] [Google Scholar]
- Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, Morrow B, Karayiorgou M, Antonarakis SE, Housman D and Kucherlapati R 1994. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives; J. Nerv. Ment. Dis 182 476–478 [DOI] [PubMed] [Google Scholar]
- Rao D, Jonsson EG, Pauss S, Ganguli R, Nothen M and Nimgaonkar VL 1998. Schizophrenia and the serotonin transporter gene; Psychiatr. Genet 8 207–212 [DOI] [PubMed] [Google Scholar]
- Riley BP, Lin MW, Mogudi-Carter M, Jenkins T, Williamson R, Powell JF, Collier D and Murray R 1998. Failure to exclude a possible schizophrenia susceptibility locus on chromosome 13q14⋅1-q32 in southern African Bantu speaking families; Psychiatr. Genet 8 155–162 [DOI] [PubMed] [Google Scholar]
- Riley BP and McGuffin P 2000. Linkage and associated studies of schizophrenia; Am. J. Med. Genet. (Semin. Med. Genet.) 97 23–44 [DOI] [PubMed] [Google Scholar]
- Riley BP, Tahir E, Rajagopalan S, Mogudi-Carter M, Faure S, Weissenbach J, Jenkins T and Williamson R 1997. A linkage study of the N-Methyl-D-aspartate receptor subunit gene loci and schizophrenia in southern African-Bantu speaking families; Psychiatric. Genet 7 57–74 [DOI] [PubMed] [Google Scholar]
- Risch N 1990. Linkage strategies for genetically complex traits. I. Multilocus models; Am. J. Hum. Genet 46 222–228 [PMC free article] [PubMed] [Google Scholar]
- Ross CA, McInnis MG, Margolis RI and Li S-H 1993. Genes with triplet repeats: Candidate mediators of neuropsychiatric disorders; Trends. Neurosci 16 254–260 [DOI] [PubMed] [Google Scholar]
- Rotando A, Schuebel K, Bergen A, Aragon R, Virkkunen M, Linnoila M, Goldman D and Nielsen D 1999. Identification of four variants in the tryptophan hydroxylase promoter and association to behaviour; Mol. Psychiatry 4 360–368 [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Leggo J, Goodburn S, Crow TJ, Lofthouse R, DeLisi LE, Barton DE and Ferguson-Smith MA 1994. Study of the Huntington’s disease (HD) gene CAG repeats in schizophrenic patients shows overlap of the normal and HD affected ranges but absence of correlation with schizophrenia; J. Med. Genet 31 690–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem Q, Ganesh S, Vijaykumar M, Reddy YCJ, Brahmachari SK and Jain S 2000a. Association analysis of 5HT transporter gene in bipolar disorder in the Indian population; Am. J. Med. Genet 96 170–172 [PubMed] [Google Scholar]
- Saleem Q, Sreevidya VS, Sudhir J, Savithri JV, Gowda Y, Rao CB, Benegal V, Majumdar PP, Anand A, Brahamchari SK and Jain S 2000b. Association analysis of CAG repeats at KCNN3 locus Indian patients with bipolar disorder and schizophrenia; Am. J. Med. Genet 96 744–748 [DOI] [PubMed] [Google Scholar]
- Saleem Q, Vijaykumar M, Mutsuddi M, Chowdhary N, Jain S and Brahmachari SK 1998. Variation at the MJD locus in the major psychoses; Am. J. Med. Genet 81 440–442 [PubMed] [Google Scholar]
- Schmauss C, Haroutunian V, Davis KL and Davidson M 1993. Selective loss of dopamine D3-type receptor mRNA expression in parietal and motor cortices of patients with chronic schizophrenia; Proc. Natl. Acad. Sci. USA 90 8942–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab SG, Albus M, Hallmayer J, Honig S, Borrmann M, Lichtermann D, Ebstein RP, Ackenheil M, Lerer B, Risch N, Maier W and Wildenauer DB 1995. Evaluation of a susceptibility gene for schizophrenia on chromosome 6p by multipoint affected sib-pair linkage analysis; Nature Genet. 11 325–327 [DOI] [PubMed] [Google Scholar]
- Schwab SG, Hallmeyer J, Albus M, Lerer B, Hanses C, Kanyas K, Segman R, Borrman M, Dreikorn B, Lichtermann D, Rietschel M, Trixler M, Maier W and Wildenauer DB 1998. Further evidence for a susceptibility locus on chromosome 10p14-p11 in 72 families with schizophrenia by non-parametric linkage analysis; Am. J. Med. Genet 81 302–307 [PubMed] [Google Scholar]
- Schwab SG, Hallmeyer J, Lerer B, Albus M, Borrmann M, Honig S, Strauss M, Segman R, Lichtermann D, Knapp M, Trixler M, Maier W and Wildenauer DB 1998. Support for a chromosome 18p locus conferring susceptibility to functional psychoses in families with schizophrenia, by association and linkage analysis; Am. J. Hum. Genet 63 1139–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedvall G and Farde L 1995. Chemical brain anatomy in schizophrenia; Lancet 346 743–749 [DOI] [PubMed] [Google Scholar]
- Semwal P, Prasad S, Bhatia T, Deshpande SN, Wood J, Nimgaonkar VL and Thelma BK 2001. Family based studies of monoaminergic gene polymorphism among Indians with schizophrenia; Mol. Psychiatry 6 220–224 [DOI] [PubMed] [Google Scholar]
- Serreti SA, Lilli R, Lorenzi C, Lattuada E, Cusin C and Smeraldi E 1999. Lack of association between tryptophan hydroxylase gene and psychotic symptamatology in schizophrenia; Schizophr. Res 40 171–172 [DOI] [PubMed] [Google Scholar]
- Shaw SH, Kelly M, Smith AB, Shields G, Hopkins PJ, Loftus J, Laval SH, Vita A, De Hert M, Cardon LR, Crow TJ, Sherrington R and DeLisi LE 1998. A genome-scan search for schizophrenia susceptibility genes; Am. J. Med. Genet 81 364–376 [DOI] [PubMed] [Google Scholar]
- Shaikh S, Collier D, Arranz M, Ball D, Gill M and Kerwin R 1994. DRD2 Ser311/Cys311 polymorphism in schizophrenia; Lancet 343 1045–1046 [PubMed] [Google Scholar]
- Sherrington R, Brynjolfsson J, Petursson H, Potter M, Dudleston K, Barraclough B, Wasmuth J, Dobbs M and Gurlin H 1988. Localization of a susceptibility locus for schizophrenia on chromosome 5; Nature (London) 336 164–167 [DOI] [PubMed] [Google Scholar]
- Shima S, Yano K, Sagiura M and Tokunaga Y 1991. Anticerebral antibodies in functional psychoses; Biol. Psychiatry 29 322–328 [DOI] [PubMed] [Google Scholar]
- Shinkai T, Ohmori O, Kojima H, Terao T, Suzuki T and Abe K 1999. Association study of the 5-HT6 receptor gene in schizophrenia; Am. J. Med. Genet 88 120–122 [PubMed] [Google Scholar]
- Sidenberg DG, Basset AS, Demchyshyn L, Niznik HB, Macciardi F, Kamble AB, Honer WG and Kennedy JL 1993. New polymorphism for the human serotonin 1D receptor variant (5HTID) beta linked to schizophrenia in five Canadian pedigrees; Hum. Hered 43 315–318 [DOI] [PubMed] [Google Scholar]
- Silvermann JM, Greenberg DA, Altsteil LD, Siever LJ, Mohs RC, Smith CJ, Zhou G, Hollander TE, Yang XP, Kedache M, Li G, Zaccario ML and Davis KL 1996. Evidence of a locus for schizophrenia and related disorders on the short arm of chromosome 5 in a large pedigree; Am. J. Med. Genet 67 162–171 [DOI] [PubMed] [Google Scholar]
- Sobell J, Sigurdson DC, Heston L and Sommer S 1994. S311C D2DR variant: No association with schizophrenia; Lancet 344 621–622 [DOI] [PubMed] [Google Scholar]
- Solomon GF 1981. Immunologic abnormalities in mental illness; in Psychoneuroimmunology (ed.) Ader R (New York: Academic Press; ) [Google Scholar]
- Sommer SS, Lind TJ, Heston LL and Sobell JL 1993. Dopamine D4 receptor variants in unrelated schizophrenic cases and controls; Am. J. Med. Genet 48 90–93 [DOI] [PubMed] [Google Scholar]
- Spurlock G, Heils A, Holmans P, Williams J, D’Souza UM, Cardno A, Murphy KC, Jones L, Buckland PR, McGuffin P, Lesch KP and Owen MJ 1998. A family based association study of T102C polymorphism in 5HT2A and schizophrenia plus identification of new polymorphisms in the promoter; Mol. Psychiatry 3 42–49 [DOI] [PubMed] [Google Scholar]
- Stahl SM and Wets K 1987. Indoleamines and schizophrenia; in Neurochemistry and neuropharacology of schizophrenia (Amsterdam: Elsevier; ) pp 257–296 [Google Scholar]
- St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, Gosden C and Evans HJ 1990. Association within a family of a balanced autosomal translocation with major mental illness; Lancet 336 13–16 [DOI] [PubMed] [Google Scholar]
- Straub RE, MacLean CJ, O’Neill FA, 1997. Support for a possible schizophrenia vulnerability locus in region 5q22–31 in Irish families; Mol. Psychiatry 2 148–155 [DOI] [PubMed] [Google Scholar]
- Straub RE, MacLean CJ, O’Neil FA, Burke J, Murphy B, Duke F, Shinkwin R, Webb BT, Zhang J, Walsh D and Kendler KS 1995. A potential vulnerability locus for schizophrenia on chromosome 6p24–22: Evidence for genetic heterogeneity; Nature Genet. 11 287–293 [DOI] [PubMed] [Google Scholar]
- Sunhara RK, Guan H-C and O’Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HH and Niznik HB 1991. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1; Nature (London) 350 614–619 [DOI] [PubMed] [Google Scholar]
- Thomson G 2001. An overview of the genetic analysis of complex diseases, with reference to type I diabetes; Best Pract. Res. Clin. Endocrinol. Metab 15 265–277 [DOI] [PubMed] [Google Scholar]
- Thomson G, Robinson W, Kuhner M, Joe S and Klitz W 1989. HLA and insulin gene associations with IDDM; Genet. Epidemiol 6 155–160 [DOI] [PubMed] [Google Scholar]
- Thomson R 1996. The brain: A neuroscience primer Second edition (New York: W H Freeman; ) [Google Scholar]
- Toyooka K, Murataka T, Tanaka T, Igarashi S, Watanabe H, Takeuchi H, Hayashi S, Maeda M, Takahashi M, Tsuji S, Kumanishi T and Takahashi Y 1999. 14–3-3 protein eta chain gene (YWHAH) polymorphism and its genetic association with schizophrenia; Am. J. Med. Genet 88 164–167 [PubMed] [Google Scholar]
- Trucco M 1992. To be or not to be Asp57, that is the question; Diabetes Care 15 705–715 [DOI] [PubMed] [Google Scholar]
- Tsai G, van Kammen D P, Chen S, Kelley ME, Grier A and Coyle JT 1998. Glutamatergic neurotransmission involves structural and clinical deficits of schizophenia; Biol. Psychiatry 44 667–674 [DOI] [PubMed] [Google Scholar]
- Vandenberg DJ, Perisco AM, Hawkins AL, Griffin CA, Li X, Jabs EW and Uhl GR 1992a. Human dopamine transporter gene (DAT1) maps to chromosome 5p15⋅3 and displays a VNTR; Genomics 14 1104–1106 [DOI] [PubMed] [Google Scholar]
- Vandenberg DJ, Perisco AM and Uhl GR 1992b. A human dopamine transporter cDNA predicts reduced glycosylation, displays a novel repetitive element and provides racially dimorphic Taq I RFLPs; Mol. Brain Res 15 161–166 [DOI] [PubMed] [Google Scholar]
- Van Tol HHM, Bunzow JR, Guan H-C, 1991. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine; Nature (London) 350 610–614 [DOI] [PubMed] [Google Scholar]
- Verga M, Macciardi F, Cohen S, Pedrini S and Smeraldi E 1997. No association between schizophrenia and the serotonin receptor 5HTR2a in an Italian population; Am. J. Med. Genet 74 21–25 [DOI] [PubMed] [Google Scholar]
- Warren JT, Peacock ML, Rodoriguez LC and Fink JK 1993. An MspI polymorphism in the human serotonin receptor gene (HTR2): Detection by DGE and RFLP analysis; Hum. Mol. Genet 2 338. [DOI] [PubMed] [Google Scholar]
- Wei J and Hemmings PG 2000. The NOTCH4 locus is associated with susceptibility to schizophrenia; Nature Genet. 25 376–377 [DOI] [PubMed] [Google Scholar]
- Williams J, McGuffin P, Nothen M and Owen MJ 1997. Meta-analysis of association between the 5-HT2a receptor T102C polymorphism and schizophrenia; Lancet 349 1221. [DOI] [PubMed] [Google Scholar]
- Williams J, Spurlock G, McGuffin P, Mallet J, Nothen MM, Gill M, Aschauer H, Nylander PO, Macciardi F and Owen MJ 1996. Association between schizophrenia and T102C polymorphism of the 5-hydroxytrptamine type 2a receptor gene; Lancet 347 1294–1296 [DOI] [PubMed] [Google Scholar]
- Williams NM, Rees MI, Holmans P, Norton N, Cardno AG, Jones LA, Murphy KC, Sanders RD, McCarthy G, Gray MY, Fenton I, McGuffin P and Owen MJ 1999. A two-stage genome scan for schizophrenia susceptibility genes in 196 affected sibling pairs; Hum. Mol. Genet 8 1729–1739 [DOI] [PubMed] [Google Scholar]
- Wittekindt O, Jauch A, Burgert E, Scharer L, Holtgreve-Grez H, Yvert G, Imbert G, Zimmer J, Hoehe MR, Macher JP, Chiaroni P, van Calker D, Crocq MA and Morris-Rosendahl DJ 1998. The human small conductance calcium-regulated potassium channel gene (hSKCa3) contains two CAG repeats in exon 1, is on chromosome 1q21⋅3, and shows a possible association with schizophrenia; Neurogenetics 1 259–265 [DOI] [PubMed] [Google Scholar]
- Wright P, Donaldson PT, Underhill JA, Choudhuri K, Doherty DG and Murray RM 1996. Genetic association of the HLADRB1 gene locus on chromosome 6p21⋅3 with schizophrenia; Am. J. Psychiatry 153 1530–1533 [DOI] [PubMed] [Google Scholar]
- Yamada K, Nanko S, Hattori S and Isurugi K 1982. Cytogenetic studies in a Y-to-X translocation observed in three members of one family, with evidence of infertility in male carriers; Hum. Genet 60 85–90 [DOI] [PubMed] [Google Scholar]
- Yolken RH, Karlsson H, Yee F, Johnston-Wilson NL and Torrey EF 2000. Endogenous retroviruses and schizophrenia; Brain Res. Rev 31 193–199 [DOI] [PubMed] [Google Scholar]
- Zamani MG, De Hert M, Spaepen M, Hermans M, Marynen P, Cassiman JJ and Peuskens J 1994. Study of the possible association of HLA class II, CD4, and CD3 polymorphisms with schizophrenia; Am. J. Med. Genet 54 372–377 [DOI] [PubMed] [Google Scholar]