Inflammation plays an important role in the genesis, progression, and manifestation of cardiovascular diseases (CVDs).1 C-reactive protein (CRP), an acute phase protein produced by the liver, is one of the most extensively studied systemic markers of inflammation. CRP increases within a few hours after an inflammatory trigger, peaks in two days, and decreases rapidly with the resolution of inflammation. Over the past three decades, extensive epidemiological and clinical evidence has linked the concentration of CRP to major CVDs.2–4 Since the 2000s, multiple cardiology organizations across the world have recommended adding CRP as an adjunct to assess or predict risks for CVDs.5–8 However, the role of CRP in CVDs among human populations, especially whether a causal relationship between them exists, remains unclear. This leads to substantial debates on whether CRP can be used as a therapeutic target for CVDs.9–13
To provide some preliminary answers to the above questions, we conducted a review of studies on the relationship between CRP and CVDs. Since multiple reviews of the relationship between CRP and CVDs have been published previously,2,3,10,14–16 our aim was neither to provide an exhaustive summary of previous studies nor to reach a consensus on the role of CRP. Instead, we want to delineate the current status of this research topic. We first summarized characteristics and findings on the relationship between CRP and CVDs from a perspective of study designs, identified their similarities and discrepancies across studies by designs, and provided our interpretations. In our opinion, the available studies do not support a causal relationship between CRP and CVDs despite a widely observed positive association between them. The comparison and synthesis of studies with different designs can facilitate appropriate interpretations of such an association.
Studies of different designs on CRP and CVDs
Table 1 summarizes the characteristics and findings of studies on CRP and CVDs by designs, including animal experiments, traditional observational studies, Mendelian Randomization (MR) studies, and clinical trials. In the table, we presented only three representative studies of each design.
Table 1.
A summary of studies on the relationship between CRP and CVDs
| Author, year | Methods to estimate associations | Subjects or study populations | Sample size (cases/all) | Main findings |
|---|---|---|---|---|
| Animal experiments | ||||
| Paul, 200417 | Compared atherosclerotic lesions between huCRPtg+/ApoE−/− and huCRPtg−/ApoE−/− | Mice | 209 | CRP↑ CVD↑ |
| Kovacs, 200722 | Compared atherosclerotic lesions between huCRPtg+/LDLR−/− and huCRPtg−/LDLR−/− | Mice | 105 | CRP↑ CVD↓ |
| Koike, 200921 | Compared the susceptibility to cholesterol-rich diet-induced aortic and coronary atherosclerosis between huCRPtg+ and huCRPtg− | Rabbits | 26 | Null association |
| Traditional observational studies | ||||
| Rutter, 200424 | Compared the 7-y incidence of CVD events between highest versus lowest CRP quartile | Framingham Offspring Study in the US | 189/3,037 | CRP↑ CVD↑ |
| Cushman, 200545 | Compared the 10-y incidence of CHD between CRP>3mg/L and CRP<1 mg/L | Cardiovascular Health Study in the US | 547/3,971 | CRP↑ CVD↑ |
| Chen, 202246 | Compared the 4.62-y incidence of CVD among quartiles of 7-y cumulative burden of CRP | Kailuan study in China | 2,118/34,959 | CRP↑ CVD↑ |
| Mendelian randomization studies | ||||
| Elliott, 200930 | MR between CRP and CHD | Multiple European studies | 28,112/128,935 | Null association |
| Wensley, 201131 | MR between CRP and CHD | Multiple European studies | 46,557/194,418 | Null association |
| Wang, 202247 | MR between CRP and CHD | Multiple European and East Asian studies | 121,072/494,478 | Null association |
| Clinical trials | ||||
| Ridker, 200835 | Compared the 1.9-y incidence of MCE between Rosuvastatin group and Placebo group | JUPITER | 393/17,802 | CRP↓ CVD↓ |
| Ridker, 201736 | Compared the 3.7-y incidence of MCE between Canakinumab group and Placebo group | CANTOS | 1,490/10,061 | CRP↓ CVD↓ |
| Ridker, 201837 | Compared the 2.3-y incidence of MCE between Low-dose methotrexate group and Placebo group | CIRT | 408/4,786 | Null association* |
Footnote: huCRPtg: human CRP transgene; ApoE: apolipoprotein E; LDLR: low-density lipoprotein receptor; CHD: coronary heart disease; MCE: major cardiovascular events
No changes in both CRP and CVD comparing treatment and control groups
First, in animal experiments, findings are controversial regarding the relationship between CRP and CVDs.17–22 These studies mainly applied genetic techniques to mice, rats, and rabbits and constructed animal models with a sample size ranging from several dozens to hundreds. For example, some studies showed that CRP can contribute to the pathological process of atherosclerosis,17,18 but more studies did not observe associations between CRP and CVDs.19–21 One study even reported that human CRP may slow the development of atherosclerosis.22 There is no clear explanation for these contradictory findings from animal experiments. In addition, it is also challenging to generalize findings from animal experiments to humans because of substantial differences in CRPs across species with respect to ligand recognition, secondary effects of ligand binding, and others.14 Therefore, animal experiments have not determined whether there is a causal association between CRP and CVDs.
Second, many traditional observational studies of human populations found positive associations between CRP concentrations and CVDs, including both cross-sectional and longitudinal studies.23–25 Our search showed that to date there are at least 60 traditional observational studies that have examined the relationship between CRP and CVDs. Their number is much larger than those of studies based on other designs. Besides studies based on individual-level data, multiple meta-analyses also showed that a higher concentration of CRP is associated with an increased risk of CVDs.26–28 These findings suggest that CRP can be a useful biomarker to assess the risk of CVDs among diverse populations. For example, the American Heart Association (AHA) previously added CRP to optimize the assessment of cardiovascular risk.5 However, it is still challenging to infer causality based on traditional observational studies due to confounding.
Third, in the late 2000s, researchers started to use the MR method to examine the potential causal relationship between CRP and CVDs.4 This approach assumes that if the causality between these two indeed exists, genetic variants in CRP that are associated with altered CRP levels should also be associated with altered risks for CVDs. Since genetic variants are unrelated to confounding factors, this approach is not as prone to confounding and reverse causation bias as traditional observational studies. It should be noted that multiple factors can still influence the strength of association observed in MR studies, such as relationships between genetic variants in CRP and CRP levels, demographic characteristics of study populations, types, and stages of CVDs.29 However, most MR studies show that there is no association between CRP levels and CVDs.30–32 Evidence from a meta-analysis of CRP and CVDs also supports the null relationship.4 Therefore, we believe that no causal relationship was identified between CRP and CVDs in MR studies.
Forth, clinical trials with different anti-inflammatory treatments shed some light on the role of CRP and its relation with CVDs. Such trials were motivated by previous observational studies of the long-term administration of statins and aspirin and tested the hypothesis that inflammation can cause CVDs.33,34 To our knowledge, four renowned clinical trials explored the effect of several anti-inflammatory treatments on CVDs and CRP, including JUPITER study,35 CANTOS study,36 CIRT study,37 and COLCOT study.38 The first three fully analyzed CRP as a secondary outcome. Specifically, JUPITER and CANTOS showed that their treatments reduce both the risk of CVDs and CRP levels. In contrast, CIRT showed no treatment effects on either CRP levels or the risk of CVDs. However, these clinical trials used a wide range of anti-inflammatory treatments that are not specific to lower CRP levels. Therefore, it is also hard to conclude whether anti-inflammatory treatments reduce the risk of CVDs by lowering CRP levels.
Inconsistent findings across studies of different designs
Based on the above examination, we found some similarities and inconsistencies in findings across studies of four designs. We found that most of the traditional observational studies and clinical trials showed a positive association between CRP and CVDs. In contrast, animal experiments showed mixed results on the association between CRP and CVDs, and most MR studies showed no positive association. These seemingly contradictory findings raised key questions in which types of studies we should believe and how their different findings can be explained. Therefore, we used Directed Acyclic Graphs (DAGs) as a conceptual representation to show how the relationship between CRP and CVDs was examined in studies of each design (Figure 1A to D).39 These DAGs may have oversimplified designs of these studies but provide a clear and straightforward summary of them.
Figure 1.
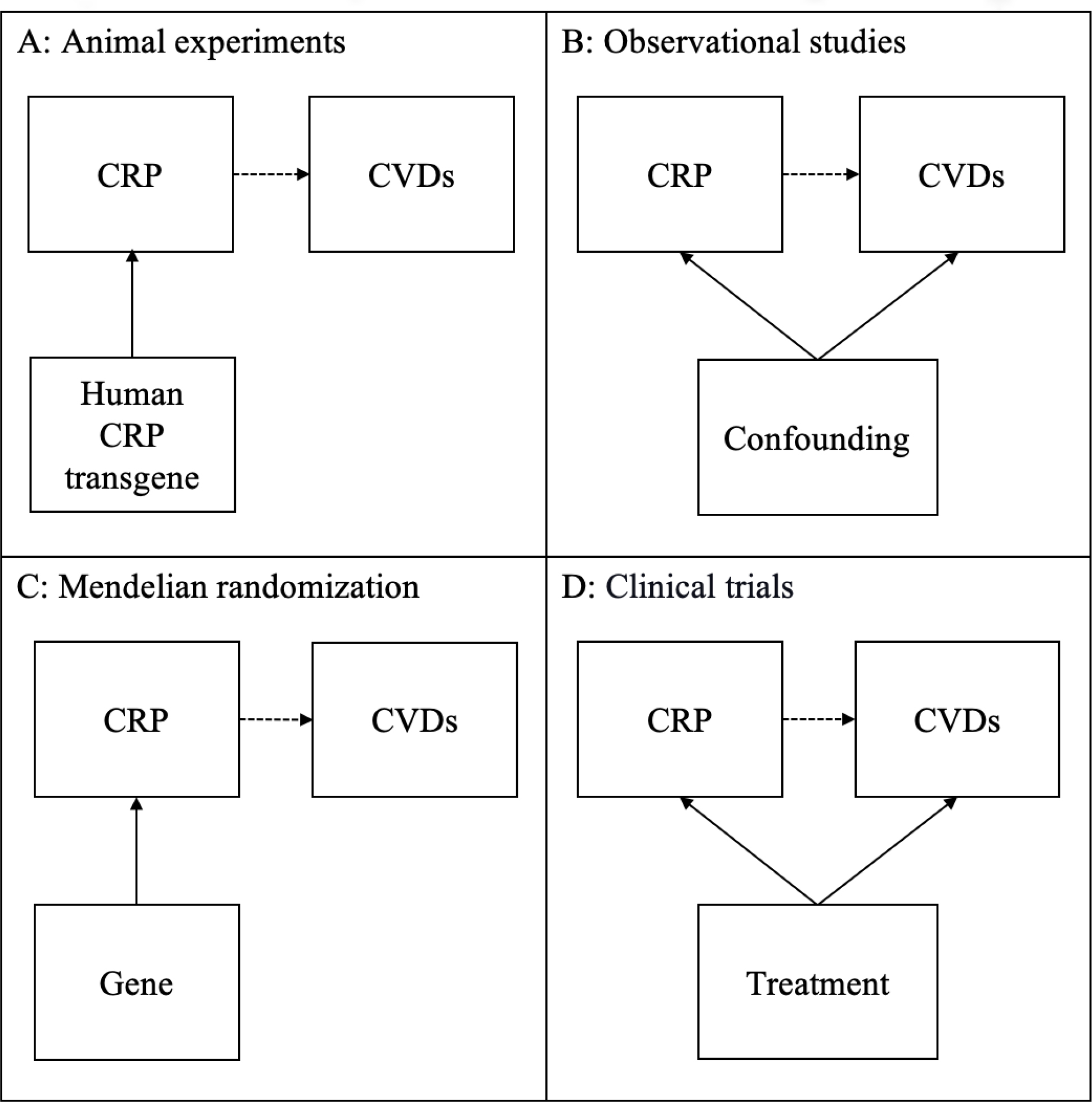
The relationship between CRP and CVDs in studies by different designs
A close examination of these DAGs suggests that studies of each design have focused on slightly different aspects of the relationship between CRP and CVDs. First, animal experiments used genetic techniques as an instrument to examine the causal relationship between CRP and CVDs (Figure 1A). Besides mixed findings of animal experiments, another challenge is whether their findings can be generalized to human populations. Second, many traditional observational studies consistently observed a positive association between CRP and CVDs in human populations, which however did not give a good answer to whether there is a causal relationship between them (Figure 1B). This is because confounding, whether measured or unmeasured, is a major issue in these studies. Third, in MR studies, researchers used CRP gene variants as an instrument to assess a causal relationship between CRP and CVDs (Figure 1C) and concluded that there is unlikely to be a causal relationship between both CRP and CVDs. MR studies also have multiple limitations, including low statistical power, reverse causation, population stratification, and others.40,41 Last, clinical trials of anti-inflammatory treatments observed positive associations between CRP and CVDs but did not directly examine the causal relationship between CRP and CVDs (Figure 1D). Their observed positive associations are likely to be explained by that CRP and CVDs shared a common cause of anti-inflammatory treatments. Besides studies of four designs summarized Figure 1A to D, we noticed a growing interest in studying the relationship between healthy behaviors, CRP, and CVDs.42,43 They had some promising findings of the potential impacts of healthy behaviors on CVD, but they did not directly assess the causal relationship between CRP and CVDs, either. These DAGs suggest that animal experiments and MR studies are more comparable to each other from a perspective of causal relationships, while traditional observational studies and clinical trials are more alike.
We believe that studies of different designs point to the same direction: CRP is more likely to be a bystander rather than a cause for CVDs. CRP is positively associated with an increased risk of CVDs due to common causes including upstream inflammatory activities. Also, it should be noted that a lack of evidence on the causal relationship between CRP and CVDs shall not preclude the use of CRP as a predictor for CVDs. What the role of CRP in the pathology of CVD is or whether CRP can be used as a therapeutic target for CVDs needs further examination in future studies.
Future directions
We want to emphasize that inflammation and immune dynamics are complex and our understanding of them is ever in flux. A major limitation of many studies on inflammation and CVDs is that the biomarkers currently used to gauge inflammation are crude and nonspecific. They reflect the downstream consequences of inflammatory activity, but do not provide information relating to the site(s) of activation and cannot be used to discriminate functionally important activation pathways. For example, while previous studies typically interpreted CRP as an inflammatory biomarker, this is not always true because it can also be elevated in the absence of a wider inflammatory response, suggesting the complex role that it plays in a range of biological processes.44 Therefore, one or two biomarkers cannot sufficiently represent the dynamic nature of inflammation and immune function. A systems-wide approach to assess inflammation and immune functions is needed for future studies. It is challenging but necessary to make measures of inflammation that are meaningful to the pathology of interest and which can guide specific and targeted therapies.
Conclusion
Our examination of available studies suggests that CRP is unlikely to be a cause for CVDs. The widely observed associations between CRP and CVDs are more likely to be explained by confounding in observational studies and by treatments in clinical trials. While CRP is a useful biomarker in CVD risk assessment, the use of it as an effective therapeutic target needs more evaluations.
Supplementary Material
Funding:
Chihua Li was supported by NIA R01AG070953 and R01AG075719.
Footnotes
Declaration of interests: All authors report no conflicts of interest.
References
- 1.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018;15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah T, Casas JP, Cooper JA, Tzoulaki I, Sofat R, McCormack V, et al. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol 2009;38:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strang F, Schunkert H. C-reactive protein and coronary heart disease: all said--is not it? Mediators Inflamm 2014;2014:757123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markozannes G, Koutsioumpa C, Cividini S, Monori G, Tsilidis KK, Kretsavos N, et al. Global assessment of C-reactive protein and health-related outcomes: an umbrella review of evidence from observational studies and Mendelian randomization studies. Eur J Epidemiol 2021;36:11–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 6.Genest J, McPherson R, Frohlich J, Anderson T, Campbell N, Carpentier A, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. Can J Cardiol 2009;25:567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers GL, Christenson RH, Cushman M, Ballantyne CM, Cooper GR, Pfeiffer CM, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice guidelines: emerging biomarkers for primary prevention of cardiovascular disease. Clin Chem 2009;55:378–384. [DOI] [PubMed] [Google Scholar]
- 8.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2010;122:e584–636. [DOI] [PubMed] [Google Scholar]
- 9.Jialal I, Devaraj S, Venugopal SK. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension 2004;44:6–11. [DOI] [PubMed] [Google Scholar]
- 10.Bisoendial RJ, Boekholdt SM, Vergeer M, Stroes ES, Kastelein JJ. C-reactive protein is a mediator of cardiovascular disease. Eur Heart J 2010;31:2087–2091. [DOI] [PubMed] [Google Scholar]
- 11.Anand SS, Yusuf S. C-reactive protein is a bystander of cardiovascular disease. Eur Heart J 2010;31:2092–2096. [DOI] [PubMed] [Google Scholar]
- 12.Nordestgaard BG, Zacho J. Lipids, atherosclerosis and CVD risk: is CRP an innocent bystander? Nutr Metab Cardiovasc Dis 2009;19:521–524. [DOI] [PubMed] [Google Scholar]
- 13.Koenig W C-reactive protein and cardiovascular risk: Will the controversy end after CANTOS? Clin Chem 2017;63:1897–1898. [DOI] [PubMed] [Google Scholar]
- 14.Casas JP, Shah T, Hingorani AD, Danesh J, Pepys MB. C-reactive protein and coronary heart disease: a critical review. J Intern Med 2008;264:295–314. [DOI] [PubMed] [Google Scholar]
- 15.Avan A, Tavakoly Sany SB, Ghayour-Mobarhan M, Rahimi HR, Tajfard M, Ferns G. Serum C-reactive protein in the prediction of cardiovascular diseases: Overview of the latest clinical studies and public health practice. J Cell Physiol 2018;233:8508–8525. [DOI] [PubMed] [Google Scholar]
- 16.Fu Y, Wu Y, Liu E. C-reactive protein and cardiovascular disease: From animal studies to the clinic (Review). Exp Ther Med 2020;20:1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul A, Ko KW, Li L, Yechoor V, McCrory MA, Szalai AJ, et al. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation 2004;109:647–655. [DOI] [PubMed] [Google Scholar]
- 18.Wang CH, Li SH, Weisel RD, Fedak PW, Dumont AS, Szmitko P, et al. C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation 2003;107:1783–1790. [DOI] [PubMed] [Google Scholar]
- 19.Hirschfield GM, Gallimore JR, Kahan MC, Hutchinson WL, Sabin CA, Benson GM, et al. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A 2005;102:8309–8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tennent GA, Hutchinson WL, Kahan MC, Hirschfield GM, Gallimore JR, Lewin J, et al. Transgenic human CRP is not pro-atherogenic, pro-atherothrombotic or pro-inflammatory in apoE−/− mice. Atherosclerosis 2008;196:248–255. [DOI] [PubMed] [Google Scholar]
- 21.Koike T, Kitajima S, Yu Y, Nishijima K, Zhang J, Ozaki Y, et al. Human C-reactive protein does not promote atherosclerosis in transgenic rabbits. Circulation 2009;120:2088–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovacs A, Tornvall P, Nilsson R, Tegnér J, Hamsten A, Björkegren J. Human C-reactive protein slows atherosclerosis development in a mouse model with human-like hypercholesterolemia. Proc Natl Acad Sci U S A 2007;104:13768–13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendall MA, Patel P, Ballam L, Strachan D, Northfield TC. C reactive protein and its relation to cardiovascular risk factors: a population based cross sectional study. BMJ 1996;312:1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB Sr., Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation 2004;110:380–385. [DOI] [PubMed] [Google Scholar]
- 25.Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 2012;367:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004;350:1387–1397. [DOI] [PubMed] [Google Scholar]
- 27.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med 2009;151:483–495. [DOI] [PubMed] [Google Scholar]
- 28.Kremers B, Wübbeke L, Mees B, Ten Cate H, Spronk H, Ten Cate-Hoek A. Plasma biomarkers to predict cardiovascular outcome in patients with peripheral artery disease: A systematic review and meta-Analysis. Arterioscler Thromb Vasc Biol 2020;40:2018–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen J, Ordovas JM. Impact of genetic and environmental factors on hsCRP concentrations and response to therapeutic agents. Clin Chem 2009;55:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC, Peden JF, et al. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 2009;302:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wensley F, Gao P, Burgess S, Kaptoge S, Di Angelantonio E, Shah T, et al. Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ 2011;342:d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008;359:1897–1908. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005;352:20–28. [DOI] [PubMed] [Google Scholar]
- 34.Rymer JA, Newby LK. Failure to launch: targeting inflammation in acute coronary syndromes. JACC Basic Transl Sci 2017;2:484–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2018;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose Colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 39.Tennant PWG, Murray EJ, Arnold KF, Berrie L, Fox MP, Gadd SC, et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: review and recommendations. Int J Epidemiol 2021;50:620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Relton CL, Davey Smith G. Mendelian randomization: applications and limitations in epigenetic studies. Epigenomics 2015;7:1239–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014;23:R89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blaum C, Brunner FJ, Kröger F, Braetz J, Lorenz T, Goßling A, et al. Modifiable lifestyle risk factors and C-reactive protein in patients with coronary artery disease: Implications for an anti-inflammatory treatment target population. Eur J Prev Cardiol 2021;28:152–158. [DOI] [PubMed] [Google Scholar]
- 43.Waldeyer C, Brunner FJ, Braetz J, Ruebsamen N, Zyriax BC, Blaum C, et al. Adherence to Mediterranean diet, high-sensitive C-reactive protein, and severity of coronary artery disease: Contemporary data from the INTERCATH cohort. Atherosclerosis 2018;275:256–261. [DOI] [PubMed] [Google Scholar]
- 44.Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun 2018;70:61–75. [DOI] [PubMed] [Google Scholar]
- 45.Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation 2005;112:25–31. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z, Mo J, Xu J, Wang A, Dai L, Cheng A, et al. Effects of individual and integrated cumulative burden of blood pressure, glucose, low-density lipoprotein cholesterol, and C-reactive protein on cardiovascular risk. Eur J Prev Cardiol 2022;29:127–135. [DOI] [PubMed] [Google Scholar]
- 47.Wang K, Shi X, Zhu Z, Hao X, Chen L, Cheng S, et al. Mendelian randomization analysis of 37 clinical factors and coronary artery disease in East Asian and European populations. Genome Med 2022;14:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


