Abstract
Background
A growing body of literature examines the relationship between peripheral immune function and Alzheimer’s Disease (AD) in human populations. Our living systematic review summarizes the characteristics and findings of these studies, appraises their quality, and formulates recommendations for future research.
Methods
We searched the electronic databases PubMed, PsycINFO, and Web of Science, and reviewed references of previous reviews and meta-analyses to identify human studies examining the relationship between any peripheral immune biomarkers and AD up to September 7th, 2023. We examined patterns of reported statistical associations (positive, negative, and null) between each biomarker and AD across studies. Evidence for each biomarker was categorized into four groups based on the proportion of studies reporting different associations: corroborating a positive association with AD, a negative association, a null association, and presenting contradictory findings. A modified Newcastle–Ottawa scale (NOS) was employed to assess the quality of the included studies.
Findings
In total, 286 studies were included in this review. The majority were cross-sectional (n=245, 85.7%) and hospital-based (n=248, 86.7%), examining relationships between 187 different peripheral immune biomarkers and AD. Cytokines were the most frequently studied group of peripheral immune biomarkers. Evidence supported a positive association with AD for six biomarkers, including IL-6, IL-1β, IFN-γ, ACT, IL-18, and IL-12, and a negative association for two biomarkers, including lymphocytes and IL-6R. Only a small proportion of included studies (n=22, 7.7%) were deemed to be of high quality based on quality assessment.
Interpretation
Existing research on peripheral immune function and AD exhibits substantial methodological variability and limitations, with a notable lack of longitudinal, population-based studies investigating a broad range of biomarkers with prospective AD outcomes. The extent and manner in which peripheral immune function can contribute to AD pathophysiology remain open questions. Given the biomarkers that we identified to be associated with AD, we posit that targeting peripheral immune dysregulation may present a promising intervention point to reduce the burden of AD.
Keywords: Peripheral immune function, Alzheimer’s Disease, systematic review, quality assessment
INTRODUCTION
Dementia is a growing global health crisis, with Alzheimer’s disease (AD) accounting for 60–70% of cases.1,2 Peripheral immunosenescence is increasingly recognized as a risk factor for brain health and AD development.3–6 Peripheral immunosenescence refers to a set of changes that occur in the peripheral immune system including increasing chronic-low inflammation (i.e., inflammaging), an inversion of the CD4+:CD8+ T cells ratios, a decrease in naïve T cells, and an accumulation of effector memory T cells with limited function. These changes are often observed as individuals age but can also be observed in response to a variety of social stressors.7–10 It was previously thought that only immune dysfunction occurring in the central nervous system (CNS) was relevant to the etiology of AD.11–18 This belief stemmed from the longstanding consideration of the peripheral and central immune systems being separated by the blood-brain barrier (BBB) and blood-cerebrospinal fluid (CSF) barrier, respectively. However, accumulating evidence suggests that peripheral immune function, defined as systemic immune signals originating outside the brain, also plays both direct and indirect roles in AD pathogenesis and progression.19–22 For example, peripheral immune cells can reside at the boundaries of the brain and actively participate in brain homeostasis through immune surveillance; they can also enter the CNS especially when the BBB is perturbated or impaired—often related to pathogen exposure and other stressors.23–34 These developments foster a growing awareness of viewing AD as a systemic condition that involves dynamic and interactive processes in both central and peripheral immune systems (Figure 1).19
Figure 1. Theoretical mechanistic framework depicting the hypothesized relationship between peripheral and central immune dysregulation and neurophysiology.
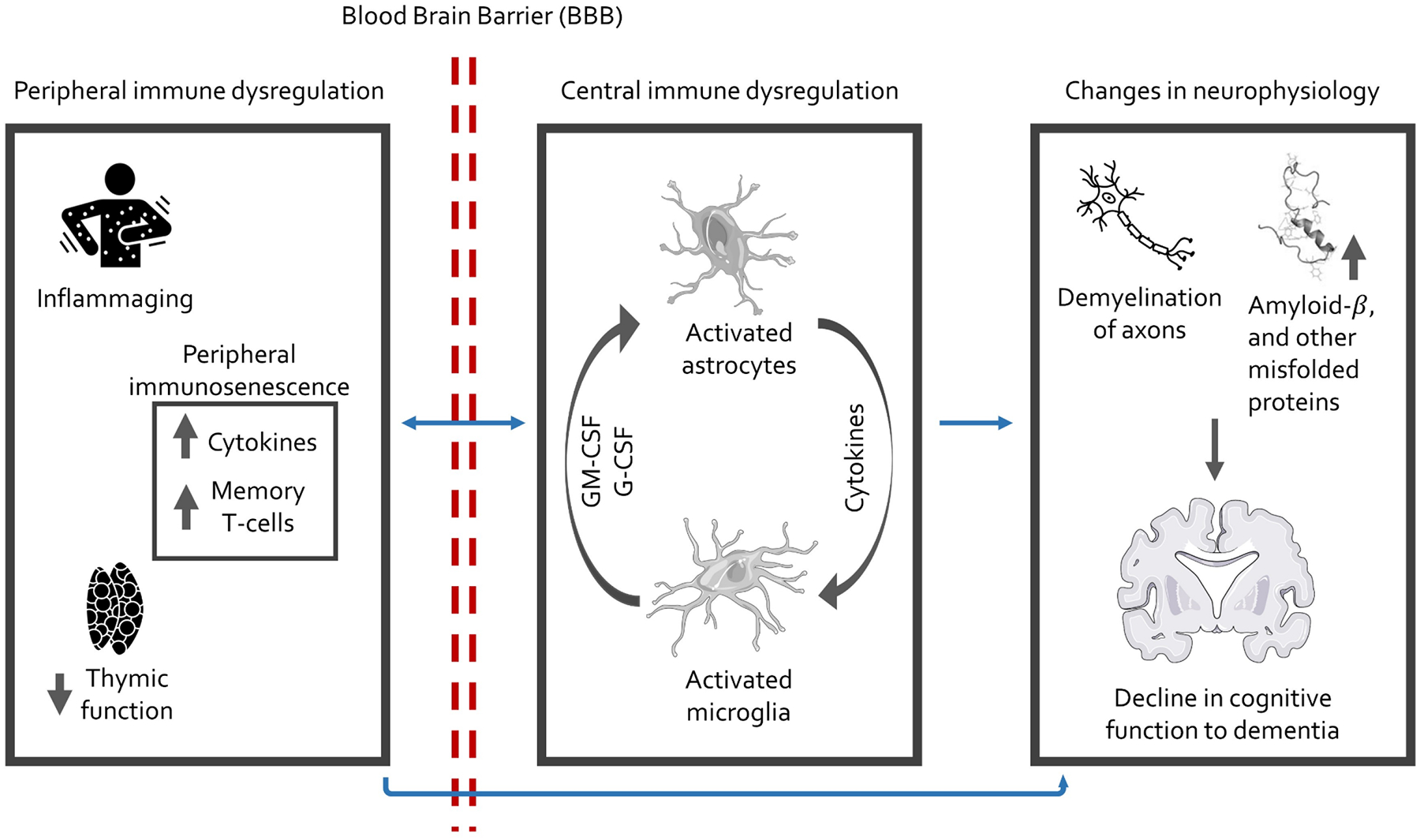
Inflammaging refers to the changes that take place in the innate immune system, distinct from the adaptive immune system, due to the aging process. This typically manifests as a rise in the levels of circulating pro-inflammatory cytokines as individuals age.
Several marked changes in peripheral immune function, occurring with aging, potentially contribute to the development of AD and are worth noting. In the innate immune system, decreased leukocyte production and function, reduced phagocytic capability of neutrophils, monocytes, and macrophages, a functional shift towards a proinflammatory phenotype in monocytes, and decreased cytotoxicity of natural killer cells are observed.35 In the adaptive immune compartment, antigenic stimulation across the life course drives the expansion of memory and effector T cell subsets increasing the number of pathogen-specific, highly differentiated T cells, which also generate a heightened inflammatory response.36,37 The thymus gland, key in the adaptive immune response, undergoes chronic involution after puberty, resulting in a continual decline in thymic output of naïve T cells with age.38 These processes coalesce to form a phenotype of immunosenescence in older age and contribute to AD development.24,39
Despite the progress mentioned above, the importance of the peripheral immune-AD relationship remains underappreciated, and many important questions need to be answered.19,24 For example, while some studies have reported evidence that patients with AD have signs of advanced peripheral immunosenescence compared to non-AD controls, it is unclear whether the advanced immunosenescence is a cause or consequence of AD, highlighting the need for longitudinal studies.30,40 Furthermore, the lack of population-based studies makes it challenging to evaluate whether findings of clinically-based studies are generalizable to the broader population and to examine how major social determinants, including race/ethnicity, sex/gender, and socioeconomic status, may modify the peripheral immune-AD relationship.41,42 Addressing these questions will contribute to understanding the development of AD, identifying diagnosis and treatment tools, and developing population-wide prevention strategies.
There are many reviews on peripheral immune function and AD. Some focus on animal and in vitro studies,23,24,26,43–47 while others center on single or few peripheral immune biomarkers.20,48–62 However, none have comprehensively documented and evaluated human studies on peripheral immune function and AD. To address these knowledge gaps, we conducted this review. Specifically, we summarize the characteristics and findings of human studies on peripheral immune biomarkers and AD and critically appraise these studies from the perspective of study designs and epidemiological principles. Furthermore, we have developed a publicly accessible online database, providing a living update of related studies to serve as a reference for researchers interested in the role of peripheral immune function in AD. The database can be accessed at the GitHub repository (https://github.com/Peripheral-immune-AD/Peripheral-immune-and-AD).
METHODS
A living systematic review (LSR) is an approach that aims to regularly update a review and incorporate new evidence as it becomes available.63,64 This approach is particularly suitable for synthesizing literature related to the rapidly growing body of studies examining the relationship between peripheral immune function and Alzheimer’s Disease (AD) among human populations.
Accordingly, this review will undergo biannual updates to integrate emerging evidence.
The study protocol, registered on the Research Registry (UIN reviewregistry1487), is provided in Supplementary Text 1. The protocol aimed to synthesize human studies on peripheral immune function and Alzheimer’s Disease and related dementias (ADRD), including both AD and non-AD dementia. However, given the substantial volume of eligible studies and the complexity of various dementia types, this review narrowed its focus primarily to AD, the most prevalent type of dementia. Studies concerning peripheral immune function and non-AD dementia will be synthesized in a separate review to delineate detailed findings. Therefore, the main outcome of interest in this review is AD. Studies on all-cause dementia are also included if the type of dementia was not specified because AD accounts for 60–70% of all dementia cases. The main exposure of interest is measurable indicators within the peripheral immune system that can provide information about immune function and inflammation. These include various direct and derived measures of cells, proteins, cytokines, and antibodies that play a role in immune responses. Reporting of this review followed the Preferred Reporting Items for Systematic Reviewers and Meta-analysis (PRISMA) guidelines (Supplementary Table 1). An institutional ethics review was not sought because the current review relied on the secondary use of reported data.
Search strategy and selection criteria
Our search strategy was designed to identify any studies relating peripheral immune function to AD. First, three electronic databases were searched, including PubMed, PsycINFO, and Web of Science. The following broad search terms were used: ((inflammation OR inflammatory OR interleukin OR lymphocyte OR acute phase protein OR cytokine OR immunity OR immune cell OR immune function OR immunosenescence OR immune dysregulation OR immune regulation) AND (cognition OR cognitive decline OR cognitive change OR cognitive aging OR dementia OR Alzheimer’s disease OR Alzheimer’s disease and related dementias OR ADRD)) AND human. The initial search was conducted in March 2018, and updated searches were performed in August 2019, September 2022, and September 2023. Second, 46 relevant reviews and/or meta-analyses were identified, and their reference lists and supplementary materials were screened for additional studies (Supplementary Table 2). Detailed search process and results can be found in Supplementary Text 2.
Eligibility criteria
Studies meeting the following criteria were included: (1) the study was an observational study based on human populations; (2) AD was the main outcome of interest; (3) peripheral immune function was the main or secondary risk factor of interest, which was measured by peripheral blood biomarkers participated in immune responses; (4) the study examined the relationship between peripheral immune biomarkers and AD; (5) the study was reported in English. Studies were excluded if: (1) the study was published as a conference abstract, commentary, protocol, or review article; (2) the study did not provide clear descriptions of the study population, study design, and analytical method; (3) the study had a total sample size of fewer than 20 subjects; (4) the study reported stimulated levels of peripheral immune biomarkers in vitro. When several studies were based on the same or overlapping study participants and they studied the same peripheral immune biomarkers, the study that had the largest sample size will be used as a representative study. However, if these studies examined different peripheral immune biomarkers, each of them would be included as an independent study even though they used the same or overlapping study participants.
Screening process and data extraction
At least two reviewers independently screened the title and abstract of each study for full-text examination. Two reviewers further conducted the full-text examination to evaluate if the study met the eligibility criteria. Any disagreements among reviewers were resolved by reaching an agreement through group discussion. For articles meeting the eligibility criteria, the following information was extracted by a single reviewer and examined by another reviewer: (1) author and publication information (author names, publication year, and PMID if available); (2) study characteristics (study setting, follow-up time, total sample size, number of AD cases, AD diagnosis tool, control selection, assessment tool of peripheral immune biomarkers, covariate assessment, main analytical method); (3) study participants characteristics by comparison groups (age, sex, education, race/ethnicity, severity of AD); (4) study results (association estimates and measures of uncertainty if available).
Quality assessment
Following the method used in our previous studies, the Newcastle–Ottawa scale (NOS) was customized from the perspective of study designs and epidemiological principles to evaluate the quality of included studies (Supplementary Text 3).65–67 Two reviewers appraised each study based on the NOS independently, and discrepancies were resolved through group discussion. The NOS had ten domains: sampling representativeness, sample size, follow-up, AD assessment, laboratory methods, biomarker assessment, comparison selection, statistical methods, confounding adjustment, and finding report. The quality of each domain was scored as ‘good’ (2), ‘fair’ (1), or ‘poor’ (0), and a total score was calculated for each study (range: 0–20). With this quality assessment tool, we aimed to identify studies with top total scores and examine whether any patterns of study quality by domains may exist. For ease of description, we further classified studies into three groups based on their total quality score. Cutoffs of classification were selected based on intervals of equal distance. A study with a total score of 14 and over was classified as of ‘high’ quality, a score of 7–13 was classified as of ‘medium’ quality, and a score of 0–6 was classified as of ‘low’ quality.
Synthesis of the evidence and data sharing
We summarized the characteristics of the included studies. We documented all peripheral immune biomarkers reported across studies. For each peripheral immune biomarker, we examined the pattern of reported findings by summarizing the number and proportion of studies reporting a positive, null, or negative association separately.
We selected the top 20 most examined peripheral immune biomarkers and classified the available evidence into the following four categories: Category 1. evidence corroborating a positive association with AD: 25% or more of studies reported a positive association with AD and less than 10% of studies reported a negative association with AD; Category 2. evidence corroborating a negative association with AD: 25% or more of studies reported a negative association with AD and less than 10% of studies reported a positive association with AD; Category 3. little or no evidence of an association with AD: less than 25% of studies reported either a positive or negative association; Category 4. contradictory evidence of an association with AD: 25% or more of studies reported a positive association and more than 10% reported a negative association. We determined these cut-off points based on previous related reviews and meta-analyses, finding that a quarter and more of a body of research tends to be a strong driver of an association in meta-analyses.20,50,53,54,56,68–71 We also classified the available evidence for other less-studied biomarkers. However, the results were less conclusive due to the limited number of studies for each. We deposited all data and code necessary to reproduce the findings of this study in the GitHub repository. The repository is publicly accessible and will be updated biannually together with this review.
RESULTS
The search of electronic databases and relevant reviews identified 15,670 records, and the removal of 7,984 duplicates yielded 7,686 unique records (Figure 2). After the screening of their titles and abstracts, 658 studies underwent full-text examination. In total, 286 studies examining the relationship between peripheral immune function and AD met eligibility criteria and were included in this living systematic review (LSR). Due to the large number of these studies, a full list of included studies with publication information was summarized in Supplementary Table 3.
Figure 2.
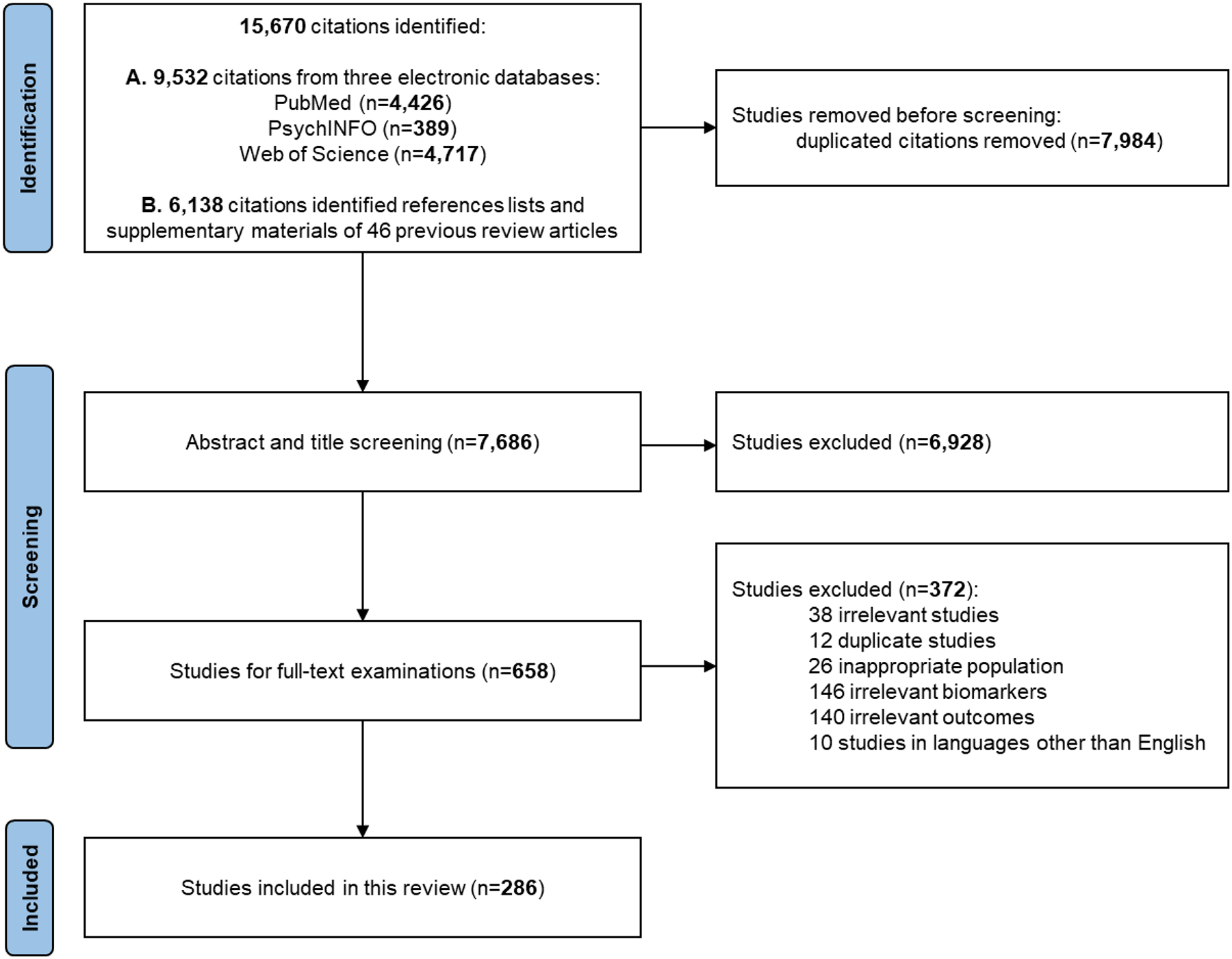
Study selection
Table 1 summarizes selected characteristics of the 286 included studies based on whether they had a follow-up of participants or not. Among them, 245 (85.7%) studies had no follow-up, and the remaining 41 (14.3%) studies did have a follow-up. The number of studies published in each decade increased from 8 studies in the 1980s to 117 studies in the 2010s, and there have been 49 studies published in the first few years of this decade. Studies were predominantly single-hospital (n=214, 74.8%) or multiple-hospital (n=34, 11.9%) studies. Over 90% of them were conducted in Europe, Asia, and North America. Most studies had a sample size of fewer than 100 participants (n=158, 55.2%) or between 100 and 999 participants (n=107, 37.4%). The most used AD diagnosis tools included NINCDS-ADRDA (n=207, 72.4%) and DSM-III/IV (n=89, 31.1%). Two hundred seventy-four studies specified the number of AD cases, while the remaining 12 studies included all-cause dementia without detailing the types or numbers of different dementia cases. Only 42 (14.7%) studies examined the severity of AD, and the evaluation criteria varied by study. Serum and/or plasma samples were both widely used, and 272 (95.1%) studies specified assays and procedures used to examine peripheral immune biomarkers. The three most widely reported sociodemographic characteristics included age, gender, and education. Most of these studies included participants aged 60 years and older, and only 36 (12.6%) studies included participants younger than 55 years old. Detailed characteristics of each study can be found in Supplementary Table 4.
Table 1.
Selected characteristics of included studies
| No. of studies by study designs | |||
|---|---|---|---|
| All | Without follow-up | With follow-up | |
| N | 286 | 245 | 41 |
| Peripheral immune biomarkers as primary exposure of interest, n (%) | |||
| Yes | 258 (90.2) | 224 (91.4) | 34 (82.9) |
| No | 28 (9.8) | 21 (8.6) | 7 (17.1) |
| Publication period, n (%) | |||
| 1980s and before | 8 (2.8) | 8 (3.3) | 0 (0.0) |
| 1990–1999 | 31 (10.8) | 31 (12.7) | 0 (0.0) |
| 2000–2009 | 81 (28.3) | 72 (29.4) | 9 (22.0) |
| 2010–2019 | 117 (40.9) | 102 (41.6) | 15 (36.6) |
| 2020 and after | 49 (17.1) | 32 (13.1) | 17 (41.5) |
| Sampling representativeness, n (%) | |||
| Single-hospital | 214 (74.8) | 206 (84.1) | 8 (19.5) |
| Multi-hospital | 34 (11.9) | 28 (11.4) | 6 (14.6) |
| Regional | 33 (11.5) | 8 (3.3) | 25 (61.0) |
| National | 5 (1.7) | 3 (1.2) | 2 (4.9) |
| Study population by region, n (%) | |||
| Asia | 53 (18.5) | 47 (19.2) | 6 (14.6) |
| Europe | 152 (53.1) | 135 (55.1) | 17 (41.5) |
| North America | 54 (18.9) | 38 (15.5) | 16 (39.0) |
| Others * | 27 (9.4) | 25 (10.2) | 2 (4.9) |
| Total sample size, n (%) | |||
| 20–99 | 158 (55.2) | 152 (62.0) | 6 (14.6) |
| 100–999 | 107 (37.4) | 90 (36.7) | 17 (41.5) |
| 1000 and over | 21 (7.3) | 3 (1.2) | 18 (43.9) |
| AD assessment # , n (%) | |||
| NINCDS-ADRDA | 207 (72.4) | 186 (75.9) | 21 (51.2) |
| DSM-III or IV | 89 (31.1) | 76 (31.0) | 13 (31.7) |
| ICD-9 or 10 | 11 (3.8) | 3 (1.2) | 8 (19.5) |
| Others | 25 (8.7) | 21 (8.6) | 4 (9.8) |
| Not reported | 25 (8.7) | 21 (8.6) | 4 (9.8) |
| AD severity examined, n (%) | |||
| Yes | 42 (14.7) | 40 (16.3) | 2 (4.9) |
| No | 244 (85.3) | 205 (83.7) | 39 (95.1) |
| Biomarker sample source, n (%) | |||
| Serum | 121 (42.3) | 108 (44.1) | 13 (31.7) |
| Plasma | 113 (39.5) | 101 (41.2) | 12 (29.3) |
| Serum and plasma | 46 (16.1) | 32 (13.1) | 14 (34.1) |
| Not reported | 6 (2.1) | 4 (1.6) | 2 (4.9) |
| Assay type reported, n (%) | |||
| Yes | 272 (95.1) | 238 (97.1) | 34 (82.9) |
| No | 14 (4.9) | 7 (2.9) | 7 (17.1) |
| Mean age of AD cases, n (%) | |||
| Middle-aged (<65 years) | 12 (4.2) | 10 (4.1) | 2 (4.9) |
| Youngest-old (65–74 years) | 112 (39.2) | 103 (42.0) | 9 (22.0) |
| Middle-old (75–84 years) | 117 (40.9) | 106 (43.3) | 11 (26.8) |
| Oldest-old (85 years) | 2 (0.7) | 2 (0.8) | 0 (0.0) |
| Not reported | 43 (15.0) | 24 (9.8) | 19 (46.3) |
| Other sociodemographic characteristics examined, n (%) | |||
| Gender | 236 (82.5) | 205 (83.7) | 31 (75.6) |
| Education | 93 (32.5) | 68 (27.8) | 25 (61.0) |
| Race/ethnicity | 31 (10.8) | 22 (9.0) | 9 (22.0) |
| None reported | 39 (13.6) | 35 (14.3) | 4 (9.8) |
Others include Africa, Australia, and South America
Some studies used more than one AD assessment tool
In total, relationships between 187 different peripheral immune biomarkers and AD were examined across 286 studies. These biomarkers mainly included adaptive immune cells (e.g., T and B cells), cytokines, and complement proteins. Cytokines were the most frequently studied group of peripheral immune biomarkers in relation to AD, especially those involved in inflammatory responses. A list of full and abbreviated names of these biomarkers is presented in Supplementary Table 5. The number of peripheral immune biomarkers examined in each study varied, ranging from one to 38. Figure 3A shows that 154 (53.8%) out of 286 studies examined the relationship between one or two peripheral immune biomarkers and AD, 83 (29.0%) studies examined three to five biomarkers, and 49 (17.1%) studies examined more than five biomarkers.
Figure 3. Summary of reported findings across included studies.
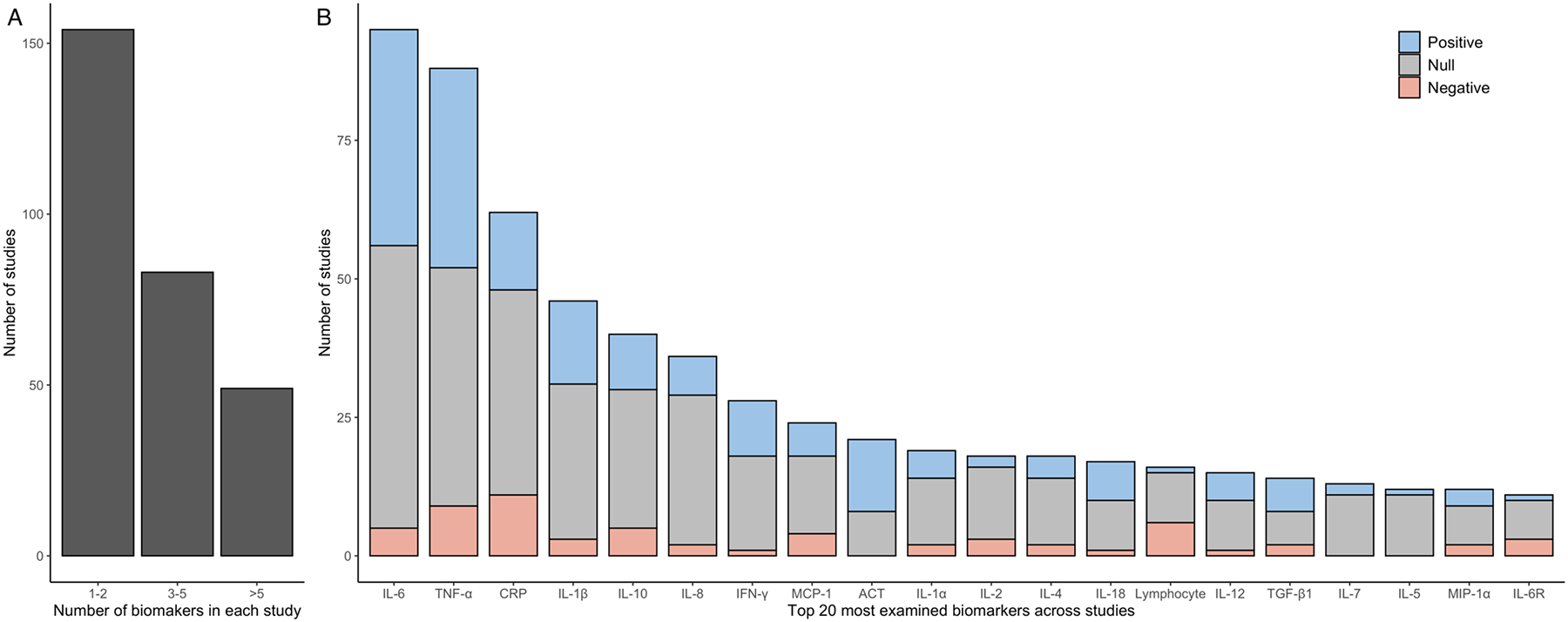
Panel A displays the number of studies that examined between one and two, three and five, or more than five peripheral immune biomarkers, underscoring that most existing studies have only investigated a limited number of such biomarkers. Panel B depicts the number of studies and their reported associations for the top 20 most frequently examined peripheral immune biomarkers. Different colors were used for studies based on their reported statistical associations with AD. Evidence supports either a positive or a negative association with AD for eight of these biomarkers, including IL-6, IL-1β, IFN-γ, ACT, IL-18, IL-12, lymphocyte, and IL-6R.
Figure 3B shows the number of studies for the top 20 most examined peripheral immune biomarkers, ranging from around 10 to over 90 studies for each biomarker. It also shows the pattern of reported associations between each biomarker and AD. The proportion of positive, null, and negative associations varied substantially by biomarkers. Evidence corroborating a positive association with AD was observed for six biomarkers: IL-6, IL-1β, IFN-γ, ACT, IL-18, and IL-12 (Supplementary Table 6). For these six biomarkers, 25% or more of the included studies reported a positive association with AD and less than 10% of studies reported a negative association with AD. Evidence corroborating a negative association with AD was observed for lymphocyte and IL-6R, with 25% or more of included studies reporting a negative association with AD and less than 10% of studies reporting a positive association with AD. Reported results for these eight biomarkers corroborating either a positive or negative association with AD across included studies were summarized in Supplementary Table 7. Four biomarkers showed little or no evidence of an association with AD because less than 25% or more of studies reported either a positive or negative association for them. These included IL-8, IL-2, IL-7, and IL-15. Seven biomarkers, TNF-α, CRP, IL-10, MCP-1, IL-1α, IL-4, and TGF-β1, had contradictory evidence, which had over 25% of studies reporting a positive association and over 10% of studies reporting a negative association.
In addition, studies that reported a null association were less likely to provide effect estimates and measures of uncertainty compared to studies that reported statistically significant results of either a positive or negative association (Supplementary Table 8). For example, among 44 studies that reported either a significant positive or negative association between IL-6 and AD, 33 (75.0%) studies provided effect estimates and measures of uncertainty; in contrast, among 51 studies that reported a null association between IL-6 and AD, only 29 (56.9%) studies provided effect estimates and measures of uncertainty. Supplementary Figure 1 describes patterns of reported associations between AD and all 187 peripheral immune biomarkers as Figure 3B. A detailed summary of reported findings by peripheral immune biomarkers in each study can be found in the GitHub repository.
Figure 4A shows quality assessment results for each study by domain based on the customized NOS. It shows that only 22 (7.7%) out of 286 included studies received a total score of 14 and over, which were classified as high quality.28,72–92 These 22 studies generally were scored as good in most domains. Figure 4B summarizes the number of studies by total quality score (score range: 0–20). The remainder of them were either of medium quality (n=200, 69.9%) or low quality (n=64, 22.4%). Figure 4C further summarizes the proportion of studies scored as good (2), fair (1), and poor (0) for each domain. Most studies scored good or fair in four domains, including AD assessment, laboratory methods, statistical methods, and finding report. In contrast, over 55% to almost 90% of studies received a score of poor in the other six domains: sampling representativeness, sample size, follow-up, biomarker assessment, comparison selection, and confounding adjustment.
Figure 4. Quality assessment of included studies on peripheral immunity and AD.
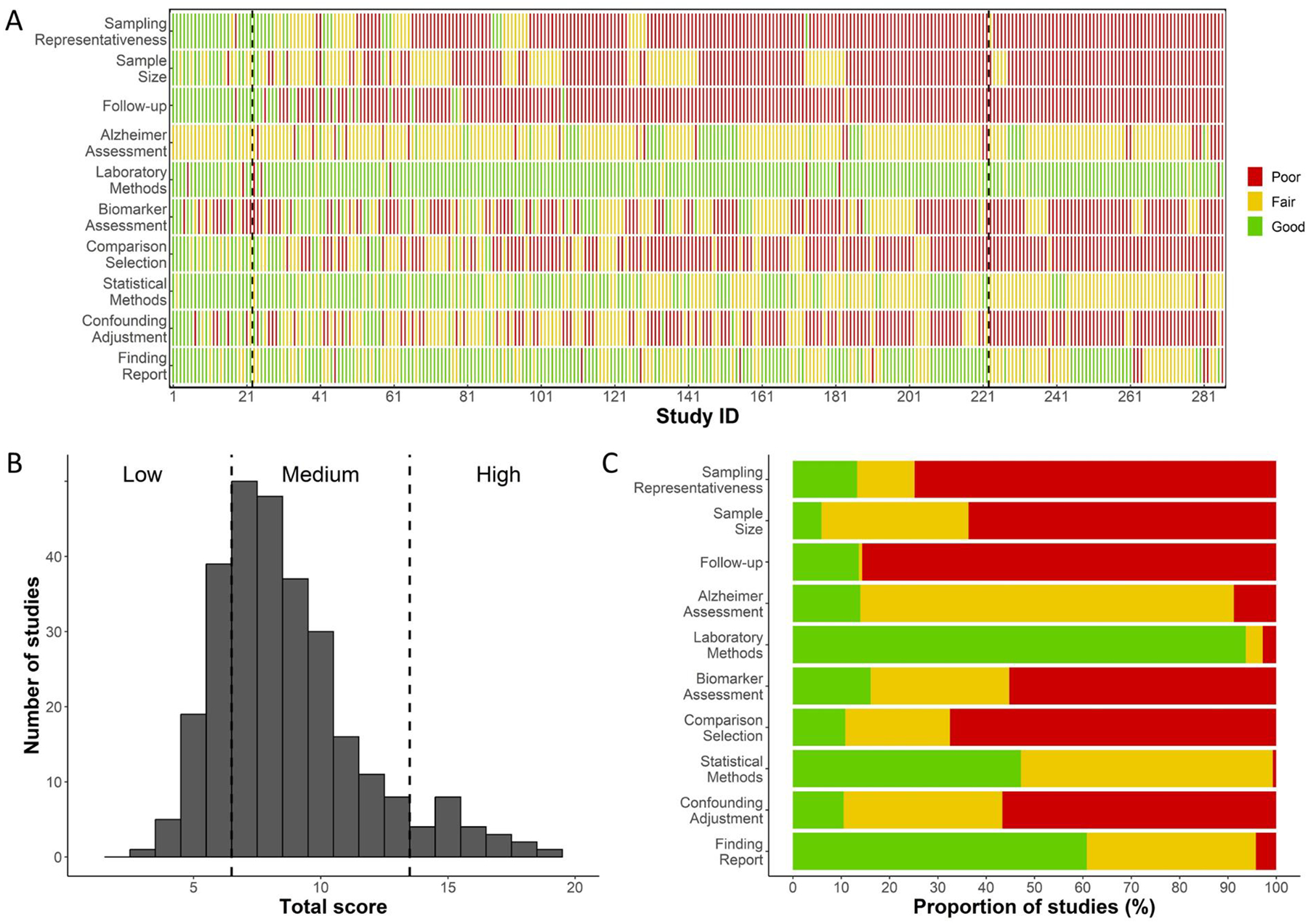
Panel A presents the detailed quality assessment results for all included studies. Studies are arranged based on their total quality assessment score, following the order documented in Supplementary Tables 3 and 4. Dashed vertical lines demarcate studies of low, medium, and high quality. This panel illustrates clusters of studies that have been scored as good, fair, or poor across different quality assessment domains. Panel B showcases the number of studies by their total quality assessment score, emphasizing the limited number of high-quality studies (n=22, 7.7%). Panel C outlines the proportion of studies that received scores of good, fair, or poor in each domain. Notably, sampling representativeness and follow-up are the two domains where the majority of studies received a score of poor.
Discussion
Our living systematic review (LSR) provides, to date, the most comprehensive overview of human studies on the relationship between peripheral immune function and AD. As of September 2023, at least 286 human studies have examined the relationships between peripheral immune biomarkers and AD. These studies were predominantly single- or multiple-hospital-based, typically did not include participant follow-ups and investigated a limited number of biomarkers. They also had substantial methodological variability in other aspects, and only 7.7% of them were deemed of high quality based on the modified Newcastle–Ottawa scale. Nonetheless, we identified a select group of immune biomarkers that showed moderately strong evidence of an association with AD. This highlights the potential of targeting peripheral immune dysregulation to reduce the burden of AD.
We found that 187 peripheral immune biomarkers have been examined in relation to AD across the included studies. We identified the most frequently examined biomarkers and examined patterns of reported findings. While a majority of studies reported a null association, seven biomarkers showed a relatively consistent positive or negative association with AD, including IL-6, IL-1β, IFN-γ, ACT, IL-18, IL-12, lymphocyte, and IL-6R. These biomarkers represent promising targets for future research. Multiple previous systematic reviews and meta-analyses have claimed a significant relationship between many of the biomarkers reviewed here and AD.20,49,50,52–54,56,68–71 However, there are also some discrepancies comparing our findings to previous reviews. For example, we found only 22.6% of studies on CRP showed a positive association with AD. In contrast, among studies included in previous meta-analyses, the proportion of studies reporting a positive association between CRP and AD is higher, ranging from 25% to 50%.20,54,56,60,69,93
The reason for our contrasting findings may be related to methodological differences and the comprehensiveness of our review compared to earlier systematic reviews and meta-analyses.20,50,53,54,56,58–62,68–71 First, it may be that previous meta-analyses failed to conduct a comprehensive screening of the literature, especially those studies that reported a null association. For example, the four previous systematic reviews and meta-analyses that focused specifically on CRP and AD included only 10 or fewer studies.20,54,56,69 However, we identified 62 original studies that examined the relationship between CRP and AD. Using the GitHub repository we created, many more studies that were overlooked by previous meta-analyses can be identified for CRP and other peripheral immune biomarkers. Second, most previous meta-analyses did not evaluate biases associated with the studies and data sources when generating summary estimates.20,50,53,54,56,68–71 We found that studies reporting a null association between peripheral immune biomarkers and AD were much less likely to provide effect estimates and/or measures of uncertainty than studies reporting a statistically significant association (Supplementary Table 8). This latter concern was observed not only for CRP but also for other peripheral immune biomarkers. Therefore, a large proportion of studies reporting a null association were not included in prior meta-analyses. Similar to our approach, future reviews should include a more comprehensive sampling of the literature, as opposed to focusing only on meta-analytical eligible studies with published effect estimates.
In the quality assessment of studies included in this review, we determined that less than 5% were of high quality. This demonstrates the urgent need to improve the quality of studies investigating the peripheral immune-AD relationship. One of the key reasons that most included studies were of medium or low quality is that they were predominantly based on small clinical samples that did not follow participants over time. While small clinical samples are useful to establish preliminary evidence of an association, they lack population representativeness and statistical power to make broader conclusions about the hypothesized relationships. Furthermore, cross-sectional studies (i.e. those that lack follow-up) cannot establish the temporality required to infer any causal relationship.
Across the ten domains of quality assessment, we identified six that are most in need of improvement: sampling representativeness, sample size, follow-up, biomarker assessment, comparison selection, and confounding adjustment. These domains and recommendations to address these methodological problems are specified in Table 2. First, it is important to recruit a study sample that is representative of the broader target population (i.e., the researcher’s target population, whatever that might be based on the research question), follow these recruited participants over time, and collect data on a broad range of peripheral immune biomarkers. Additionally, recruiting participants in early or mid-adulthood and tracking them longitudinally is essential. This approach will illuminate the timing of changes in these biomarkers and how they contribute to AD development. These design improvements will help answer many important research questions and establish the temporal sequence of the exposure-outcome relationship. For example, it remains unclear to what extent peripheral immunosenescence reflects normal aging or progression of AD. Ongoing studies in the U.S., such as the Health and Retirement Study (HRS) and the National Longitudinal Study of Adolescent to Adult Health (AddHealth), are particularly suited for this exploration. They possess the longitudinal framework to study dementia risk and a repository of biospecimens for testing multiple peripheral immune biomarkers.9,94,95 There are myriad additional research questions that could be explored and answered once the research community embraces these opportunities for the improvement of data sources in this area.
Table 2.
Key domains for improvement and recommendations for future research
| Quality assessment domain | Recommendation for future research | Rationale |
|---|---|---|
| Sampling representativeness | Conduct systematic sampling resulting in a study sample that is representative of a well-defined population | To be able to generalize results to the population and make inferences that will be borne out in real-world scenarios |
| Sample size | Prioritize larger study samples | To reduce random error in study results and to ensure representation of the wide range of biomarker levels in the population; to provide statistical power for investigation of biological and social interactions and effect modification |
| Follow-up | Invest in longitudinal studies that allow for investigation of trends in exposures and outcomes over time; invest in studies that enroll younger participants, thus allowing researchers to capture the onset of disease | Cross-sectional studies limit our ability to understand causal relationships and short-term or later-in-life cohorts are limited in the ability to detect the onset of dementia |
| Biomarker assessment | Employ a systems-wide approach to biomarker assessment and analysis; increase the number of biomarkers measured | The immune system is complex, and no single biomarker of immunity captures the dynamic processes underlying immune aging |
| Confounding adjustment | Craft a precise research question and select the appropriate covariate adjustment set based on a strong theoretical understanding of how the variables may relate to one another | Controlling for potential colliders and/or mediators can generate biased results |
In contrast, small clinical samples often hinder the interpretation of study findings, the generalizability of results to the target population, and the transportability of the results to other populations. For example, small clinically-based samples often differ from the broader population on several sociodemographic characteristics including age, sex, racial and ethnic makeup, and socioeconomic status.96,97 Differences in the relative proportions of these groups within the sample and target population can lead to estimates that are not true to the target population, particularly in the case where these sociodemographic characteristics may be modifiers of the exposure-outcome relationship in question. Designs of small clinical studies also made it challenging to have appropriate comparison selections and confounding adjustments.
Second, most of the included studies predominantly focused on a few inflammatory cytokines widely examined in social science research. It is important to look beyond these few biomarkers of inflammatory immune function, and we can do so by including comprehensive assays for other biomarkers of the peripheral immune compartment, such as T cell surface markers, in large, population-based studies. In the analysis stage, studies of the etiology of AD need to incorporate a more comprehensive mechanistic understanding of peripheral immune function. Immune dynamics are complex and our understanding of them is ever in flux. For example, while previous studies typically interpreted IL-6 and CRP as inflammatory biomarkers, this is not always the truth because they both can also be elevated in the absence of a wider inflammatory response, suggesting the complex role that these biomarkers play in a range of biological processes and different chronic conditions.57,98 Therefore, one or two biomarkers cannot sufficiently represent the dynamic nature of immune function. Indeed, peripheral immune function might best be captured by employing a systems-wide approach.
Furthermore, the majority of the existing literature focuses on biomarkers of innate immunity. However, the adaptive system, particularly the T cell compartment, undergoes critical changes as individuals age and in response to a number of social, environmental, and biological stressors.8 Antigenic stimulation across the life course drives the expansion of memory and effector T cell subsets, increasing the number of pathogen-specific, highly differentiated T cells which then generate a greater inflammatory response.36,37 Studies comparing the immune profile of individuals with AD to those of healthy controls have found evidence of advanced immunosenescence, particularly in the adaptive immune compartment, in the blood of individuals with AD, specifically decreases in naïve T cells and increases in memory T cells.30,40 This suggests that changes in the adaptive peripheral immune compartment may be critical biological intermediaries in the etiology of AD. As such, with the increasing ease of measuring these biomarkers, future studies should endeavor to include measures related to the adaptive immune system as well as the innate.
Additionally, while this review does not focus on viral pathogens, their potential role in the etiology of AD warrants further investigation. This is because multiple viral pathogens can burden the immune system and contribute to AD development. For example, emerging studies examining immune response to viral infections offer compelling evidence suggestive of a role of latent viral infections including CMV99 and HSV-1100–102 in AD, though a 2021 paper found no relationship between several latent infections and all-cause dementia incidence.88 A more recent extensive human study revealed a significant and meaningful association between the Epstein-Barr Virus (EBV) and Multiple Sclerosis (MS).103 This additional evidence highlights that the peripheral immune system and infections can have profound downstream clinical consequences on the central nervous system. Future studies should examine both the impact of having a latent viral infection and individuals’ immunological responses to such infections.
The strengths of our LSR are the comprehensive coverage of the body of existing studies, the careful synthesis of their reported findings and identification of related patterns, and the systematic evaluation of their study quality. Our publicly accessible database on GitHub will serve as a reference for clinicians and medical researchers who are interested in studying the peripheral immune-AD relationship. We also plan to update this LSR and related data online biannually as new information becomes available and expand our databases to include different types of non-AD dementias.
However, our study does have several limitations warranting careful consideration. First, we did not conduct any meta-analyses to produce summary estimates of the relationships between specific peripheral immune biomarkers and AD. We are exploring ways to quantify reported findings of included studies and address two main problems that were overlooked or poorly addressed in previous meta-analyses: selective reporting of findings and large heterogeneity across studies on peripheral immune function and AD. Second, our review included studies on both AD and all-cause dementia. Out of 286 studies, 12 reported findings on all-cause dementia without specifying the type, leading to potential misclassification of the outcome of interest. However, excluding these 12 studies would not alter our study’s main findings. Third, we focused on patterns of reported associations between peripheral immune biomarkers and AD across studies without considering their overall quality. Ideally, evaluations of reported findings across studies should weigh high-quality studies more than medium- and low-quality ones. However, due to the small number of high-quality studies, we observed no specific patterns when we stratified our analyses. We intend to update our analysis with emerging high-quality studies in the future. Fourth, our LSR included only studies published in English, and we identified 10 studies in other languages. Given the limited number of studies in other languages, we do not anticipate that including them would alter our main findings. We plan to incorporate these studies in future biannual updates.
In conclusion, we have identified the most extensively examined peripheral immune biomarkers in relation to AD. We found relatively consistent evidence of an association with AD for eight biomarkers: IL-6, IL-1β, IFN-γ, ACT, IL-18, IL-12, lymphocyte, and IL-6R. This suggests that these may be integral biomarkers associated with the development or pathology of AD. However, other biomarkers demonstrated less consistent associations with AD and even yielded contradictory evidence in the existing literature, suggesting that these biomarkers may not play a significant role in AD risk. Our review also highlights key methodological limitations in existing studies, the majority of which are clinical studies featuring small sample sizes and lacking longitudinal follow-up of participants. This work is timely given the rapidly growing interest in the peripheral immune system as a target for AD therapeutics. A deeper understanding of the causal roles that these consistently identified peripheral immune biomarkers have with AD may pave the way for the development of new diagnostic tools and medications for AD and other related neurogenerative diseases.
Supplementary Material
Acknowledgments
Chihua Li was supported by NIA R01AG070953 and R01AG075719; Grace A. Noppert was supported by NIA R00AG062749 and R01AG075719; Rebecca C. Stebbins and was supported by NIA R01AG075719; Allison E. Aiello was supported by NIA R01AG075719.
Footnotes
Declaration of interests
We declare no competing interests.
Data sharing
The data used in this study are all presented in the main tables or supplementary materials. Additional data and code to generate findings reported in this study are available from the GitHub repository (https://github.com/Peripheral-immune-AD/Peripheral-immune-and-AD).
REFERENCES
- 1.2021 Alzheimer’s disease facts and figures. Alzheimers Dement 2021; 17(3): 327–406. [DOI] [PubMed] [Google Scholar]
- 2.Collaborators GDF. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022; 7(2): e105–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawelec G, Solana R. Immunosenescence. Immunol Today 1997; 18(11): 514–6. [DOI] [PubMed] [Google Scholar]
- 4.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology 2007; 120(4): 435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pawelec G. Age and immunity: What is “immunosenescence”? Exp Gerontol 2018; 105: 4–9. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y, Zhan JK, Liu Y. A Perspective on Roles Played by Immunosenescence in the Pathobiology of Alzheimer’s Disease. Aging Dis 2020; 11(6): 1594–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noppert GA, Stebbins RC, Dowd JB, Hummer RA, Aiello AE. Life Course Socioeconomic Disadvantage and the Aging Immune System: Findings From the Health and Retirement Study. J Gerontol B Psychol Sci Soc Sci 2021; 76(6): 1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klopack ET, Crimmins EM, Cole SW, Seeman TE, Carroll JE. Social stressors associated with age-related T lymphocyte percentages in older US adults: Evidence from the US Health and Retirement Study. Proc Natl Acad Sci U S A 2022; 119(25): e2202780119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noppert GA, Stebbins RC, Dowd JB, Aiello AE. Socioeconomic and race/ethnic differences in immunosenescence: Evidence from the Health and Retirement Study. Brain Behav Immun 2022; 107: 361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noppert GA, Duchowny KA, Stebbins R, Aiello AE, Dowd JB, Clarke P. Biological expressions of early life trauma in the immune system of older adults. PLoS One 2023; 18(6): e0286141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 2012; 12(9): 623–35. [DOI] [PubMed] [Google Scholar]
- 12.Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol 2015; 36(10): 569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fakhoury M Role of immunity and inflammation in the pathophysiology of neurodegenerative diseases. Neurodegener Dis 2015; 15(2): 63–9. [DOI] [PubMed] [Google Scholar]
- 14.Rawji KS, Mishra MK, Michaels NJ, Rivest S, Stys PK, Yong VW. Immunosenescence of microglia and macrophages: Impact on the ageing central nervous system. Brain 2016; 139(3): 653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.VanItallie TB. Alzheimer’s Disease: Innate immunity gone awry? Metabolism 2017; 69S: S41–9. [DOI] [PubMed] [Google Scholar]
- 16.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s Disease. Alzheimers Dement (N Y) 2018; 4: 575–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasqualetti G, Brooks DJ, Edison P. The role of neuroinflammation in dementias. Curr Neurol Neurosci Rep 2015; 15(4): 17. [DOI] [PubMed] [Google Scholar]
- 18.Bright F, Werry EL, Dobson-Stone C, Piguet O, Ittner LM, Halliday GM, et al. Neuroinflammation in frontotemporal dementia. Nat Rev Neurol 2019; 15(9): 540–55. [DOI] [PubMed] [Google Scholar]
- 19.Bettcher BM, Tansey MG, Dorothée G, Heneka MT. Peripheral and central immune system crosstalk in Alzheimer disease - a research prospectus. Nat Rev Neurol 2021; 17(11): 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyama A, O’Brien J, Weuve J, Blacker D, Metti AL, Yaffe K. The role of peripheral inflammatory markers in dementia and Alzheimer’s Disease: A meta-analysis. J Gerontol A Biol Sci Med Sci 2013; 68(4): 433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris G, Berk M, Maes M, Puri BK. Could Alzheimer’s Disease originate in the periphery and if so how so? Mol Neurobiol 2019; 56(1): 406–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Luigi A, Pizzimenti S, Quadri P, Lucca U, Tettamanti M, Fragiacomo C, et al. Peripheral inflammatory response in Alzheimer’s disease and multiinfarct dementia. Neurobiol Dis 2002; 11(2): 308–14. [DOI] [PubMed] [Google Scholar]
- 23.Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci 2017; 20(2): 136–44. [DOI] [PubMed] [Google Scholar]
- 24.Cao W, Zheng H. Peripheral immune system in aging and Alzheimer’s disease. Mol Neurodegener 2018; 13(1): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korin B, Ben-Shaanan TL, Schiller M, Dubovik T, Azulay-Debby H, Boshnak NT, et al. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat Neurosci 2017; 20(9): 1300–9. [DOI] [PubMed] [Google Scholar]
- 26.Yang Q, Wang G, Zhang F. Role of peripheral immune cells-mediated inflammation on the process of neurodegenerative diseases. Front Immunol 2020; 11: 582825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer Disease and other neurodegenerative disorders. Nat Rev Neurol 2018; 14(3): 133–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang YR, Wang JJ, Chen SF, Wang HF, Li YZ, Ou YN, et al. Peripheral immunity is associated with the risk of incident dementia. Mol Psychiatry 2022; 27(4): 1956–62. [DOI] [PubMed] [Google Scholar]
- 29.Wu KM, Zhang YR, Huang YY, Dong Q, Tan L, Yu JT. The role of the immune system in Alzheimer’s Disease. Ageing Res Rev 2021; 70: 101409. [DOI] [PubMed] [Google Scholar]
- 30.Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s Disease. Nature 2020; 577(7790): 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altendorfer B, Unger MS, Poupardin R, Hoog A, Asslaber D, Gratz IK, et al. Transcriptomic Profiling Identifies CD8(+) T Cells in the Brain of Aged and Alzheimer’s Disease Transgenic Mice as Tissue-Resident Memory T Cells. J Immunol 2022; 209(7): 1272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng W, Zhang Y, Ding S, Chen S, Wang T, Wang Z, et al. B lymphocytes ameliorate Alzheimer’s disease-like neuropathology via interleukin-35. Brain Behav Immun 2023; 108: 16–31. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz-Castro C, Mejias-Ortega M, Sanchez-Mejias E, Navarro V, Trujillo-Estrada L, Jimenez S, et al. Monocyte-derived cells invade brain parenchyma and amyloid plaques in human Alzheimer’s disease hippocampus. Acta Neuropathol Commun 2023; 11(1): 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med 2015; 21(8): 880–6. [DOI] [PubMed] [Google Scholar]
- 35.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol 2013; 13(12): 875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging. Longev Healthspan 2013; 2(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol 2007; 211(2): 144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer DB. The effect of age on thymic function. Front Immunol 2013; 4: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heavener KS, Bradshaw EM. The aging immune system in Alzheimer’s and Parkinson’s diseases. Semin Immunopathol 2022; 44(5): 649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, et al. Occurrence of T cells in the brain of Alzheimer’s Disease and other neurological diseases. J Neuroimmunol 2002; 124(1–2): 83–92. [DOI] [PubMed] [Google Scholar]
- 41.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev 2008; 18(3): 223–54. [DOI] [PubMed] [Google Scholar]
- 42.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 2016; 12(3): 216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franco Bocanegra DK, Nicoll JAR, Boche D. Innate immunity in Alzheimer’s Disease: the relevance of animal models? J Neural Transm (Vienna) 2018; 125(5): 827–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Page A, Dupuis G, Frost EH, Larbi A, Pawelec G, Witkowski JM, et al. Role of the peripheral innate immune system in the development of Alzheimer’s Disease. Exp Gerontol 2018; 107: 59–66. [DOI] [PubMed] [Google Scholar]
- 45.Shi Y, Holtzman DM. Interplay between innate immunity and Alzheimer Disease: APOE and TREM2 in the spotlight. Nat Rev Immunol 2018; 18(12): 759–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webers A, Heneka MT, Gleeson PA. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s Disease. Immunol Cell Biol 2020; 98(1): 28–41. [DOI] [PubMed] [Google Scholar]
- 47.Rossi B, Santos-Lima B, Terrabuio E, Zenaro E, Constantin G. Common peripheral immunity mechanisms in multiple Sclerosis and Alzheimer’s Disease. Front Immunol 2021; 12: 639369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perry RT, Collins JS, Wiener H, Acton R, Go RC. The role of TNF and its receptors in Alzheimer’s Disease. Neurobiol Aging 2001; 22(6): 873–83. [DOI] [PubMed] [Google Scholar]
- 49.Lee KS, Chung JH, Choi TK, Suh SY, Oh BH, Hong CH. Peripheral cytokines and chemokines in Alzheimer’s Disease. Dement Geriatr Cogn Disord 2009; 28(4): 281–7. [DOI] [PubMed] [Google Scholar]
- 50.Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s Disease. Biol Psychiatry 2010; 68(10): 930–41. [DOI] [PubMed] [Google Scholar]
- 51.Hedges DW, Farrer TJ, Brown BL. Association between C-reactive protein and cognitive deficits in elderly men and women: a meta-analysis. Int Psychogeriatr 2012; 24(9): 1387–92. [DOI] [PubMed] [Google Scholar]
- 52.Brosseron F, Krauthausen M, Kummer M, Heneka MT. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol Neurobiol 2014; 50(2): 534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang J, Fan C, Pan L, Xie M, He Q, Li D, et al. C-reactive protein plays a marginal role in cognitive decline: A systematic review and meta-analysis. Int J Geriatr Psychiatry 2015; 30(2): 156–65. [DOI] [PubMed] [Google Scholar]
- 54.Gong C, Wei D, Wang Y, Ma J, Yuan C, Zhang W, et al. A meta-analysis of C-Reactive Protein in patients with Alzheimer’s Disease. Am J Alzheimers Dis Other Demen 2016; 31(3): 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esteras N, Alquézar C, de la Encarnación A, Martín-Requero Á. Lymphocytes in Alzheimer’s Disease pathology: Altered signaling pathways. Curr Alzheimer Res 2016; 13(4): 439–49. [DOI] [PubMed] [Google Scholar]
- 56.Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, et al. IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s Disease: Systematic review and meta-analysis. Sci Rep 2018; 8(1): 12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun 2018; 70: 61–75. [DOI] [PubMed] [Google Scholar]
- 58.Plantone D, Pardini M, Locci S, Nobili F, De Stefano N. B Lymphocytes in Alzheimer’s Disease-A Comprehensive Review. J Alzheimers Dis 2022; 88(4): 1241–62. [DOI] [PubMed] [Google Scholar]
- 59.Anuradha U, Kumar A, Singh RK. The clinical correlation of proinflammatory and anti-inflammatory biomarkers with Alzheimer disease: a meta-analysis. Neurol Sci 2022; 43(1): 285–98. [DOI] [PubMed] [Google Scholar]
- 60.Long S, Chen Y, Meng Y, Yang Z, Wei M, Li T, et al. Peripheral high levels of CRP predict progression from normal cognition to dementia: A systematic review and meta-analysis. J Clin Neurosci 2023; 107: 54–63. [DOI] [PubMed] [Google Scholar]
- 61.Gautam AS, Pulivarthi CB, Singh RK. Proinflammatory IL-17 levels in serum/cerebrospinal fluid of patients with neurodegenerative diseases: a meta-analysis study. Naunyn Schmiedebergs Arch Pharmacol 2023; 396(3): 577–88. [DOI] [PubMed] [Google Scholar]
- 62.Chihara N, Tsuji A, Matsumoto R. Neuroinflammation and neuroimmunology in Alzheimer’s disease: The role of T-lymphocytes in Alzheimer’s disease. Clin Exp Neuroimmunol 2023; 14(2): 92–9. [Google Scholar]
- 63.Elliott JH, Synnot A, Turner T, Simmonds M, Akl EA, McDonald S, et al. Living systematic review: 1. Introduction-the why, what, when, and how. J Clin Epidemiol 2017; 91: 23–30. [DOI] [PubMed] [Google Scholar]
- 64.Simmonds M, Elliott JH, Synnot A, Turner T. Living systematic reviews. Methods Mol Biol 2022; 2345: 121–34. [DOI] [PubMed] [Google Scholar]
- 65.Li C, Lumey LH. Exposure to the Chinese famine of 1959–61 in early life and long-term health conditions: a systematic review and meta-analysis. Int J Epidemiol 2017; 46(4): 1157–70. [DOI] [PubMed] [Google Scholar]
- 66.Li C, Lumey LH. Early-Life Exposure to the Chinese Famine of 1959–1961 and Type 2 Diabetes in Adulthood: A Systematic Review and Meta-Analysis. Nutrients 2022; 14(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo B, Zhao C, He MZ, Senter C, Zhou Z, Peng J, et al. Identifying patterns of reported findings on long-term cardiac complications of COVID-19: a systematic review and meta-analysis. BMC Medicine 2023; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai KSP, Liu CS, Rau A, Lanctôt KL, Köhler CA, Pakosh M, et al. Peripheral inflammatory markers in Alzheimer’s Disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry 2017; 88(10): 876–82. [DOI] [PubMed] [Google Scholar]
- 69.Darweesh SKL, Wolters FJ, Ikram MA, de Wolf F, Bos D, Hofman A. Inflammatory markers and the risk of dementia and Alzheimer’s disease: A meta-analysis. Alzheimers Dement 2018; 14(11): 1450–9. [DOI] [PubMed] [Google Scholar]
- 70.Shen XN, Niu LD, Wang YJ, Cao XP, Liu Q, Tan L, et al. Inflammatory markers in Alzheimer’s Disease and mild cognitive impairment: a meta-analysis and systematic review of 170 studies. J Neurol Neurosurg Psychiatry 2019; 90(5): 590–8. [DOI] [PubMed] [Google Scholar]
- 71.Su C, Zhao K, Xia H, Xu Y. Peripheral inflammatory biomarkers in Alzheimer’s Disease and mild cognitive impairment: A systematic review and meta-analysis. Psychogeriatrics 2019; 19(4): 300–9. [DOI] [PubMed] [Google Scholar]
- 72.van Oijen M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Fibrinogen is associated with an increased risk of Alzheimer Disease and vascular dementia. Stroke 2005; 36(12): 2637–41. [DOI] [PubMed] [Google Scholar]
- 73.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, et al. Inflammatory markers and the risk of Alzheimer Disease: the Framingham Study. Neurology 2007; 68(22): 1902–8. [DOI] [PubMed] [Google Scholar]
- 74.Gu Y, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer’s Disease. J Alzheimers Dis 2010; 22(2): 483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hendrie HC, Hake A, Lane K, Purnell C, Unverzagt F, Smith-Gamble V, et al. Statin use, incident dementia and Alzheimer disease in elderly African Americans. Ethn Dis 2015; 25(3): 345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ritchie K, Carrière I, Berr C, Amieva H, Dartigues JF, Ancelin ML, et al. The clinical picture of Alzheimer’s Disease in the decade before diagnosis: clinical and biomarker trajectories. J Clin Psychiatry 2016; 77(3): e305–11. [DOI] [PubMed] [Google Scholar]
- 77.André P, Samieri C, Buisson C, Dartigues JF, Helmer C, Laugerette F, et al. Lipopolysaccharide-binding protein, soluble CD14, and the long-term risk of Alzheimer’s disease: A nested case-control pilot study of older community dwellers from the three-city cohort. J Alzheimers Dis 2019; 71(3): 751–61. [DOI] [PubMed] [Google Scholar]
- 78.Thomas AJ, Hamilton CA, Donaghy PC, Martin-Ruiz C, Morris CM, Barnett N, et al. Prospective longitudinal evaluation of cytokines in mild cognitive impairment due to AD and Lewy body disease. Int J Geriatr Psychiatry 2020; 35(10): 1250–9. [DOI] [PubMed] [Google Scholar]
- 79.Hao J, Qiao Y, Li T, Yang J, Song Y, Jia L, et al. Investigating changes in the serum inflammatory factors in Alzheimer’s Disease and their correlation with cognitive function. J Alzheimers Dis 2021; 84(2): 835–42. [DOI] [PubMed] [Google Scholar]
- 80.Sundelöf J, Kilander L, Helmersson J, Larsson A, Rönnemaa E, Degerman-Gunnarsson M, et al. Systemic inflammation and the risk of Alzheimer’s Disease and dementia: A prospective population-based study. J Alzheimers Dis 2009; 18(1): 79–87. [DOI] [PubMed] [Google Scholar]
- 81.Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol 2004; 61(5): 668–72. [DOI] [PubMed] [Google Scholar]
- 82.Ravaglia G, Forti P, Maioli F, Chiappelli M, Montesi F, Tumini E, et al. Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiol Aging 2007; 28(12): 1810–20. [DOI] [PubMed] [Google Scholar]
- 83.Fohner AE, Sitlani CM, Buzkova P, Doyle MF, Liu X, Bis JC, et al. Association of Peripheral Lymphocyte Subsets with Cognitive Decline and Dementia: The Cardiovascular Health Study. J Alzheimers Dis 2022; 88(1): 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen J, Doyle MF, Fang Y, Mez J, Crane PK, Scollard P, et al. Peripheral inflammatory biomarkers are associated with cognitive function and dementia: Framingham Heart Study Offspring cohort. Aging Cell 2023: e13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo J, Thomassen JQ, Nordestgaard BG, Tybjærg-Hansen A, Frikke-Schmidt R. Blood Leukocyte Counts in Alzheimer Disease. JAMA Netw Open 2022; 5(10): e2235648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fang Y, Doyle MF, Chen J, Alosco ML, Mez J, Satizabal CL, et al. Association between inflammatory biomarkers and cognitive aging. PLoS One 2022; 17(9): e0274350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang X, Sanders JL, Boudreau RM, Arnold AM, Justice JN, Espeland MA, et al. Association of a blood-based aging biomarker index with death and chronic disease: Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stebbins RC, Edwards JK, Plassman BL, Yang YC, Noppert GA, Haan M, et al. Immune function, cortisol, and cognitive decline & dementia in an aging latino population. Psychoneuroendocrinology 2021; 133: 105414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walker KA, Chen J, Shi L, Yang Y, Fornage M, Zhou L, et al. Proteomics analysis of plasma from middle-aged adults identifies protein markers of dementia risk in later life. Sci Transl Med 2023; 15(705): eadf5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van der Willik KD, Fani L, Rizopoulos D, Licher S, Fest J, Schagen SB, et al. Balance between innate versus adaptive immune system and the risk of dementia: a population-based cohort study. J Neuroinflammation 2019; 16(1): 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chou OHI, Zhou J, Li L, Chan JSK, Satti DI, Chou VHC, et al. The Association Between Neutrophil-Lymphocyte Ratio and Variability with New-Onset Dementia: A Population-Based Cohort Study. J Alzheimers Dis 2023; 94(2): 547–57. [DOI] [PubMed] [Google Scholar]
- 92.Kravitz BA, Corrada MM, Kawas CH. High levels of serum C-reactive protein are associated with greater risk of all-cause mortality, but not dementia, in the oldest-old: results from The 90+ Study. J Am Geriatr Soc 2009; 57(4): 641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cooper J, Pastorello Y, Slevin M. A meta-analysis investigating the relationship between inflammation in autoimmune disease, elevated CRP, and the risk of dementia. Front Immunol 2023; 14: 1087571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Farina MP, Kim JK, Hayward MD, Crimmins EM. Links between inflammation and immune functioning with cognitive status among older Americans in the Health and Retirement Study. Brain Behav Immun Health 2022; 26: 100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Renson A, Mullan Harris K, Dowd JB, Gaydosh L, McQueen MB, Krauter KS, et al. Early signs of gut microbiome aging: Biomarkers of inflammation, metabolism, and macromolecular damage in young adulthood. J Gerontol A Biol Sci Med Sci 2020; 75(7): 1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kukull WA. The association between smoking and Alzheimer’s Disease: effects of study design and bias. Biol Psychiatry 2001; 49(3): 194–9. [DOI] [PubMed] [Google Scholar]
- 97.Kukull WA, Ganguli M. Generalizability: the trees, the forest, and the low-hanging fruit. Neurology 2012; 78(23): 1886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu C, Li C. C-reactive protein and cardiovascular diseases: a synthesis of studies based on different designs. Eur J Prev Cardiol 2023; 30(15): 1593–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barnes LL, Capuano AW, Aiello AE, Turner AD, Yolken RH, Torrey EF, et al. Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J Infect Dis 2015; 211(2): 230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harris SA, Harris EA. Herpes Simplex Virus Type 1 and Other Pathogens are Key Causative Factors in Sporadic Alzheimer’s Disease. J Alzheimers Dis 2015; 48(2): 319–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Itzhaki RF. Herpes and Alzheimer’s Disease: Subversion in the central nervous system and how it might be halted. J Alzheimers Dis 2016; 54(4): 1273–81. [DOI] [PubMed] [Google Scholar]
- 102.Itzhaki RF, Cosby SL, Wozniak MA. Herpes simplex virus type 1 and Alzheimer’s Disease: the autophagy connection. J Neurovirol 2008; 14(1): 1–4. [DOI] [PubMed] [Google Scholar]
- 103.Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022; 375(6578): 296–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are all presented in the main tables or supplementary materials. Additional data and code to generate findings reported in this study are available from the GitHub repository (https://github.com/Peripheral-immune-AD/Peripheral-immune-and-AD).


