Abstract
Background
Clinical trial participation can improve overall survival and mitigate healthcare disparities for gynecologic cancer patients in low-volume community centers. This study aimed to assess the effectiveness of a centrally regulated but administratively decentralized electronic screening log system to identify eligible patients across a large catchment area for a National Cancer Institute (NCI)-designated cancer center's open clinical trials.
Methods
Electronic screening log data collected between 2014 and 2021 from ten community partner sites in a single NCI-designated cancer center's catchment area were reviewed retrospectively. Clinical factors assessed included cancer site, primary versus recurrent disease status, and histology. Identification efficiency (the ratio of patients screened identified with an available trial) was calculated. Identification inefficiencies (failures to identify patients with a potentially relevant trial) were assessed, and etiologies were characterized.
Results
Across ten community partner sites, 492 gynecologic cancer patients were screened for seven open clinical trials during the study period. This included 170 (34.5 %) ovarian cancer patients, 156 (31.7 %) endometrial cancer patients, and 119 (24.2 %) cervical cancer patients. Over 40 % had advanced stage disease, and 10.6 % had recurrent disease. Only three patients were identified as having a relevant open trial; none ultimately enrolled due to not meeting trial eligibility criteria. An additional 2–52 patients were retrospectively found to have a relevant trial available despite not being identified as such within the electronic screening log system. Up to 14.4 % of patients had one or more missing minimum data elements that hindered full evaluation of clinical trial availability. Re-screening patients when new trials open may identify 12-15 additional patients per recurrent disease trial.
Conclusions
An electronic screening log system can increase awareness of gynecologic oncology clinical trials at a NCI-designated cancer center's community partner sites. However, it is inadequate as a single intervention to increase clinical trial enrollment. Providing adequate support staff, documenting clinical factors consistently, re-screening patients at relevant intervals, and coordinating with central study personnel may increase its utility.
Keywords: Clinical trials, Gynecologic oncology, Patient recruitment, Electronic screening
1. Introduction
The American Cancer Society reported an estimated 114,810 new diagnoses and 34,020 deaths from gynecologic cancers in the United States in 2023 [1]. Despite the rapid development of new and efficacious treatments for endometrial, ovarian, and cervical cancers, only ovarian cancer population-level overall survival has improved in recent decades [1]. Many gynecologic cancer patients continue to face disparate access to evidence-based, high-quality care [2]. Overall survival and receipt of guidelines-concordant care are significantly associated with the involvement of a gynecologic oncologist in care planning [3,4]. At least 40 % of health referral areas do not have a practicing gynecologic oncologist, and over 14 million patients live in low-access counties [5]. This is a structural consequence of the prevailing “Centers of Excellence” model that concentrates gynecologic cancer care at urban tertiary medical centers [6,7].
However, the drivers of persistent barriers to access to gynecologic cancer care are complex, multi-factorial, and context-dependent. In New York, greater distance to a gynecologic oncologist was associated with increased mortality for cervical and endometrial cancer patients [8]. Similarly, in Iowa, distance to a gynecologic oncologist was associated with a lower likelihood of optimal cytoreduction and less frequent receipt of guideline-concordant adjuvant chemotherapy [9,10]. By contrast, in Kansas, patients who lived fewer than ten miles from an NCI-designated cancer center had lower overall survival and lower rates of optimal cytoreduction [11]. This finding is striking, given that Kansas also has the lowest gynecologic oncologist-to-beneficiary ratio in the United States [10].
Reports of varied and persistent disparities in receipt of evidence-based gynecologic cancer care demonstrate that ongoing efforts to combat disparities in the delivery of usual cancer care are inadequate to improve overall survival for underserved gynecologic cancer populations. Clinical trial participation has been shown to increase the delivery of guideline-concordant care and mitigate survival differences by race/ethnicity in gynecologic cancers [[11], [12], [13], [14]]. Similarly, rural ovarian cancer patients enrolled in Phase 2–3 SWOG trials had non-inferior cancer-specific, progression-free, and overall survival relative to their urban patients when receiving care as part of a clinical trial [15,16].
The untapped opportunity for increased clinical trial participation is of particular urgency in the context of the ongoing “clinical trials crisis” in gynecologic oncology. Since 2010, there has been a decrease in accrual in all National Clinical Trials Network (NCTN) trials by 33 % [17]. However, trials in gynecologic oncology have disproportionately dropped off, including a 90 % reduction in accrual in Phase III studies and a 64 % decrease in the number of gynecologic oncology trials sponsored by the NCI's Cancer Therapy Evaluation Program (CTEP) [18]. This crisis threatens to deleteriously impact clinical outcomes for all gynecologic oncology patients but disproportionately affects patients receiving care at low-volume community sites. This imposes a moral imperative to understand ways to enhance clinical trial recruitment in gynecologic oncology [19].
The University of Kansas Cancer Center (KUCC) established the Masonic Cancer Alliance (MCA, formerly known as the Midwest Cancer Alliance) in 2008 to facilitate systematic outreach to rural and underserved hospitals, cancer centers, and clinics in KUCC's catchment area. This includes increasing local access to KUCC's specialized cancer care resources, cancer screening and patient programs, and professional education. More specifically, since 2010, KUCC began working with twelve partner organizations to offer KUCC clinical trials at MCA community partner sites so patients could enroll and participate in clinical trials locally. The MCA serves the state of Kansas and 18 counties in Missouri. Of the 123 counties represented in KUCC and MCA's shared catchment area, 76 % are considered rural by rural-urban continuum codes. Since its creation, MCA's clinical trial network has decreased out-of-state migration for care by 20 % and kept over $3.3M of medical spending in the state [20].
Unger et al. [18] introduced a uniform conceptual framework defining steps in the decision-making process supporting cancer patient clinical trial enrollment, including ensuring the availability of clinical trials, assessment of patient eligibility, trial discussion, and offer, and the patient's decision on whether to enroll [21]. KUCC developed an electronic screening log system to assess the availability of eligible patient cohorts and understand reasonable accrual goals for open KUCC clinical trials across ten MCA community partner sites [Supplemental Appendix 1]. A prior study demonstrated that the electronic screening log system facilitated broader patient recruitment across KUCC and MCA's shared catchment area for breast and lung cancer clinical trials [22]. The objective of the present study was to evaluate whether an electronic screening log system was adequate and effective in identifying available clinical trials for which gynecologic cancer patients at low-volume community sites should be screened for eligibility.
2. Methods
2.1. Screening system and workflow
The University of Kansas Medical Center Institutional Review Board (IRB) approved the study as the central IRB and governing body for all MCA institutions (STUDY00002341). As part of the IRB approval, minimum data use agreements were obtained from individual MCA cancer treatment sites. The University of Kansas Medical Center Department of Biostatistics and Informatics created an electronic screening log system on behalf of the MCA using the Research Electronic Data Capture (REDCap) program [23,24]. The MCA administrative team designed a screening log survey (Supplemental Appendix 1) including basic mandatory questions about a patient's cancer to prompt MCA local CRCs to screen patients meeting criteria for any open relevant clinical trial for eligibility. Survey responses constituted the data stored in the MCA's electronic screening log system's database. The screening log survey instrument was revised in response to feedback from MCA local CRCs to minimize the administrative burdens of data entry.
Demographic factors collected were screening site, screening date, and new versus established patient status. Additional demographic factors that did not directly impact clinical trial eligibility (age, race/ethnicity, gender, and city of residence) were not collected to minimize unnecessary administrative burdens for MCA sites. Given that inadequate recruitment staff and data collection infrastructure previously have been identified as barriers to clinical trial participation [[25], [26], [27], [28]], we actively sought to avoid creating problematic secondary effects on MCA community partner sites from our intervention. Moreover, the collection of only a minimum of necessary patient information remains an important tenet in safeguarding patient health information in research studies. All patients diagnosed with gynecologic cancers are female sex assigned at birth. Clinical factors collected included cancer type (e.g., gynecologic), cancer location (ovary, fallopian tube, primary peritoneal, uterine, cervix, vagina or vulva), histology (squamous, adenocarcinoma, clear cell, serous, mucinous, endometrioid, transitional cell, mesenchymal, mixed), TNM staging including tumor stage (T1-T4), nodal status (N0-N3), and presence or absence of distant metastasis (M0 or M1), recurrent or progressive disease (yes/no), stage (I-IV), and CA-125 level.
All screening was conducted using the REDCap system [29,30]. MCA community partner site members who signed a data use agreement were onboarded to the electronic screening log system and were trained on how to input data into the system. Each MCA community partner site's clinical research coordinator (CRC) was added as a user in the REDCAP electronic screening log system. A CRC's data access was limited to their only individual cancer center's participant records using role-based access permissions in REDCap. The KUCC central administrative team could view all the records across the MCA network. An instance of REDCap is installed within the KUCC data center for research use purposes. The REDCap instance is HIPAA certified, allowing KUCC researchers to capture and store patient health information. The system also has an InCommon built-in feature that allows users to use their university credentials to log onto the system securely.
Local MCA CRCs entered data either from their desktop or mobile devices as they reviewed potential participants for clinical trials at their community partner site. The CRC inputted data into the electronic screening log database when a cancer patient was first evaluated, either at the time of diagnosis or at the time of cancer recurrence. While entering data into the electronic screening log system based on the participant's diagnosis, the CRC was instructed to cross reference entered data to assess if any clinical trial open through the MCA was available that fits the participant's diagnostic criteria. If a potentially relevant clinical trial was available, the CRC queried the patient's treating physician to confirm whether the clinical trial was correctly identified as relevant and clinically appropriate. If this was confirmed, then the CRC scheduled a time to contact the patient to discuss the clinical trial and inquire if the patient was interested in proceeding with full screening for trial eligibility. This conversation was conducted in person with timing coordinated with a patient's other clinical appointments. Sufficient time was given for patients to review informational material about the clinical trial and consider participation. If the physician determined that the patient did not meet relevant clinical criteria for any existing open available clinical trials and/or if the patient declined or withdrew from the full clinical trial eligibility screening process, then the reason that the patient did not matriculate to study screening was documented within the electronic screening log system. All clinical trial screening and consent processes were designed and maintained in accordance with Good Clinical Practice (GCP) standards to ensure that human subjects' rights, safety, and well-being were protected and that trials were conducted consistent with approved plans with rigor, integrity, and reliability in data collection.
The lack of study availability allowed KUCC leadership to evaluate if a certain type of study needed to be designed or launched to help treat patients across the KUCC catchment area. Cases in which patients were incorrectly deemed not to have a relevant open clinical trial available prompted the MCA administrative team to hold a brainstorming session with study principal investigators to evaluate what factors about clinical trial eligibility criteria may be overly confusing or restrictive and thereby inadvertently functioning to limit patient identification. MCA sites were encouraged to 1) review all new patients for potential clinical trial eligibility, 2) enter data routinely into the MCA electronic screening log system, and 3) discuss potential available clinical trial opportunities with the treating physician. Deidentified reports summarizing electronic screening log system data could be run by local MCA site CRCs, the central MCA administrative team, and clinical trial principal investigators and were reviewed at meetings and in communications between these groups to identify failures and inefficiencies in the electronic screening log system. Reports also formed the basis for a retrospective quality improvement review of the electronic screening log system. Individual patient records could be updated or reviewed again within the electronic screening log system when new clinical information and/or new clinical trials became available.
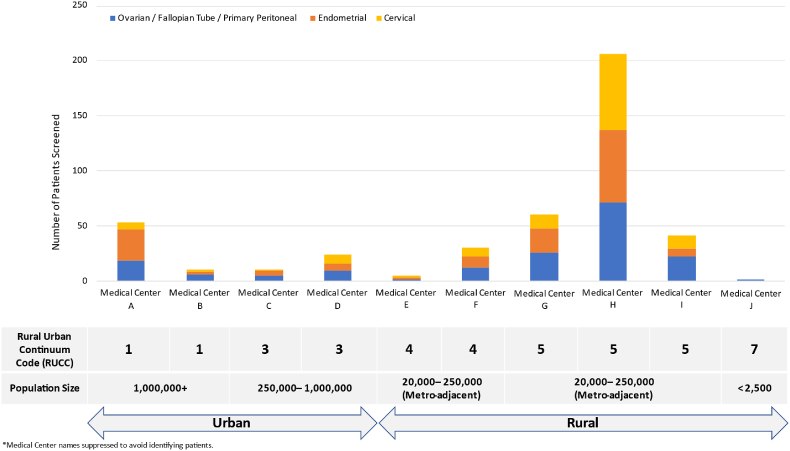
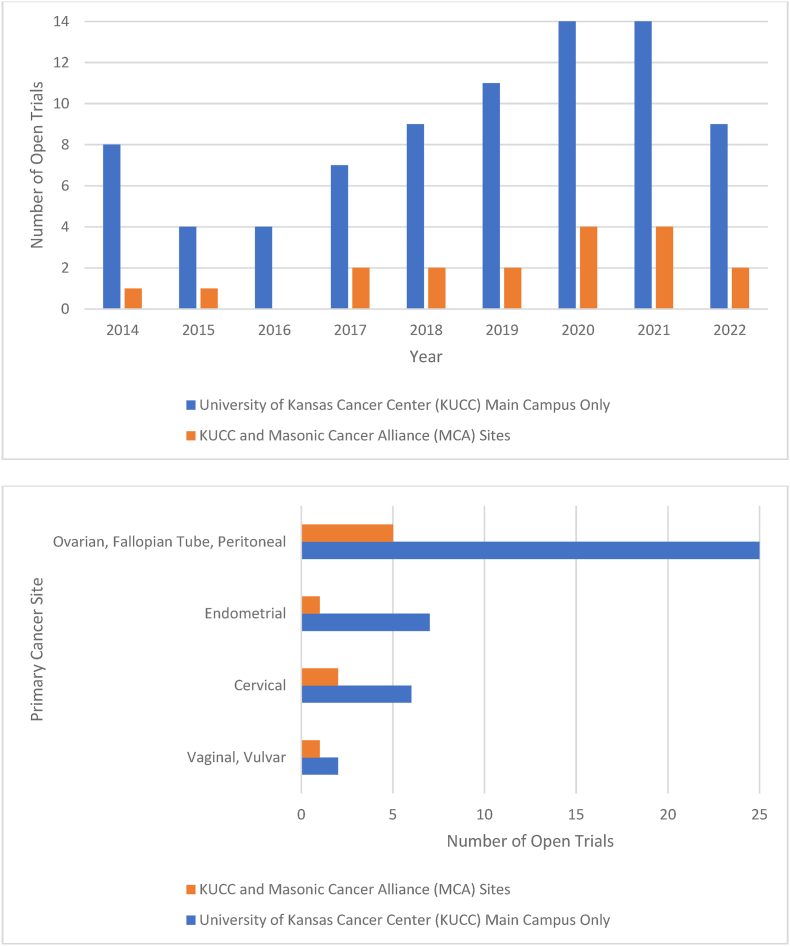
Screening occurred for gynecologic cancer patients across ten MCA community sites between October 2014 and December 2022 (Fig. 1). MCA community partner sites were defined as rural or urban using 2023 Rural-Urban Continuum Codes, which assess counties based on population size, adjacency to a metropolitan area, and degree of urbanization [31]. There was no limit on the number of open clinical trials for which a patient could be considered, though studies were limited to trials opened at MCA through the NCTN mechanism. This analysis does not include 25 gynecologic oncology trials enrolled only at KUCC Main Campus, including 11 clinical trials from the gynecologic oncology Multiple Early Phase Clinical Trials (EPCT) program (Fig. 2), as MCA patients were not screened for clinical trials offered only at KUCC Main Campus.
Fig. 1.
Number of patients screened by Masonic Cancer Alliance (MCA) sites, classified by Rural-Urban Continuum Code (RUCC).
Fig. 2.
Gynecologic oncology clinical trials open by site by year (A) and by primary cancer site (B).
2.2. Statistical analysis
Formal statistical analyses are limited given the retrospective cohort study design with data derived from an electronic screening log database developed for operational rather than research purposes. Due to low sample sizes, we could not compare demographic or clinical factors between cancer types. We also could not assess associations between demographic or clinical factors and the likelihood of having an available clinical trial. However, the identification efficiency (ratio of patients screened to patients identified with available clinical trial) and identification inefficiencies (ratio of patients screened to patients with a possible available clinical trial who were not identified) are described [32].
3. Results
Between January 2014 and December 2022, 492 gynecologic cancer patients completed screening across ten MCA community partner sites. Ninety-eight (20 %) patients completed screening at one of six rural MCA community partner sites, and 394 (80 %) patients completed screening at one of four urban MCA community partner sites (Fig. 1). Most patients were screened at a new patient visit (395 or 80.3 %), whereas 79 (16 %) were screened at an established patient visit, and 18 (3.7 %) patients had unknown status (Table 1). A variety of gynecologic cancer sites were represented, as 170 (34.5 %) of screened patients had ovarian, fallopian tube, or primary peritoneal cancer, 156 (31.7 %) had endometrial cancer, and 119 (24.2 %) had cervical cancer (Fig. 2). Over half of patients had advanced-stage disease, with 108 (22 %) and 136 (27.6 %) of screened patients having stage III and IV disease, respectively. Only a subset (52 or 10.6 %) of patients had recurrent disease at the time of screening, including 20 patients with recurrent ovarian, fallopian tube, or primary peritoneal cancer.
Table 1.
Clinical characteristics of screened gynecologic cancer patients.
| Clinical Factor | N, % |
|---|---|
| Primary Gynecologic Cancer Site | |
| Ovarian/Fallopian tube/primary peritoneal | 170 (34.5 %) |
| Endometrial | 156 (31.7 %) |
| Cervical | 119 (24.2 %) |
| Vaginal/Vulvar | 24 (4.9 %) |
| Unknown |
23 (4.7 %) |
| Stage | |
| I | 142 (28.9 %) |
| II | 46 (9.3 %) |
| III | 108 (22 %) |
| IV | 136 (27.6 %) |
| Unknown |
60 (12.2 %) |
| Cancer Status | |
| Primary | 369 (75 %) |
| Recurrent/Progressive | 52 (10.6 %) |
| Unknown |
71 (14.4 %) |
| Histology | |
| Adenocarcinoma | 174 (35.4 %) |
| Squamous cell | 66 (13.4 %) |
| Endometrioid | 105 (21.3 %) |
| Serous | 48 (9.8 %) |
| Clear cell | 16 (3.3 %) |
| Mucinous | 2 (0.4 %) |
| Mixed | 12 (2.4 %) |
| Unknown |
69 (14 %) |
| Patient Status | |
| New | 395 (80.3 %) |
| Established | 79 (16 %) |
| Unknown | 18 (3.7 %) |
Seven gynecologic oncology clinical trials were open for recruitment at MCA community partner sites during the study period (Fig. 2). This included three Phase II trials (43 %), one Phase II/III trial (14 %), and three Phase III trials (43 %). No Phase I KUCC trials were open for recruitment at MCA community partner sites. Trials were open for a median of 18 months. Between one to four clinical trials were open per year at MCA community partner sites. The largest number of open clinical trials was available between 2020 and 2021. Two of seven trials (28.6 %) investigated radiation treatments; these two trials were open only at four of ten (40 %) MCA community partner sites with Radiation Oncology services on-site (two urban and two rural sites). Of the remaining five ovarian cancer trials, two trials (40 %) were histology-specific (one in recurrent clear cell carcinoma and one in primary low-grade serous carcinoma). Trials for recurrent disease were available only for ovarian cancer patients, including one trial in platinum-sensitive disease and two trials in platinum-resistant disease.
Only three patients were identified as having a relevant clinical trial available (Table 2); two in 2014 (one endometrial cancer patient, one cervical cancer patient) and one in 2019 (vulvar cancer patient). None of the three patients ultimately enrolled. In all three cases, this was due to not meeting the eligibility criteria for the identified trial. Of note, none of the three patients were noted to have incompatible age, performance status, co-morbid conditions, organ dysfunction, pregnancy and/or breastfeeding status, prior chemotherapy, radiotherapy and/or surgery, psychiatric conditions, or inadequate tissue available.
Table 2.
Screening log system implementation outcomes by trial type.
| Trial Characteristic (Cancer Types Included in Trials) | Number of Trials in Category (N) | Patients Screened During Trial Open Window Identified as Potentially Eligible by Electronic Screening Log System (N) | Patients with Available Eligible Trial Screened During Trial Open Window Missed by Electronic Screening Log System (Identified on Manual Retrospective Re-Review)) (N if blanks excluded) (N if blanks included) |
Patients Screened Prior to Trial Open Window that would be Classified as Eligible if Re-Screened when Trial Opened (N if blanks excluded) (N if blanks included) |
KUCC Clinical Research Coordinator Implementation Notes and Feedback on Relevant Electronic Screening Log System Deficiencies |
|---|---|---|---|---|---|
| Chemotherapy Only (Ovarian/Fallopian Tube/Peritoneal) | 5 | 0 | 21 13 |
113 72 |
|
| Radiation Included (Endometrial, Cervical, Vaginal/Vulvar) |
2 | 3 | 75 70 |
138 121 |
|
| Primary Disease (Endometrial, Cervical, Ovarian/Fallopian Tube/Peritoneal) |
4 | 0 | 84 78 |
180 160 |
|
| Recurrent Disease (Ovarian/Fallopian Tube/Peritoneal) | 4 | 0 | 12 5 |
77 39 |
|
| Histology-Specific (Ovarian/Fallopian Tube/Peritoneal) | 3 | 0 | 10 8 |
50 39 |
|
Overall, the identification efficiency of the electronic screening log system was low, as only 3 of 492 (0.6 %) of screened patients were identified as having a relevant open clinical trial in typical clinical use. A secondary retrospective review of the electronic screening log system database was undertaken by the study team for quality improvement purposes (Supplemental Appendix 2). When the electronic screening log system database was sorted by 2–3 categories [primary cancer site, primary versus recurrent disease status, and histology (where relevant)] and filtered to capture patients screened at clinic visits during the study open enrollment periods for each of the seven offered clinical trials, an additional 2–52 patients per trial were identified that may have potentially been eligible for the trial. Individual patient entries in the electronic screening log system could and should have been updated when new relevant clinical information arose. However, in the initial implementation period, there were no predefined triggers to standardize or prompt updating or re-screening of individual patient records. When the same retrospective review (patients sorted by primary cancer site, primary versus recurrent disease status, and histology) was expanded with filtering set to include additional patients screened prior to the trial open enrollment period (as would have been the case if previously screened patients were automatically re-screened any time a new clinical trial was opened at a MCA community partner site), an additional 2–44 patients per trial (on top of the additional potentially eligible patients screened during the study open enrollment period) were identified that may have potentially been eligible for the trial. Thus, setting automated quality improvement retrospective reviews of electronic screening log data by centralized KUCC staff would have increased the identification efficiency of the electronic screening log system from 0.6 % to 16.9 %. Automated re-screening of previously screened recurrent disease patients upon opening of a new clinical trial by centralized KUCC staff would have further increased the identification efficiency of the electronic screening log system to 75 % in this sub-population.
Missing data were identified in the electronic screening log system. This included 23 (4.7 %) patients with unknown primary cancer sites, 71 (14.4 %) patients with unknown primary versus recurrent disease status, 69 (14 %) patients with unknown histology, and 60 (12.2 %) with unknown stage. As a result, 84 (17 %) of patients could not be screened effectively for potentially relevant open clinical trials. This corresponds to an adjusted identification efficiency of the electronic screening log system of 0.7 %. Local target enrollment goals for individual clinical trials ranged between 1 and 10 patients per trial. Across KUCC and MCA sites, total accrual ranged from 20 to 50 % of enrollment goal per trial. Patients accrued from outside of the electronic screening log system were either identified at KUCC main campus (primary academic medical center site of the catchment area) or via direct patient referral by a local oncologist. Of note, there were an additional 17 trials offered at MCA community partner sites during the study period for multiple solid organ tumors with pre-defined immunohistochemical, molecular, and/or genetic profiling criteria. These trials may have been relevant for a subset of the 492 screened gynecologic cancer patients had additional necessary data for eligibility assessment been collected within the electronic screening log system.
4. Discussion
Our results describe the real-world implementation of an electronic screening log system at community partner sites within an NCI-designated cancer center's catchment area to identify local gynecologic cancer patients who should be screened for open clinical trials. Three patients were identified as having a relevant open gynecologic oncology clinical trial. However, in all cases, patients ultimately failed screening due to not meeting the eligibility criteria for the identified clinical trial. The electronic screening log system had a 0.6 % identification efficiency for matching gynecologic cancer patients to open clinical trials in the context of routine decentralized local clinical practice at MCA community partner sites. A retrospective quality improvement review identified a potential 16.3 % increase in identification efficiency that could be achieved with a secondary review of electronic screening log system data by centralized KUCC CRCs and/or study primary investigators. We also identified the potential for an over 70 % increase in identification efficiency with automated re-screening of MCA patients with recurrent disease at the time a new clinical trial is opened. Use of the electronic screening log system was further limited for 84 (17 %) patients with incomplete or missing clinical data. Though an electronic screening log system holds the potential to increase the identification of community-based patients for screening for NCI-designated cancer center clinical trials, routine data auditing by primary site CRCs, automated rescreening of local patients, and targeted outreach to community sites are needed to fulfill the promise of this tool.
This study addresses an area of critical unmet need in the new and evolving mandate for NCI-designated cancer centers. Since 2016, the NCI Funding Opportunity Announcement (FOA) has defined requirements for NCI-designated cancer centers to 1) describe their catchment area, 2) increase clinical trial participation among the catchment area population, and 3) engage in systematic community outreach [31,32]. However, to date, little research has been published with specific implementation data on operationalizing these mandates toward tangible clinical and quality improvement outcome measures [33]. Our research describes a specific protocol for engagement with community partner sites to decentralize clinical trial screening and enrollment and work toward improving clinical trial accrual to match the overall demographics of the catchment area. Our case study, like other previously published investigations [[34], [35], [36], [37]], is not without necessary areas for improvement. However, there remains lingering confusion on how to describe and evaluate the effectiveness of clinical trial screening and accrual in broad catchment areas. Therefore, a real-world evaluation of the practical challenges encountered in initiating and evaluating a centralized monitoring mechanism helps chart a path forward for future research endeavors. Our study represents a departure from most past case studies that have focused solely on population health-level descriptive analysis of cancer burden in the catchment area and/or interventions related to cancer screening and prevention only. We also leveraged novel implementation outcomes, such as the identification efficiency ratio, that are both relevant and feasible for collection at community partner sites, as was recently assessed in Australia by Tew et al. (2023).
Calculation of the identification efficiency ratio of the electronic screening log system and sensitivity analysis assessing the impact of various system deficiencies on identification efficacy proved to be a useful tool. These analyses permitted evaluation of how the electronic screening log system failed, what specific challenges were encountered, and what lessons can be learned from these failures. Three patients were correctly identified as having a relevant open and available clinical trial, and zero patients ultimately enrolled in the trial. These determinations required manual and subjective evaluation of the electronic screening log system data by the local MCA CRC based on their individual and independent review of a list of clinical trials available through the KUCC-MCA network that fit patients' diagnostic criteria. When data from the electronic screening log system were extracted automatically into statistical software and analyzed using consistent decision rules based on patients’ primary cancer site, primary versus recurrent disease status, and histology criteria, over ten-fold more patients were identified as having relevant available clinical trials. This suggests that in its current state of implementation, the electronic screening log system leaves room for many type II errors or false negative screenings. We hope to assess the impact of adding an automated decision rule support tool to the electronic screening log system on identification efficiency in a future study. It remains to be seen to what extent this type of decision-rule support introduces new type I error (or false positive screenings) into the system and the impact of associated increases in administrative burden.
Only trials that included radiation for endometrial, cervical, and vaginal cancers identified potentially eligible patients in the electronic screening log system without use of a clinical decision support rule, though use of the automated decision-rule support tool still identified additional patients. This may reflect greater buy in for clinical trials from local oncologists when they are working with radiation oncologists rather than gynecologic oncologists from academic medical centers. Alternatively, all radiation trials sought out newly diagnosed patients who often have a more consistent visit schedule with lesser risk of loss to follow-up compared to patients with recurrent disease or in disease surveillance. Chemotherapy trials and trials in recurrent disease did not have any potentially eligible patients identified in the electronic screening log system. This may be due to the presence of multiple possible guidelines-concordant chemotherapy regimens already available and a preference among local oncologists for known guidelines-concordant chemotherapy regimens over patient participation in clinical trials. Alternatively, identification of patients for clinical trials investigating chemotherapy and other disease-directed targeted therapies can be limited by overly narrow and stringent eligibility criteria [[38], [39], [40]]. Prior studies have demonstrated that increases in exclusion criteria based on comorbidities and performance status actively increased rates of trial non-participation and limited generalizability of clinical trial results [[41], [42], [43]]. Increasing numbers of eligibility criteria in academic and pharmaceutical-sponsored clinical trials can create undue administrative and logistical barriers for patient participation in clinical trials [44]. Moreover, increasing complexity of clinical trial eligibility criteria can impede clarity and understanding of required criteria and increase the perception of barriers to patient feasibility and appropriateness for trial participation among physicians, CRCs, and patients [[45], [46], [47]].
A second source of system failure was the lack of systematic re-evaluation of previously screened patients when new clinical trials became available at MCA community partner sites. In our quality improvement assessment, we expanded the automated decision rule criteria to include not only patients who were screened in a clinic visit during a given trial's open enrollment period but also patients who were screened in a clinic visit prior to the trial opening. As a result, an additional 2–16 patients per trial who were potentially eligible were identified. Importantly, these numbers likely overestimate the gain in identification efficiency from reassessment of previously screened patients given the lack of systematic data collection about subsequent changes in clinical status (e.g., patients may have died, recurred, received additional treatment lines, or had other relevant changes in their clinical history since their screening encounter). We also posit that systematic re-review of previously screened patients holds the greatest utility and incremental identification efficiency benefit for patients with recurrent disease. Many primary disease trials in gynecologic oncology address a narrow window in time within the primary disease course (e.g., adjuvant treatment following primary surgical intervention, primary and/or definitive chemoradiation). Primary disease patients are highly likely to fall outside of this narrow window at the time of later re-evaluation and be deemed ineligible for primary disease clinical trials opened long after their initial screening encounter. As a result, we recommend instituting both systematic updating of patient records within the electronic screening log system at the time of disease recurrence and an automatic data re-review of previously screened recurrent disease patients whenever a new recurrent disease clinical trial opens. This recommendation is of particular importance given local trends in the timing of clinical trial availability assessment we observed at the MCA community partner sites. Three-fourths of patients were evaluated at the time of primary diagnosis, while only one-tenth of patients were evaluated at the time of recurrence, despite the availability of twice as many trials for recurrent disease relative to primary disease. This parallels screening patterns seen by Manders et al. [48], where patients offered a clinical trial at the time of disease recurrence were 66 % more likely to accept than patients who had this discussion at the time of primary diagnosis [45].
Missing and incomplete data were a third key source of system failure in our study. A significant proportion of patients had missing data in the electronic screening log system that precluded comprehensive evaluation of clinical trial availability. This would have occurred due to the failure of the local MCA CRC to input all data correctly into the electronic screening log system and/or failure to update the patient record when new pertinent information became available. Ultimately 17 % of patients had one or more blank or incomplete data fields in the electronic screening log system that precluded full evaluation of possible available clinical trials. This included 4.7 % of patients with unknown primary cancer sites, 14.4 % with unknown primary versus recurrent disease status, 14 % of patients with unknown histology, and 12.2 % of patients with unknown stage, all of which are frequent and clinically significant inclusion criteria in gynecologic oncology clinical trials. Additionally, no data were collected on histologic grade (e.g., low-grade versus high-grade serous histology), which further limited the specificity of patient evaluation and consideration for histology-specific trials.
Further research is needed to assess factors contributing to incomplete electronic screening log data entry. Potential contributing factors include a lack of awareness of the clinical significance of histology type; final pathology reports not being accessible at the time of data entry, insufficient support from gynecologic pathology subspecialists, lack of confirmatory immunohistochemical or molecular testing, and/or inconsistent documentation of clinical and pathologic staging by oncologists at MCAcommunity partner sites. Failure to complete the screening log in its entirety could also reflect time constraints for the local MCA CRCs versus the subconscious effect of physician and/or patient bias against clinical trials [46]. This effect was demonstrated previously in a trial investigating screening failures for gynecologic oncology trials in Texas [12]. Results showed that 16 % of patients were excluded from clinical trial consideration by their physician based on perceived “medical unfitness” or “prior non-compliance” even though none of the patients met any of the pre-defined exclusion criteria for the trials [49]. Radiation oncology trials could only be offered at a subset of MCA sites with local radiation oncology facilities, which may have created disparities in awareness of and access to these trials within the catchment area. This also represents a potential role for centralized KUCC CRCs to increase their involvement and outreach with the shared MCA catchment area. Though re-screening may create too much undue burden for community physicians, if centralized KUCC CRCs intimately familiar with all available gynecologic oncology trials could review screening logs and identify needs for updated data for potential candidates, this could increase our ability to identify available clinical trials for patients. Such centralized review paired with the currently decentralized electronic screening log system likely also would increase the specificity of the screening log system.
These results are significant in the context of the ongoing “clinical trials crisis” in gynecologic oncology [19] as both the number of trials available and accrual continue to decrease. A recent study demonstrated that 21 % of gynecologic oncology clinical trials were terminated, suspended, or withdrawn primarily due to low accrual [47]. More specifically, there were increased odds of non-completion for trials enrolling at 2–5 sites [47]. This emphasizes the importance of increasing the feasibility of patient identification and recruitment for clinical trials at community partner sites.
Yet, we continue to lack robust implementation science data specific to clinical trials in gynecologic oncology. Mudaranthakam et al. (2022) previously published on the use of the same electronic screening log system described in the present study to facilitate the identification and enrollment of MCA community site patients in KUCC clinical trials for lung and breast cancer. In the prior study, the same electronic screening log system had a 19.9 % identification efficiency ratio for lung cancer patients and an 11.3 % identification efficiency ratio for breast cancer patients seeking relevant open clinical trials, which far exceeds the 0.6 % identification efficiency ratio for gynecologic cancer patients we reported. The identification efficiency for breast and lung cancers is likely helped by the greater number of available clinical trials for breast and lung cancers relative to gynecologic cancers. However, it is interesting that other cancer types that also often restrict trial eligibility based on primary versus recurrent disease status and/or histology were not also limited in identification efficiency by missing data to the same extent that trials for gynecologic cancers were impacted. The two studies of the KUCC electronic screening log system from our group support its potential generalizability and scalability across cancer types. It is notable that we were not able to use the electronic screening log system for phase I multi-organ cancer trials with inclusion criteria defined by genetic and/or molecular testing. This is likely due to lack of data collection on these variables in the electronic screening log system (which is readily modifiable) as well as greater difficulty in including patients at MCA community partner sites in Phase I trials given the intensity and frequency of clinic visits and laboratory assessments required in many trial protocols (which is a more difficult barrier to overcome). Our results also underscore the need for further qualitative and quantitative research on barriers to clinical trial eligibility screening and trial participation specific to gynecologic cancer patients and trials.
What little data exist in gynecologic oncology largely came from past efforts to improve patient identification and enrollment in screening and prevention trials. Rimel et al., [47], showed that an online patient-facing clinical trial registry matched 82 % of patients to an available clinical trial. However, 15 % of patients who ultimately enrolled in an identified trial participated in a cancer screening, imaging, and/or tissue bank study [49]. Of note, no patients enrolled in a cancer therapeutic trial, over 50 % of patients did not have a cancer diagnosis at the time of screening, and all screenings were performed at a single, urban academic center without rural or community outreach [49]. In contrast, Gynecologic Oncology Group (GOG) investigators evaluated enrollment in GOG therapeutic trials. 38 % of participants had a clinical trial available, and 91 % of patients were enrolled. Most enrolling gynecologic oncologists (58 %) practiced at an academic center or hospital-based practice [50]. Additionally, institutions without GOG data management staff less commonly had open trials available for patients. Further, both physician and patient attitudes impacted clinical trial enrollment [50]. These studies demonstrate that gynecologic oncology therapeutics trials historically have limited penetration beyond academic centers to community and rural practices, further emphasizing the importance of successfully operationalizing a functional screening system at community partner sites within the catchment areas of academic medical centers in the United States.
Unger et al. [18] operationalized the process of clinical trial accrual by defining its discrete steps including identification of available trials, assessment of patient eligibility, patient counseling, and patient decision. This process map effectively shifted the evaluation of stagnating clinical trial enrollment rates from individual-level factors to systemic barriers limiting access to clinical trials. Our results parallel the findings of this systematic review in that the majority of patients did not participate in a clinical trial due to a lack of trial availability followed by ineligibility. Multiple studies support this shift in paradigm away from an emphasis on individual-level factors, as prior reviews show that more than half of patients offered a clinical trial agree to participate without significant differences by age, race, or rurality (19). Prior studies emphasizing individual barriers have variable statistical significance and notable potential for bias [15,51]. More recently, Caston et al. [52] retrospectively assessed outcomes for underrepresented breast and ovarian cancer populations against the Unger et al. [18] clinical trial accrual process map. The investigators noted that differences in trial eligibility did not drive differences in enrollment among patients who were black, rural, or living in areas of higher disadvantage. However, black patients’ lower odds of enrollment were mitigated when they were counseled and offered the chance to enroll. In contrast, patients from disadvantaged neighborhoods had lower odds of enrollment and higher odds of declining enrollment when offered [52].
In the present study, no patients were found to be eligible for a clinical trial to permit assessment of individual-level factors impacting enrollment decisions. Our study illustrates the challenges in defining the minimum necessary data collection parameters to adequately permit the identification of relevant clinical trials and address areas of unmet need. Other studies have characterized demographic disparities in clinical trial recruitment specifically as they relate to racial/ethnic minorities and rural patients [14,25]. However, these same reviews have noted that many disparities in clinical trial enrollment and acceptability based on demographic factors are eliminated when all patients are informed and queried about clinical trials in a systematic, standardized, and patient-centered fashion. Our electronic screening log system benefited from reduced administrative burden by eliminating the collection of demographic variables that are time-consuming to collect and that can create a sense of “othering” and defensiveness in the process of their collection while not being directly relevant to clinical trial eligibility. It was reassuring that no patients’ screening failed due to incompatible age, race/ethnicity, performance status, co-morbid conditions and/or organ function, pregnancy and/or breastfeeding status, or psychiatric conditions. This begins to define areas where other existing electronic screening and enrollment systems can streamline data collection to improve system efficiency.
In our population, 80 % of patients were screened at community sites in rural counties. Though clinical trial participation is one path to increasing equity in cancer care for rural patients, many rural patients face greater barriers to clinical trial participation than their urban counterparts. Mudaranthakam et al. [22] evaluated clinical trial availability in the same catchment area as the present study and found that urban patients with breast and lung cancer were 3.56 and 4.27 times more likely to have an available clinical trial, respectively. This aligns with prior studies that report a median participation rate of 9 % for rural patients in clinical trials [[53], [54]]]. Provider-level barriers to clinical trial enrollment for rural patients included lack of awareness about available clinical trials, workload/time required to counsel patients on trials, lack of supporting professional networks, and concerns about potential patient non-compliance and/or ineligibility due to comorbid conditions and/or challenging social situations [[53], [54]]. Patient-level barriers to clinical trial participation for rural patients included knowledge and attitudes regarding the amount of effort and financial resources required to participate in trials and associations between clinical trials and “being in an experiment,” “gambling on a game of chance,” and/or an option of “last resort” [53]. Our high rates of patient screening at rural MCA community partner sites demonstrate some ability to overcome these challenges. Notably, this was driven by high screening rates at one rural MCA community partner site where CRCs self-reported significant protected time and high interest in clinical trial participation. This suggests that adequate staffing and infrastructure remain critical.
Prior studies have investigated interventions to increase clinical trial recruitment at community sites, including physician awareness/education campaigns [55], direct-to-patient recruitment letters and surveys [56,57], patient navigation programs [58,59], and institutional policy shifts [60]. Though some gains were realized at individual institutions with the above interventions, incremental increases were modest, and a recent study still demonstrated a four-fold difference in recruitment between NCI-designed tertiary care centers and partnered community sites [61]. Ultimately, a multimodal group of interventions, quality improvement processes, and regular program assessment is needed to affect meaningful and sustained increases in clinical trial participation for community-dwelling cancer patients. New models for trial decentralization have emerged, including public-private partnerships and community practice oncology alliances that have increased the penetration of clinical trials into community settings, even independent of a supporting NCI-designated tertiary care center [62]. Telemedicine may also help in this endeavor [63]. Clinical trial delivery is highly context-dependent [64]; further implementation science research is warranted to understand how to determine which model(s) of clinical trial delivery best fit within different geographic and practice settings. As Shalowitz et al. [65] recently outlined, ethical outreach in cancer care requires specific localized needs assessment. This allows us to determine when and how to deploy clinical trial infrastructure to maintain clinical safeguards for an adequate standard of cancer care while adequately addressing patients’ priorities and unmet needs [65].
The strengths of our study lie in the collection and comprehensive reporting of real-world data across multiple diverse community settings in a NCI-designated cancer center's large catchment area and in detailing a clinical trial screening process leveraging an established clinical trial implementation science framework [14]. Montes de Oca et al. [66] noted that only 1–14 % of clinical trials reported race and ethnicity data; to date, few studies provide any data to assess the enrollment of rural patients. Publication bias also limits reporting and rigorous analysis of negative results in studies with small sample sizes. However, this is an ongoing reality for gynecologic cancer clinical trials. Rigorous needs assessment is only effective with ongoing quality improvement and implementation science analysis to identify areas for improvement and to design tailored interventions to address them. These strengths also represent the study's limitations, including the small sample size limiting statistical analysis and comparison among subgroups, incomplete dataset, and the absence of contextualizing clinical (e.g., current treatment regimen, performance status, comorbidities) and qualitative data to support root cause analysis of screening failures. This is a planned area of future study. Additionally, the final two years of data collection occurred during the COVID-19 pandemic when clinical trial resources became further strained. This likely negatively impacted the efficacy and reliability of existing protocols for screening patients for clinical trials. The COVID-19 pandemic also limited resource allocation to clinical trials and created unique barriers for patients to participate in clinical trials [67].
5. Conclusions
An electronic screening log system can help increase awareness around gynecologic oncology clinical trials for primary and recurrent disease. However, it is inadequate as a stand-alone intervention to increase the identification of available clinical trials and facilitate enrollment at community partner sites in a NCI-designated cancer center's large and geographically diverse catchment area. Use of standardized decision-making supports for community site CRCs, regular centralized auditing, and scheduled recurring updates of patient records can increase identification effectiveness and efficiency of the system. Increased inclusion of histologic and molecular factors, rescreening patients at relevant intervals, and greater coordination with the primary academic medical center study CRCs may increase the utility of this tool. Further research is required to understand the challenges to comprehensive screening and recruitment of gynecologic cancer patients for clinical trials at community practices. This research is imperative to ensure equity in cancer care for all gynecologic cancer patients.
Ethics approval and consent to participate
The University of Kansas Medical Center Institutional Review Board (IRB) approved the study as the central IRB and governing body for all MCA institutions (STUDY00002341). The original study was approved as a quality improvement project, and the University of Kansas Medical Center Institutional Review Board waived the need for patient consent. There were no identifiers or patient information was collected as part of the screening log.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
Not applicable.
CRediT authorship contribution statement
Rubina Ratnaparkhi: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Gary C. Doolittle: Supervision, Methodology, Investigation, Conceptualization. Hope Krebill: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Michelle Springer: Writing – original draft, Project administration, Data curation. Elizabeth Calhoun: Writing – original draft, Supervision, Formal analysis, Conceptualization. Andrea Jewell: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Dinesh Pal Mudaranthakam: Writing – review & editing, Writing – original draft, Supervision, Software, Resources, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2024.101379.
List of Abbreviations
- NCTN -
National Clinical Trials Network
- NCI -
National Cancer Institute
- CTEP -
Cancer Therapy Evaluation Program
- KUCC -
The University of Kansas Cancer Center
- MCA -
Masonic Cancer Alliance
- IRB -
Institutional Review Board
- REDCap -
Research Electronic Data Capture
- GCP -
Good Clinical Practice
- EPCT -
Early Phase Clinical Trials
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Reade C., Elit L. Trends in gynecologic cancer care in North America. Obstet. Gynecol. Clin. N. Am. 2012;39:107–129. doi: 10.1016/j.ogc.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Tan W., Stehman F.B., Carter R.L. Mortality rates due to gynecologic cancers in New York state by demographic factors and proximity to a Gynecologic Oncology Group member treatment center: 1979–2001. Gynecol. Oncol. 2009;114:346–352. doi: 10.1016/j.ygyno.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Petersen S., Shahiri P., Jewell A., Spoozak L., Chapman J., Fitzgerald-Wolff S., Lai S.M., Khabele D. Disparities in ovarian cancer survival at the only NCI-designated cancer center in Kansas. Am. J. Surg. 2021 Apr;221(4):712–717. doi: 10.1016/j.amjsurg.2020.12.009. Epub 2020 Dec 7. PMID: 33309256; PMCID: PMC8052277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shalowitz D.I., Vinograd A.M., Giuntoli R.L., 2nd Geographic access to gynecologic cancer care in the United States. Gynecol. Oncol. 2015 Jul;138(1):115–120. doi: 10.1016/j.ygyno.2015.04.025. Epub 2015 Apr 25. PMID: 25922191. [DOI] [PubMed] [Google Scholar]
- 6.Ricci S., Tergas A.I., Long Roche K., et al. Geographic disparities in the distribution of the U.S. gynecologic oncology workforce: a Society of Gynecologic Oncology study. Gynecol Oncol Rep. 2017;22:100–104. doi: 10.1016/j.gore.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart S.L., Cooney D., Hirsch S., et al. The effect of gynecologic oncologist availability on ovarian cancer mortality. World J. Obstet. Gynecol. 2014;3(2):71–77. doi: 10.5317/wjog.v3.i2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talbott J., Khurana A., Wasson M. Supply of obstetrician-gynecologists and gynecologic oncologists to the US Medicare population: a state-by-state analysis. Am. J. Obstet. Gynecol. 2023 Feb;228(2):203.e1–203.e9. doi: 10.1016/j.ajog.2022.09.005. Epub 2022 Sep 8. PMID: 36088988. [DOI] [PubMed] [Google Scholar]
- 9.Patel K.B.P.A., Williams H., Coste H., Zhang L.F., Sadek R., Wallbillich J.J., Ghamande S., Rungruang B.J. 2018. Participation in Clinical Trials May Overcome Health Disparities in the Treatment of Advanced or Recurrent Epithelial Ovarian Cancer. [Google Scholar]
- 10.Sud S., Holmes J., Eblan M., Chen R., Jones E. Clinical characteristics associated with racial disparities in endometrial cancer outcomes: a surveillance, epidemiology and end results analysis. Gynecol. Oncol. 2018;148(2):349–356. doi: 10.1016/j.ygyno.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Unger J.M., Moseley A., Symington B., Chavez-MacGregor M., Ramsey S.D., Hershman D.L. Geographic distribution and survival outcomes for rural patients with Cancer treated in Clinical trials. JAMA Netw. Open. 2018;1(4) doi: 10.1001/jamanetworkopen.2018.1235. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones N., Wilhite A., Paladugu R., Tinker N., Hude C., Scalici J., et al. Eliminating racial disparities in endometrial cancer clinical trial enrollment in the deep south: a pathway to equity. Gynecol. Oncol. 2021;162:S6. [Google Scholar]
- 13.Unger J.M., Moseley A., Symington B., Chavez-MacGregor M., Ramsey S.D., Hershman D.L. Geographic distribution and survival outcomes for rural patients with cancer treated in clinical trials. JAMA Netw. Open. 2018;1(4) doi: 10.1001/jamanetworkopen.2018.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Participation in clinical trials may overcome health disparities in ovarian cancer. 2018. https://www.sgo.org/news/participation-in-clinical-trials-may-overcome-health-disparities-in-ovarian-cancer/
- 15.Unger J.M., Hershman D.L., Till C., et al. "When offered to participate": a systematic review and meta-analysis of patient agreement to participate in cancer clinical trials. J. Natl. Cancer Inst. 2021;113(3):244–257. doi: 10.1093/jnci/djaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sateren W.B., Trimble E., Abrams J., et al. How socio-economic, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J. Clin. Oncol. 2002;20:2019–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 17.Mills E.J., Seely D., Rachlis B., et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7(2):141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 18.Unger J.M., Vaidya R., Hershman D.L., Minasian L.M., Fleury M.E. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J. Natl. Cancer Inst. 2019;111(3):245–255. doi: 10.1093/jnci/djy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Carmen M.G., Annunziata C.M., Rice L.W. The clinical trials crisis in gynecologic oncology. Gynecol. Oncol. 2017;145(3):481–482. doi: 10.1016/j.ygyno.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 20.The crisis in gynecologic cancer clinical trial access. 2017. https://www.sgo.org/wp-content/uploads/2012/09/SGO-Clinical-Trial-Crisis-FINAL.pdf
- 21.Gafford J.A., Gurley-Calvez T., Krebill H., Lai S.M., Christiadi Doolittle GC. Expanding local cancer clinical trial options: analysis of the economic impact of the midwest cancer alliance in Kansas. Acad. Med. 2017;92(9):1274–1279. doi: 10.1097/ACM.0000000000001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mudaranthakam D.P., Gajewski B., Krebill H., et al. Barriers to clinical trial participation: comparative study between rural and urban participants. JMIR Cancer. 2022;8(2) doi: 10.2196/33240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inf. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahnd W.E., Davis M.M., Rotter J.S., et al. Rural-urban differences in financial burden among cancer survivors: an analysis of a nationally representative survey. Support. Care Cancer. 2019;27:4779–4786. doi: 10.1007/s00520-019-04742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nass S.J., Cogle C.R., Brink J.A., et al. Improving cancer diagnosis and care: patient access to oncologic imaging expertise. J. Clin. Oncol. 2019;37:1690–1694. doi: 10.1200/JCO.18.01970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virani S., Burke L., Remick S.C., et al. Barriers to recruitment of rural patients in cancer clinical trials. J Oncol Pract. 2011;7:172–177. doi: 10.1200/JOP.2010.000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levit Laura A., et al. Closing the rural cancer care gap: three institutional approaches. JCO Oncol Pract. 2020;16:422–430. doi: 10.1200/OP.20.00174. [DOI] [PubMed] [Google Scholar]
- 29.Sanders A and Cromartie J, Rural-Urban Continuum Codes. Economic Research Service, U.S. Department of Agriculture. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/.
- 30.Tew M., Catchpool M., Furler J., et al. Site-specific factors associated with clinical trial recruitment efficiency in general practice settings: a comparative descriptive analysis. Trials. 2023;24(1):164. doi: 10.1186/s13063-023-07177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institutes of Health, National Cancer Institute "Cancer Center SupportGrants(CCSGs)forNCI-designatedCancerCenters(P30)(PAR-12298)". Bethesda, MD: NIH. Available from: https://grants.nih.gov/grants/guide/pa-files/PAR-12-298.html.
- 32.National Institutes of Health, National Cancer Institute, "Cancer Center Support Grants (CCSGs) for NCI-designated Cancer Centers (P30) (PAR-17-095). "Bethesda, MD: NIH. Available from: https://grants.nih.gov/grants/guide/pa-files/PAR-17-095.html.29716925.
- 33.Paskett E.D., Hiatt R.A. Catchment areas and community outreach and engagement: the new mandate for NCI-designated cancer centers. Cancer Epidemiol. Biomarkers Prev. 2018 May;27(5):517–519. doi: 10.1158/1055-9965.EPI-17-1050. [DOI] [PubMed] [Google Scholar]
- 34.Hiatt R.A., Sibley A., Fejerman L., Glantz S.A., Nguyen T., Pasick R., et al. The San Francisco CancerInitiative: a communityinitiative to reducethe population burden of cancer. Health Aff. 2018;37:54–61. doi: 10.1377/hlthaff.2017.1260. [DOI] [PubMed] [Google Scholar]
- 35.Paskett E.D., Hiatt R.A. Catchment areas and community outreach and engagement: the new mandate for NCI-designated cancer centers. Cancer Epidemiol. Biomarkers Prev. 2018 May;27(5):517–519. doi: 10.1158/1055-9965.EPI-17-1050. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen L.H., Cook E.D. A primer for cancer research programs on defining and evaluating the catchment area and evaluating minority clinical trials recruitment. Adv. Cancer Res. 2020;146:219–226. doi: 10.1016/bs.acr.2020.02.001. Epub 2020 Mar 14. PMID: 32241390. [DOI] [PubMed] [Google Scholar]
- 37.Spinosa D., Howel E., Montes de Oca M.K., et al. Gynecologic oncology clinical trials: study the studies to terminate the terminations. Gynecol. Oncol. 2021 doi: 10.1016/S0090-8258(21)00960-4. [DOI] [Google Scholar]
- 38.Klabunde C.N., Springer B.C., Butler B., et al. Factors influencing enrollment in clinical trials for cancer treatment. South. Med. J. 1999;92:1189–1193. doi: 10.1097/00007611-199912000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Hunter C.P., Frelick R.W., Feldman A.R., et al. Selection factors in clinical trials: results from the community clinical oncology program physician's patient log. Cancer Treat Rep. 1987;71:559–565. [PubMed] [Google Scholar]
- 40.Simon M.S., Brown D.R., Du W., et al. Accrual to breast cancer clinical trials at a university-affiliated hospital in metropolitan Detroit. Am. J. Clin. Oncol. 1999;22:42–46. doi: 10.1097/00000421-199902000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Newhouse J.P., McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu. Rev. Publ. Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 42.Unger J.M., Barlow W.E., Martin D.P., et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J. Natl. Cancer Inst. 2014;106 doi: 10.1093/jnci/dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He J., Morales D.R., Guthrie B. Exclusion rates in randomized controlled trials of treatments for physical conditions: a systematic review. Trials. 2020;21:228. doi: 10.1186/s13063-020-4139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clisant S., Clermont A., Adenis A., et al. Inflation in the number of eligibility criteria for industry-sponsored phase II cancer clinical trial: illustration over a 20-year period. Contemp. Clin. Trials. 2012;33:459. doi: 10.1016/j.cct.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Cragg W.J., McMahon K., Oughton J.B., et al. Clinical trial recruiters' experiences working with trial eligibility criteria: results of an exploratory, cross-sectional, online survey in the UK. Trials. 2021;22:736. doi: 10.1186/s13063-021-05723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross J., Tu S., Carini S., Sim I. Analysis of eligibility criteria complexity in clinical trials. Summit on Translat Bioinforma. 2010;2010:46–50. http://www.ncbi.nlm.nih.gov/pubmed/21347148 [PMC free article] [PubMed] [Google Scholar]
- 47.Statler A., Othus M., Erba H.P., Chauncey T.R., Radich J.P., Coutre S., et al. Comparable outcomes of patients eligible vs ineligible for SWOG leukemia studies. Blood. 2018;131(25):2782–2788. doi: 10.1182/blood-2018-01-826693. Rimel BJ, Lester J, Sabacan L, et al. A novel clinical trial recruitment strategy for women's cancer. Gynecol Oncol. 2015;138(2):445-2788. doi:10.1016/j.ygyno.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falk D., Tooze J.A., Winkfield K.M., et al. A comparison of survey incentive methods to recruit rural cancer survivors into cancer care delivery research studies. Cancer Causes Control. 2022;33(11):1381–1386. doi: 10.1007/s10552-022-01621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manders D.B., Paulsen A., Richardson D.L., Kehoe S.M., Miller D.S., Lea J.S. Factors associated with clinical trial screening failures in gynecologic oncology. Gynecol. Oncol. 2014;134(3):450–454. doi: 10.1016/j.ygyno.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 50.Ford J.G., Howerton M.W., Lai G.Y., et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 51.Brooks S.E., Carter R.L., Plaxe S.C., et al. Patient and physician factors associated with participation in cervical and uterine cancer trials: an NRG/GOG247 study. Gynecol. Oncol. 2015;138(1):101–108. doi: 10.1016/j.ygyno.2015.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McPhee N.J., Nightingale C.E., Harris S.J., Segelov E., Ristevski E. Barriers and enablers to cancer clinical trial participation and initiatives to improve opportunities for rural cancer patients: a scoping review. Clin. Trials. 2022;19(4):464–476. doi: 10.1177/17407745221090733. [DOI] [PubMed] [Google Scholar]
- 53.Caston N.E., Lalor F., Wall J., et al. Ineligible, unaware, or uninterested? Associations between underrepresented patient populations and retention in the pathway to cancer clinical trial enrollment. JCO Oncol Pract. 2022 doi: 10.1200/OP.22.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bell J.A.H., Kelly M.T., Gelmon K., et al. Gatekeeping in cancer clinical trials in Canada: the ethics of recruiting the "ideal" patient. Cancer Med. 2020;9(12):4107–4113. doi: 10.1002/cam4.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paskett E.D., Cooper M.R., Stark N., et al. Clinical trial enrollment of rural patients with cancer. Cancer Pract. 2002;10:28–35. doi: 10.1046/j.1523-5394.2002.101006.x. [DOI] [PubMed] [Google Scholar]
- 56.Brown S.D., Partee P.N., Feng J., et al. Outreach to diversify clinical trial participation: a randomized recruitment study. Clin. Trials. 2015;12(3):205–211. doi: 10.1177/1740774514568125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghebre R.G., Jones L.A., Wenzel J.A., Martin M.Y., Durant R.W., Ford J.G. State-of-the-science of patient navigation as a strategy for enhancing minority clinical trial accrual. Cancer. 2014;120(Suppl 7):1122–1130. doi: 10.1002/cncr.28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fouad M.N., Partridge E., Dignan M., et al. A community-driven action plan to eliminate breast and cervical cancer disparity: successes and limitations. J. Cancer Educ. 2006;21(Suppl 1):91–100. doi: 10.1207/s15430154jce2101s_16. [DOI] [PubMed] [Google Scholar]
- 59.Anwuri V.V., Hall L.E., Mathews K., et al. An institutional strategy to increase minority recruitment to therapeutic trials. Cancer Causes Control. 2013;24(10):1797–1809. doi: 10.1007/s10552-013-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Unger J.M., Fleury M. Nationally representative estimates of the participation of cancer patients in clinical research studies according to the commission on cancer. J. Clin. Oncol. 2021;39(Suppl 28) Abstr 74. [Google Scholar]
- 61.Virgil H. Decentralized clinical trials conducted in community oncology practices may unlock opportunities to address disparities. 2023. https://www.cancernetwork.com/view/decentralized-clinical-trials-conducted-in-community-oncology-practices-may-unlock-opportunities-to-address-disparities
- 62.Sundquist S., Batist G., Brodeur-Robb K., et al. CRAFT-A proposed framework for decentralized clinical trials participation in Canada. Curr. Oncol. 2021;28(5):3857–3865. doi: 10.3390/curroncol28050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doll K.M. Minority enrollment on clinical trials enhances scientific rigor but requires structural changes and commitment. Gynecol. Oncol. 2020;157(2):301–302. doi: 10.1016/j.ygyno.2020.04.682. [DOI] [PubMed] [Google Scholar]
- 64.Shalowitz D.I., Magalhaes M., Miller F.G. Ethical outreach for rural cancer care in the United States: balancing access with optimal clinical outcomes. JCO Oncol Pract. 2023 doi: 10.1200/OP.22.00629. [DOI] [PubMed] [Google Scholar]
- 65.Montes de Oca M.K., Howell E.P., Spinosa D., et al. Diversity and transparency in gynecologic oncology clinical trials. Cancer Causes Control. 2023;34(2):133–140. doi: 10.1007/s10552-022-01646-y. [DOI] [PubMed] [Google Scholar]
- 66.Pothuri B., Alvarez Secord A., Armstrong D.K., et al. Anti-cancer therapy and clinical trial considerations for gynecologic oncology patients during the COVID-19 pandemic crisis. Gynecol. Oncol. 2020;158(1):16–24. doi: 10.1016/j.ygyno.2020.04.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data will be made available on request.