Abstract
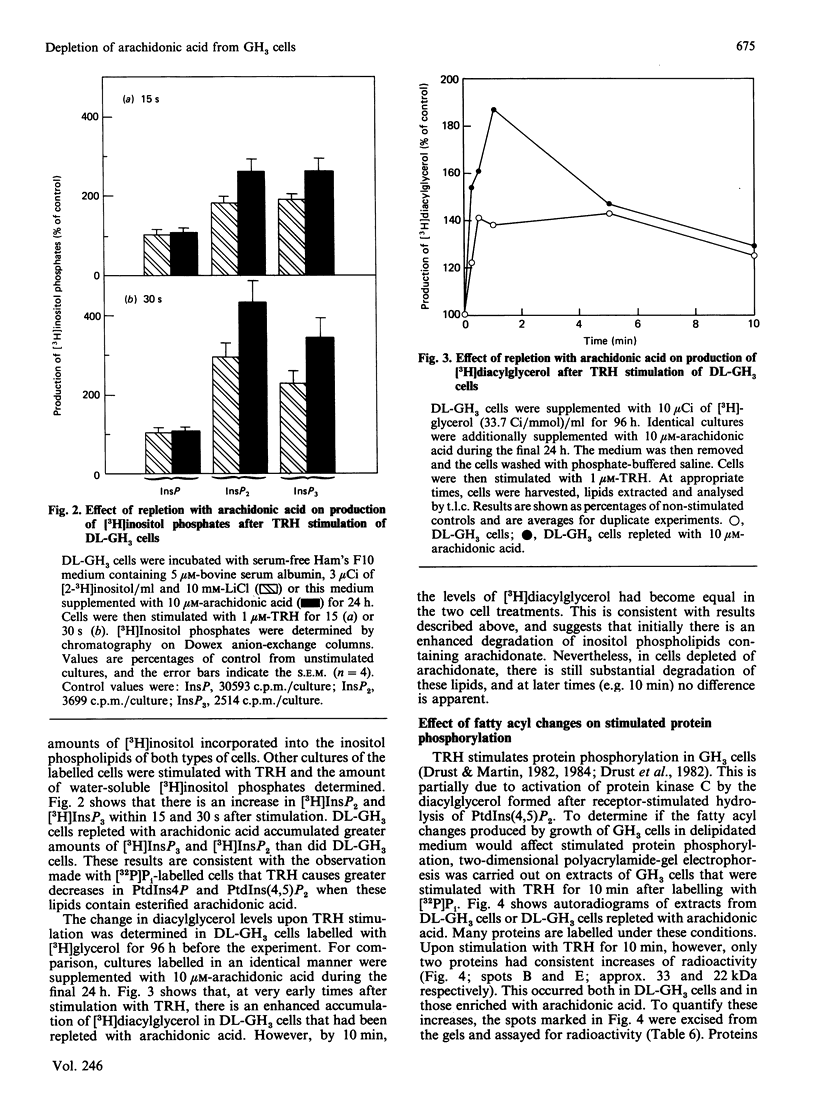
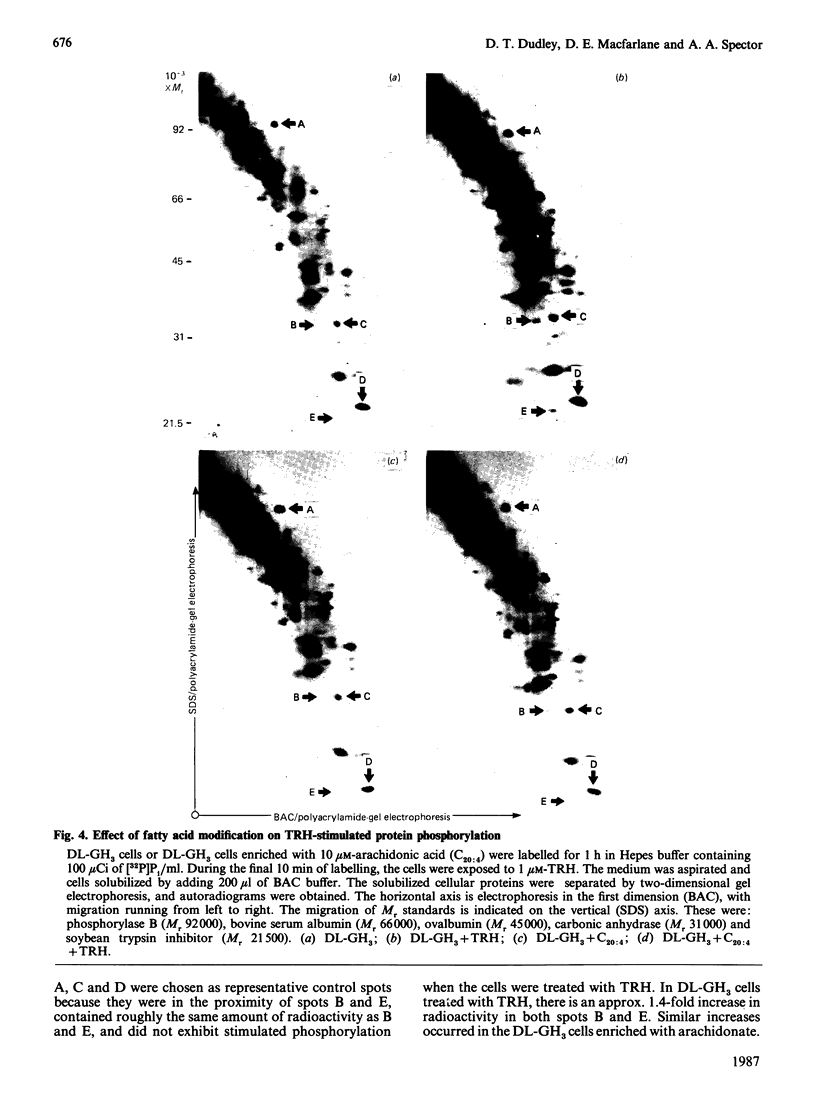
We have adapted rat pituitary GH3 cells to grow in delipidated culture medium. In response, esterfied linoleic acid and arachidonic acid become essentially undetectable, whereas eicosa-5,8,11-trienoic acid accumulates and oleic acid increases markedly. These changes occur in all phospholipid classes, but are particularly pronounced in inositol phospholipids, where the usual stearate/arachidonate profile is replaced with oleate/eicosatrienoate (n - 9) and stearate/eicosatrienoate (n - 9). Incubation of arachidonate-depleted cells with 10 microM-arachidonic acid for only 24 h results in extensive remodelling of phospholipid fatty acids, such that close-to-normal compositions and arachidonic acid content are achieved for the inositol phospholipids. In comparison studies with arachidonic acid-depleted or -repleted cells, it was found that the arachidonate content does not affect thyrotropin-releasing-hormone (TRH)-stimulated responses measured at long time points, including [32P]Pi labelling of phosphatidylinositol and phosphatidic acid, stimulation of protein phosphorylation, and basal or TRH-stimulated prolactin release. However, transient events such as stimulated breakdown of inositol phospholipids and an initial rise in diacylglycerol are enhanced by the presence of arachidonate. These results show that arachidonic acid itself is not required for operation of the phosphatidylinositol cycle and is not an obligatory intermediate in TRH-mediated GH3 cell activation. It is possible that any structural or functional role of arachidonic acid in these processes is largely met by replacement with eicosatrienoate (n - 9). However, since arachidonate in inositol phospholipids facilitates their hydrolysis upon stimulation by TRH, arachidonic acid apparently may have a specific role in the recognition of these lipids by phospholipase C.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Tashjian A. H., Jr Thyrotropin-releasing hormone-induced spike and plateau in cytosolic free Ca2+ concentrations in pituitary cells. Relation to prolactin release. J Biol Chem. 1984 May 10;259(9):5827–5832. [PubMed] [Google Scholar]
- Allan D., Cockcroft S. The fatty acid composition of 1,2-diacylglycerol and polyphosphoinositides from human erythrocyte membranes. Biochem J. 1983 Aug 1;213(2):555–557. doi: 10.1042/bj2130555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M. Essential fatty acid requirements and metabolism of cells in tissue culture. Adv Prostaglandin Thromboxane Res. 1980;6:549–565. [PubMed] [Google Scholar]
- Baker R. R., Thompson W. Positional distribution and turnover of fatty acids in phosphatidic acid, phosphinositides, phosphatidylcholine and phosphatidylethanolamine in rat brain in vivo. Biochim Biophys Acta. 1972 Aug 11;270(4):489–503. doi: 10.1016/0005-2760(72)90114-2. [DOI] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Phosphatidylinositol hydrolysis: a multifunctional transducing mechanism. Mol Cell Endocrinol. 1981 Nov;24(2):115–140. doi: 10.1016/0303-7207(81)90055-1. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 activity specific for phosphatidic acid. A possible mechanism for the production of arachidonic acid in platelets. J Biol Chem. 1981 Jun 10;256(11):5399–5403. [PubMed] [Google Scholar]
- Canonico P. L., Cronin M. J., MacLeod R. M. Diacylglycerol lipase and pituitary prolactin release in vitro: studies employing RHC 80267. Life Sci. 1985 Mar 11;36(10):997–1002. doi: 10.1016/0024-3205(85)90397-2. [DOI] [PubMed] [Google Scholar]
- Canonico P. L., Judd A. M., Koike K., Valdenegro C. A., MacLeod R. M. Arachidonate stimulates prolactin release in vitro: a role for the fatty acid and its metabolites as intracellular regulator(s) in mammotrophs. Endocrinology. 1985 Jan;116(1):218–225. doi: 10.1210/endo-116-1-218. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chalvardjian A., Rudnicki E. Determination of lipid phosphorus in the nanomolar range. Anal Biochem. 1970 Jul;36(1):225–226. doi: 10.1016/0003-2697(70)90352-0. [DOI] [PubMed] [Google Scholar]
- Chau L. Y., Tai H. H. Release of arachidonate from diglyceride in human platelets requires the sequential action of a diglyceride lipase and a monoglyceride lipase. Biochem Biophys Res Commun. 1981 Jun;100(4):1688–1695. doi: 10.1016/0006-291x(81)90713-0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Baldwin J. M., Allan D. The Ca2+-activated polyphosphoinositide phosphodiesterase of human and rabbit neutrophil membranes. Biochem J. 1984 Jul 15;221(2):477–482. doi: 10.1042/bj2210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drust D. S., Martin T. F. Thyrotropin-releasing hormone rapidly activates protein phosphorylation in GH3 pituitary cells by a lipid-linked, protein kinase C-mediated pathway. J Biol Chem. 1984 Dec 10;259(23):14520–14530. [PubMed] [Google Scholar]
- Drust D. S., Martin T. F. Thyrotropin-releasing hormone rapidly and transiently stimulates cytosolic calcium-dependent protein phosphorylation in GH3 pituitary cells. J Biol Chem. 1982 Jul 10;257(13):7566–7573. [PubMed] [Google Scholar]
- Drust D. S., Sutton C. A., Martin T. F. Thyrotropin-releasing hormone and cyclic AMP activate distinctive pathways of protein phosphorylation in GH pituitary cells. J Biol Chem. 1982 Mar 25;257(6):3306–3312. [PubMed] [Google Scholar]
- Dudley D. T., Spector A. A. Inositol phospholipid arachidonic acid metabolism in GH3 pituitary cells. Biochem J. 1986 May 15;236(1):235–242. doi: 10.1042/bj2360235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULCO A. J., MEAD J. F. Metabolism of essential fatty acids. VIII. Origin of 5,8,11-eicosatrienoic acid in the fat-deficient rat. J Biol Chem. 1959 Jun;234(6):1411–1416. [PubMed] [Google Scholar]
- Fine J. B., Sprecher H. Unidimensional thin-layer chromatography of phospholipids on boric acid-impregnated plates. J Lipid Res. 1982 May;23(4):660–663. [PubMed] [Google Scholar]
- Gershengorn M. C., Geras E., Purrello V. S., Rebecchi M. J. Inositol trisphosphate mediates thyrotropin-releasing hormone mobilization of nonmitochondrial calcium in rat mammotropic pituitary cells. J Biol Chem. 1984 Sep 10;259(17):10675–10681. [PubMed] [Google Scholar]
- Gershengorn M. C., Thaw C. Calcium influx is not required for TRH to elevate free cytoplasmic calcium in GH3 cells. Endocrinology. 1983 Oct;113(4):1522–1524. doi: 10.1210/endo-113-4-1522. [DOI] [PubMed] [Google Scholar]
- Ghosh D., Williams M. A., Tinoco J. The influence of lecithin structure on their monolayer behavior and interactions with cholesterol. Biochim Biophys Acta. 1973 Jan 26;291(2):351–362. doi: 10.1016/0005-2736(73)90488-4. [DOI] [PubMed] [Google Scholar]
- Gitler C. Use of ANS to detect phospholipids and apolar molecules in chromatograms. Anal Biochem. 1972 Nov;50(1):324–325. doi: 10.1016/0003-2697(72)90512-x. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Morton D. L. Double-antibody method and the protein-A-bearing Staphylococcus aureus cells method compared for separating bound and free antigen in radioimmunoassay. Clin Chem. 1979 May;25(5):752–756. [PubMed] [Google Scholar]
- Hammarström S. Conversion of 5,8,11-eicosatrienoic acid to leukotrienes C3 and D3. J Biol Chem. 1981 Mar 10;256(5):2275–2279. [PubMed] [Google Scholar]
- Hirata M., Suematsu E., Hashimoto T., Hamachi T., Koga T. Release of Ca2+ from a non-mitochondrial store site in peritoneal macrophages treated with saponin by inositol 1,4,5-trisphosphate. Biochem J. 1984 Oct 1;223(1):229–236. doi: 10.1042/bj2230229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Holub B. J., Kuksis A., Thompson W. Molecular species of mono-, di-, and triphosphoinositides of bovine brain. J Lipid Res. 1970 Nov;11(6):558–564. [PubMed] [Google Scholar]
- Jakschik B. A., Sams A. R., Sprecher H., Needleman P. Fatty acid structural requirements for leukotriene biosynthesis. Prostaglandins. 1980 Aug;20(2):401–410. doi: 10.1016/s0090-6980(80)80057-8. [DOI] [PubMed] [Google Scholar]
- Jolles J., Zwiers H., Dekker A., Wirtz K. W., Gispen W. H. Corticotropin-(1--24)-tetracosapeptide affects protein phosphorylation and polyphosphoinositide metabolism in rat brain. Biochem J. 1981 Jan 15;194(1):283–291. doi: 10.1042/bj1940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kaibuchi K., Sano K., Hoshijima M., Takai Y., Nishizuka Y. Phosphatidylinositol turnover in platelet activation; calcium mobilization and protein phosphorylation. Cell Calcium. 1982 Oct;3(4-5):323–335. doi: 10.1016/0143-4160(82)90020-3. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Klenk E. The metabolism of polyenoic fatty acids. Adv Lipid Res. 1965;3:1–23. doi: 10.1016/b978-1-4831-9939-9.50007-3. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. N., Gershengorn M. C. Ca2+ ionophores affect phosphoinositide metabolism differently than thyrotropin-releasing hormone in GH3 pituitary cells. J Biol Chem. 1984 Aug 10;259(15):9514–9519. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Billah M. M., Cuatrecasas P. Rapid acylation and deacylation of arachidonic acid into phosphatidic acid of horse neutrophils. J Biol Chem. 1980 Nov 25;255(22):10966–10970. [PubMed] [Google Scholar]
- Laposata M., Prescott S. M., Bross T. E., Majerus P. W. Development and characterization of a tissue culture cell line with essential fatty acid deficiency. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7654–7658. doi: 10.1073/pnas.79.24.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg W. O. The significance of cis, cis, cis 5,8,11 eicosatrienoic acid in essential fatty acid deficiency. Nutr Rev. 1980 Jul;38(7):233–235. doi: 10.1111/j.1753-4887.1980.tb05910.x. [DOI] [PubMed] [Google Scholar]
- MEAD J. F., SLATON W. H., Jr Metabolism of essential fatty acids. III. Isolation of 5,8,11-eicosatrienoic acid from fat-deficient rats. J Biol Chem. 1956 Apr;219(2):705–709. [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Macfarlane D. E. Inhibitors of cyclic nucleotide phosphodiesterases inhibit protein carboxyl methylation in intact blood platelets. J Biol Chem. 1984 Jan 25;259(2):1357–1362. [PubMed] [Google Scholar]
- Macfarlane D. E. Phorbol diester-induced phosphorylation of nuclear matrix proteins in HL60 promyelocytes. Possible role in differentiation studied by cationic detergent gel electrophoresis. J Biol Chem. 1986 May 25;261(15):6947–6953. [PubMed] [Google Scholar]
- Macfarlane D. E. Use of benzyldimethyl-n-hexadecylammonium chloride ("16-BAC"), a cationic detergent, in an acidic polyacrylamide gel electrophoresis system to detect base labile protein methylation in intact cells. Anal Biochem. 1983 Jul 15;132(2):231–235. doi: 10.1016/0003-2697(83)90001-5. [DOI] [PubMed] [Google Scholar]
- Macphee C. H., Drummond A. H. Thyrotropin-releasing hormone stimulates rapid breakdown of phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 4-phosphate in GH3 pituitary tumor cells. Mol Pharmacol. 1984 Mar;25(2):193–200. [PubMed] [Google Scholar]
- Martin T. F. Thyrotropin-releasing hormone rapidly activates the phosphodiester hydrolysis of polyphosphoinositides in GH3 pituitary cells. Evidence for the role of a polyphosphoinositide-specific phospholipase C in hormone action. J Biol Chem. 1983 Dec 25;258(24):14816–14822. [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- PELUFFO R. O., BRENNER R. R., MERCURI O. ACTION OF LINOLEIC AND ARACHIDONIC ACIDS UPON THE EICOSATRIENOIC ACID LEVEL IN RAT HEART AND LIVER. J Nutr. 1963 Oct;81:110–116. doi: 10.1093/jn/81.2.110. [DOI] [PubMed] [Google Scholar]
- Prescott S. M., Majerus P. W. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J Biol Chem. 1983 Jan 25;258(2):764–769. [PubMed] [Google Scholar]
- Rebecchi M. J., Gershengorn M. C. Thyroliberin stimulates rapid hydrolysis of phosphatidylinositol 4,5-bisphosphate by a phosphodiesterase in rat mammotropic pituitary cells. Evidence for an early Ca2+-independent action. Biochem J. 1983 Nov 15;216(2):287–294. doi: 10.1042/bj2160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecchi M. J., Kolesnick R. N., Gershengorn M. C. Thyrotropin-releasing hormone stimulates rapid loss of phosphatidylinositol and its conversion to 1,2-diacylglycerol and phosphatidic acid in rat mammotropic pituitary cells. Association with calcium mobilization and prolactin secretion. J Biol Chem. 1983 Jan 10;258(1):227–234. [PubMed] [Google Scholar]
- Rothblat G. H., Arbogast L. Y., Ouellette L., Howard B. V. Preparation of delipidized serum protein for use in cell culture systems. In Vitro. 1976 Aug;12(8):554–557. doi: 10.1007/BF02797438. [DOI] [PubMed] [Google Scholar]
- Schacht J. Extraction and purification of polyphosphoinositides. Methods Enzymol. 1981;72:626–631. doi: 10.1016/s0076-6879(81)72054-8. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Kaduce T. L., Figard P. H., Norton K. C., Hoak J. C., Czervionke R. L. Eicosapentaenoic acid and prostacyclin production by cultured human endothelial cells. J Lipid Res. 1983 Dec;24(12):1595–1604. [PubMed] [Google Scholar]
- Stoffel W., Pruss H. D. Monolayer studies with synthetic saturated, mono- and polyunsaturated mixed 1,2-diglycerides, 1,2-diacylphosphatidylethanolamines and phosphatidylcholines at the air-water-interface. Hoppe Seylers Z Physiol Chem. 1969 Nov;350(11):1385–1393. doi: 10.1515/bchm2.1969.350.2.1385. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Vincent J. E., Zijlstra F. J. Formation by phospholipase A2 of prostaglandins and endoperoxides in platelets of normal and essential fatty acid-deficient rats. Adv Prostaglandin Thromboxane Res. 1978;3:143–146. [PubMed] [Google Scholar]
- Ziboh V. A., Hsia S. L. Effects of prostaglandin E 2 on rat skin: inhibition of sterol ester biosynthesis and clearing of scaly lesions in essential fatty acid deficiency. J Lipid Res. 1972 Jul;13(4):458–467. [PubMed] [Google Scholar]