Abstract
Enzyme kinetic parameters for aflatoxin B1 metabolism have been reported for chicken, quail, turkey and duck, but an integrated in silico model has not been proposed. Both enzyme-catalyzed reactions and spontaneous reactions were modeled in the CellDesigner software and results were adjusted to Hill, Rational and Hoerl models. Results revealed that the higher amount of aflatoxin B1 epoxide produced in a short lapse of time and a low production of epoxide conjugated to glutathione explains the severe genotoxic effect of aflatoxin B1 in duck. Also, the higher amount of aflatoxicol produced is time-associated to aflatoxin B1 resistance in chicken. Finally, the cytotoxic effects in quail and duck are caused by a large aflatoxin B1 dialdehyde production in a short period of time.
Keywords: Xenobiotic metabolism simulation, in silico simulation, Aflatoxin B1 metabolism, Cytotoxic pathway, Genotoxic pathway
Graphical Abstract
Highlights
-
•
High AFB1-Gua production is related to a high production of AFBO in a short time.
-
•
Poor AFB1-GSH production enhances AFBO adduction to DNA.
-
•
Storing of AFB1 as AFL is related to resistance to AFB1 in chicken.
-
•
Cytotoxic effects in quail and duck are related to a deficient AFB1 dialdehyde reduction.
1. Introduction
Since the “X disease” outbreak in 1960, where thousands of turkey poults died because of the intake of a Brazilian peanut cake contaminated with high levels of aflatoxin B1 (AFB1; Blount, Turkey [6]), great advances have been achieved in the study of the metabolism of this mycotoxin. Large differences have been found in the adverse effects of AFB1 among commercial poultry species, being the duck the more sensitive, followed by quail>turkey>chicken [9]. Further, differences in the biotransformation rate of AFB1 into different products like the 8,9-dihydro-8-(S-glutathionyl)-9-hydroxy aflatoxin B1 (AFB1-GSH) have been found in poultry (Murcia et al., 2021), where glutathione S-transferase (GST) is the enzyme that catalyzes the nucleophilic trapping of the bioactivated form of AFB1, the aflatoxin B1-8,9-epoxide (AFBO) with glutathione (GSH; [26]). Neutralization of AFBO restricts spontaneous adduction to guanine in DNA, preventing the production of the DNA-AFB1 adduct (AFB1-Gua; [18]) and consequently preventing genotoxicity. It is the case of rodents like rats and mice, where GST activity has a strong association with AFB1 resistance [18], [61]. In addition to GST enzyme activity, other enzyme kinetic parameters have been determined for poultry, as it was found for the aflatoxin B1 dihydrodiol production (AFB1-dhd), which is the hydrolyzed form of AFBO and in turn is able to rearrange into the AFB1 dialdehyde, producing spontaneous adducts with lysine in proteins causing cytotoxicity [15]. Moreover, enzyme kinetic parameters of the reduced form of AFB1 called aflatoxicol (AFL) have already been determined. The formation of AFL allows highly resistant birds, such as the chicken, to resist high AFB1 concentrations, by storing the mycotoxin in a non-toxic form such as AFL [43]. In the same way, enzyme kinetic parameters for aflatoxin B1 monoalcohol and AFB1 dialcohol already have been reported [44].
Integrated models of the metabolism of aflatoxin B1 in commercial poultry species have been proposed by Diaz and Murcia [16] and a kinetic model for human AFB1 metabolism with kinetic rates has been proposed by Guengerich et al. [25], but integration of the different enzyme kinetic parameters or kinetic rates in a simulation over time has not been performed in poultry, neither a non-linear model has been associated with each of the AFB1 biotransformation products. The use of New Approach Methodologies (NAMs) has been raised as a new trend, in order to avoid animal experiments and to assess the adverse effects of candidate xenobiotics [53]. According to this, the use of function models and the selection of a model that fit to data has become a fundamental scientific approach to find out the principles that explain a series of observations and to predict these observations [63] with no dependency on in vivo samples. Different function models have been proposed, and the choose of a model depends on the goodness-of-fit of the dataset to the selected model. For example, the Hill equation is a function model designed to adjust data that manifest a sigmoid behavior, as the binding of O2 to heamoglobin [27]. A modified version of this equation is presented by Gadagkar and Call [21], were a four-parameter logistic nonlinear regression model can be adjusted, where “Cm” is the metabolite concentration at time X, “a” is the minimum asymptote or the response when time = 0, “b” is the maximum asymptote or the stabilized metabolite concentration for an infinite time, “c” is the time at which 50 % of the maximal concentration is reached and “d” is the slope at the steepest part of the curve (also known as the Hill slope). Eq. 1 presents the modified Hill equation.
| (1) |
In the other hand, Rational models are the ratio of two polynomial functions, that can take on an extremely wide range of shapes, accommodating to a much wider range of shapes than does the polynomial family, have better interpolatory and extrapolatory properties than polynomial models and are a particularly easy nonlinear models to fit (NIST/SEMATECH, 2023). Eq. 2 present a Rational model of the type linear/quadratic.
| (2) |
Another function model is the Hoerl function (Eq. 3). This model is part of the power law family, which are a group of equations that raises one or more parameters to the power of the independent variable and can be draw as convex or concave curves with or without inflection points or maxima/minima [30]. According to Wieczerzak et al. [63] the “a” parameter of the Hoerl model can be compared with that of the Gaussian model, representing the sensitivity, the impact or the effect of the system in consideration.
| (3) |
In the case of those function models that produce Gaussian curves as the modified Hill equation or the Hoerl model, the area under the plot of concentration of reaction product versus time after dosage represents the extent of exposure to reaction products and their clearance rate from the body. By integrating over time, a more accurate estimate of the overall exposure is obtained [57]. On the other hand, the time of peak concentration (tmax) of the reaction product shows the time course of drug concentration and the effect of the reaction product, such that the highest magnitude shows up at approximately the time of peak concentration [24].
Because an in silico simulation would allow to compare the production of these metabolites in a time-dependent manner and to associate this time-dependent metabolite behavior with poultry sensitivity, the present study aims at comparing the emulation of the time-dependent production of AFBO, AFL, AFB1-GSH, AFB1-dhd, AFB1 monoalcohol, and AFB1 dialcohol and to find the best-fitting models for each metabolite production reaction to finally associate differences in metabolite production with poultry species sensitivity to AFB1.
2. Materials and methods
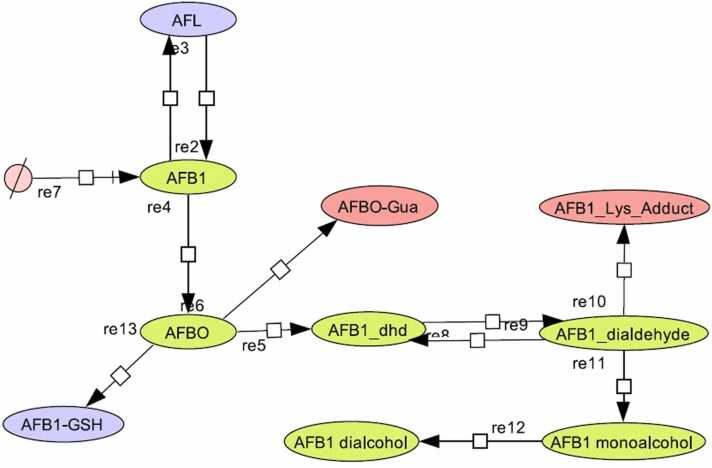
An integrated model of AFB1 metabolism (Fig. 1) was generated for each of twelve individuals from five poultry commercial species, including two chicken breeds (n = 60) in the CellDesigner software version 4.4.2 [19], [20]. The integrated model was constructed with the biotransformation enzyme kinetic parameters Km and Vmax of the Michaelis-Menten model (v = Vmax[S]/Km + [S]) obtained from Murcia and Diaz [42], [43], [44] and Diaz and Murcia [15], for Ross and Rhode Island Red (RIR) chicken breeds (Gallus gallus ssp. domesticus), Nicholas turkeys (Meleagris gallopavo), Japanese quails (Coturnix Coturnix japonica) and Pekin ducks (Anas platyrhynchos ssp. domesticus). Table 1 presents the average value ±standard deviation (SD) of these parameters by poultry species and by reaction. Reactions evaluated are as follows: AFB1 → AFBO reaction is driven by the cytochrome P450 (CYP) enzyme superfamily (E.C. number 1.14.–.-; [1]), specifically the CYP1A1 and CYP1A2 (E.C. number 1.14.1.1), the CYP2A6 (E.C. number 1.14.14.1) and the CYP3A4 (E.C. number 1.14.14.55, 1.14.14.56). Enzyme kinetic parameters for AFBO production are obtained indirectly from enzyme kinetic parameters obtained for AFB1-dhd [12], [13], [14], [15], [39], because AFBO is highly unstable in aqueous solutions (t1/2 = <1 s) and spontaneously the epoxide group in the AFBO is hydrolyzed, producing the AFB1 dihydrodiol [32]. AFB1 → AFL reaction is driven by an AFB1 cytosolic NADPH + H+ reductase and AFL → AFB1 reaction is driven by an AFL cytosolic dehydrogenase. AFB1 dialdehyde → AFB1 monoalcohol and AFB1 monoalcohol → AFB1 dialcohol reactions are driven by the aflatoxin B1 aldehyde reductase (AFAR; EC number 1.1.1.2) and the AFB1 → AFB1-GSH reaction is driven by a glutathione S-transferase (EC number 2.5.1.18).
Fig. 1.
Aflatoxin B1 CellDesigner metabolism model for commercial poultry species.
Table 1.
Average enzyme kinetic parameters Vmax and Km from different metabolic steps of AFB1 hepatic metabolism in commercial poultry species.
| Species | Reaction | Vmax(µM substrate/mg protein/minute) | SD | Km (µM substrate) | SD |
|---|---|---|---|---|---|
| Ross chickens | AFB1 → AFB1-dhd | 23,0 | 7,8 | 131,8 | 26,2 |
| AFB1 → AFL | 2,3 | 0,9 | 2,7 | 0,7 | |
| AFL → AFB1 | 60,8 | 22,8 | 11,8 | 2,6 | |
| AFB1 dialdehyde → AFB1 monoalcohol | 8,6 | 4,5 | 80,2 | 46,5 | |
| AFB1 dialdehyde → AFB1 dialcohol | 1,3 | 0,7 | 19,4 | 11,6 | |
| AFB1 → AFB1-GSH | 0005 | 0001 | 65,6 | 14,4 | |
| RIR chickens | AFB1 → AFB1-dhd | 44,8 | 5,9 | 112,5 | 33,4 |
| AFB1 → AFL | 2,2 | 0,72 | 2,9 | 0,6 | |
| AFL → AFB1 | 56,9 | 13,9 | 11,6 | 2,3 | |
| AFB1 dialdehyde → AFB1 monoalcohol | 40,2 | 22,0 | 393,2 | 227,0 | |
| AFB1 dialdehyde → AFB1 dialcohol | 1,2 | 0,66 | 21,6 | 14,4 | |
| AFB1 → AFB1-GSH | 0,0056 | 0,0005 | 47,4 | 7,1 | |
| Quail | AFB1 → AFB1-dhd | 38,3 | 12,3 | 77,8 | 22,1 |
| AFB1 → AFL | 2,0 | 1,1 | 5,6 | 2,5 | |
| AFL → AFB1 | 92,8 | 31,6 | 29,8 | 6,8 | |
| AFB1 dialdehyde → AFB1 monoalcohol | 9,3 | 6,8 | 231,4 | 208,1 | |
| AFB1 dialdehyde → AFB1 dialcohol | 0,4 | 0,2 | 13,9 | 6,1 | |
| AFB1 → AFB1-GSH | 0003 | 0,001 | 92,6 | 25,2 | |
| Turkey | AFB1 → AFB1-dhd | 23,4 | 8,3 | 49,3 | 7,6 |
| AFB1 → AFL | 3,7 | 1,2 | 13,6 | 4,5 | |
| AFL → AFB1 | 636,9 | 281,2 | 146,8 | 72,4 | |
| AFB1 dialdehyde → AFB1 monoalcohol | 10,5 | 6,1 | 72,8 | 45,9 | |
| AFB1 dialdehyde → AFB1 dialcohol | 1,5 | 0,7 | 12,6 | 6,1 | |
| AFB1 → AFB1-GSH | 0,0007 | 0,0003 | 87,6 | 24,5 | |
| Duck | AFB1 → AFB1-dhd | 22,2 | 5,3 | 3,8 | 1,0 |
| AFB1 → AFL | 11,8 | 3,1 | 46,8 | 7,7 | |
| AFL → AFB1 | 762,7 | 666,5 | 84,0 | 16,5 | |
| AFB1 dialdehyde → AFB1 monoalcohol | 7,4 | 6,3 | 139,5 | 177,2 | |
| AFB1 dialdehyde → AFB1 dialcohol | 0,7 | 0,3 | 15,6 | 5,4 | |
| AFB1 → AFB1-GSH | 00013 | 0,0007 | 61,1 | 47,7 |
Reaction rates for non-enzymatically catalyzed reactions in the integrated model, as the AFBO adduction to guanine in DNA (k = 1.5 μM−1min−1), spontaneous hydrolysis of AFBO into AFB1-dhd (k = 42 min−1 + 0.126 μM−1min−1), rearrangement of AFB1-dhd into AFB1 dialdehyde forward (k = 0.12 μM−1min−1) and reverse (k = 0.012 min−1) reactions, and adduction of AFB1 dialdehyde with lysine (AFB1-Lys adduct; k = 2.4 ×10−4 μM−1min−1) were obtained from a scheme from Guengerich et al. [25], where k represents the reaction rate coefficient of the first-order reaction rate (rate = k[P]) in min−1 units, or the reaction rate coefficient of the second-order reaction rate (rate = k[P]2) in μM−1min−1 units, and P is the product concentration.
The simulation starts at the source (Fig. 1), where the initial concentration of AFB1 in serum plasma is 96 nM. This plasma AFB1 concentration is used according to estimates of maximum AFB1 serum plasma levels found in chicken after an oral administration dose of 2 mg/kg of body weight [36]. This serum plasma concentration used is lethal for duck (LD50 = 0.34 mg/kg BW) but not for turkey (LD50 = 3.2 mg/kg BW) or chicken (LD50 = 18.0 mg/kg BW; [9]). Transmembrane transport of AFB1 from plasma to hepatocyte cytosol (reaction re7 shown in the integrated model) is estimated to be 0.6 μM/mg cellular protein/minute and is independent of membrane carriers (simple diffusion [41]).
Simulation was run in the following software conditions: error tolerance = −6 and the solver chosen was SOSlib. In the species tab, the values for all chemical species were: compartment = default, quantity type = concentration, initial quantity = 0.000 except for the source = 0.096, boundary condition = false, constant = false. Parameters tab values were set according to the enzyme kinetic parameters for each individual, with units = substance and constant = true for all species. The simulation was run assuming an AFB1 single doses and a simulation time of <1440 minutes (1 day - acute exposure).
The dataset of “concentration vs time” obtained for the AFB1 and for the biotransformation products obtained from the integrated model per bird per poultry species in the CellDesigner software was then subjected to the CurveExpert Professional Software version 2.7.3 [30], to search in all available regressions for the function model with the lowess smoothing and the best fit to data. The criterion for selection of the function model was the score value obtained after data fitting to the set of models supplied by the software (the highest score value) and the goodness-of-fit of the data to the function model represented by the coefficient of determination (R2 [40]). The R2 value was calculated for function model and poultry species. After function model selection, the function model parameters were determined by non-linear regression using the Marquardt method. In the same way, the “time to peak” was determined by the ordinary differential equation (ODE) of the Hoerl and Rational function models (supplementary material) and the Area Under the Curve (AUC) was determined by the numerical method, implementing a Romberg-type integration scheme (numerical integration method that uses extrapolation of trapezoidal sums to approximate an integral over a domain) under a QUAD subroutine [55], by integrating the area of the model function in the time range of 0–400 minutes for AFB1 dialdehyde production, 0–500 minutes for AFB1 monoalcohol production, 0 – 15 minutes for AFBO production and 0 – 20 minutes for AFL production. Normal distribution of residuals was tested by the Shapiro-Wilk test, homogeneous variance with a Leven’s test, and residual independence was performed with a “residual versus value” graph [2]. Inter-species differences in Hill, Hoerl, or Rational model parameters were determined by using the ANOVA test and multiple comparisons were made by a Tukey test. All analyses were performed using the Statistical Analysis System software [55].
3. Results
According to the highest score and the goodness-of-fit for the “Cm vs time” dataset obtained from AFB1-Gua, AFB1-GSH, AFB1-lysine, and AFB1 dialcohol products, the function model with the best adjustment was the modified Hill equation (Eq. 1) and the parameters determination is shown in Table 2. For the dataset obtained from AFB1 dialdehyde and AFB1 monoalcohol products, the best model was the Rational model (Eq. 2), and the parameters determination is presented in Table 3. In the case of the dataset obtained from AFBO and AFL products, the model with the best score was the Hoerl model (Eq. 3), and model parameters are presented in Table 4. After function model selection, results are presented by reaction step as follows.
Table 2.
Comparison of model parameters obtained by non-linear regression of AFB1-Gua, AFB1-GSH, AFB1-Lys, and AFB1 dialcohol (modified Hill model). R2: coefficient of determination; a: minimum asymptote or the response when time = 0; b: maximum asymptote or the stabilized metabolite concentration; c: time at which 50 % of the maximal concentration is reached; d: slope at the steepest part of the curve. All values are presented as the mean of 12 individuals ± standard deviation. Values with different letters are statistically significant.
| Reaction product | Poultry species | Model parameters |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | R2 | ||||||
| AFB1-Gua | Duck | c | −0.0039 ± 0.003 | a | 2.1 ± 0.2 | c | 0.9 ± 0.1 | c | 2.2 ± 0.18 | 0.9997 |
| RIR | ab | 0.0028 ± 0.001 | b | 1.0 ± 0.1 | b | 2.9 ± 0.3 | a | 2.7 ± 0.07 | 0.9998 | |
| Quail | a | 0.0035 ± 0.002 | b | 1.0± 0.3 | b | 2.7 ± 0.7 | a | 2.7 ± 0.10 | 0.9997 | |
| Ross | b | −0.0002 ± 0.001 | c | 0.5± 0.2 | a | 5.1 ± 1.5 | b | 2.3 ± 0.20 | 0.9999 | |
| Turkey | a | 0.0035 ± 0.002 | b | 1.0 ± 0.2 | b | 2.7 ± 0.4 | a | 2.7 ± 0.04 | 0.9997 | |
| AFB1-GSH | Duck | bc | 1.6E−04 ± 1.2E−04 | b | 0.09 ± 0.07 | c | 1.4 ± 0.1 | c | 1.7 ± 0.1 | 0.9996 |
| RIR | a | 8.0E−04 ± 4.6E−04 | a | 0.35 ± 0.23 | b | 3.9 ± 0.7 | a | 2.0 ± 0.2 | 0.9998 | |
| Quail | ab | 3.4E−04 ± 2.7E−04 | b | 0.09 ± 0.01 | b | 3.7 ± 1.0 | ab | 2.0 ± 0.1 | 0.9998 | |
| Ross | c | −2.3E−04 ± 1.9E−04 | ab | 0.18 ± 0.03 | a | 7.7 ± 2.5 | bc | 1.7 ± 0.1 | 0.9999 | |
| Turkey | bc | 8.6E−05 ± 4.8E−05 | b | 0.02 ± 0.01 | b | 3.6 ± 0.5 | ab | 2.0 ± 0.1 | 0.9998 | |
| AFB1-Lys | Duck | a | −0.02 ± 0.010 | ab | 1.6 ± 1.2 | b | 50.7 ± 9.9 | c | 2.0 ± 0.1 | 0.9998 |
| RIR | a | −0.02 ± 0.003 | bc | 0.6 ± 1.7 | bc | 46.0 ± 3.2 | bc | 2.1 ± 0.1 | 0.9995 | |
| Quail | a | −0.04 ± 0.012 | a | 2.8 ± 1.0 | a | 64.6 ± 7.2 | ab | 2.2 ± 0.1 | 0.9998 | |
| Ross | a | −0.01 ± 0.005 | bc | 0.6 ± 0.3 | bc | 54.2 ± 8.7 | a | 2.4 ± 0.2 | 0.9990 | |
| Turkey | a | −0.01 ± 0.003 | c | 0.3 ± 0.1 | c | 40.6 ± 2.7 | bc | 2.0 ± 0.1 | 0.9994 | |
| AFB1 dialcohol | Duck | ab | −3.5 ± 1.2 | bc | 89.9 ± 0.9 | b | 136.0 ± 20.6 | ab | 1.5 ± 0.1 | 0.9995 |
| RIR | b | −4.7 ± 0.9 | b | 90.2 ± 0.7 | bc | 117.4 ± 10.1 | bc | 1.5 ± 0.1 | 0.9992 | |
| Quail | a | −2.5 ± 0.7 | c | 88.6 ± 0.4 | a | 165.5 ± 18.3 | a | 1.7 ± 0.1 | 0.9995 | |
| Ross | bc | −4.8 ± 1.3 | b | 89.7 ± 1.1 | c | 116.8 ± 13.7 | bc | 1.5 ± 0.1 | 0.9989 | |
| Turkey | c | −6.9 ± 0.6 | a | 92.1 ± 0.7 | c | 98.3 ± 4.2 | c | 1.3 ± 0.1 | 0.9994 | |
Table 3.
Comparison of model parameters obtained by non-linear regression of AFB1 dialdehyde, and AFB1 monoalcohol (Rational model). tmax: time to peak; Cmax: concentration at tmax; AUC: area under the curve; R2: coefficient of determination. All values are presented as the mean of 12 individuals ± standard deviation. Values with different letters are statistically significant.
| Reaction product | Poultry species | Model parameters |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | tmax (minutes) | Cmax (pM) | AUC (pmol.minute/L) | R2 | ||||||||||||
| AFB1 dialdehyde | Duck | a | −0.2 ± 0.02 | a | 0.5 ± 0.04 | a | −0.02 ± 0.003 | ab | 0.0016 ± 0.0007 | b | 27.2 ± 5.6 | a | 9.0 ± 2.5 | b | 1201.5 ± 501.3 | 0.9943 | |||
| RIR | c | −0.5 ± 0.05 | c | 0.3 ± 0.03 | b | −0.03 ± 0.002 | ab | 0.0015 ± 0.0003 | b | 27.3 ± 2.4 | b | 6.6 ± 0.8 | c | 748.8 ± 115.8 | 0.9933 | ||||
| Quail | d | −0.7 ± 0.14 | b | 0.4 ± 0.07 | a | −0.02 ± 0.012 | c | 0.0008 ± 0.0002 | a | 37.6 ± 4.4 | a | 11.1 ± 2.6 | a | 1721.1 ± 376.7 | 0.9925 | ||||
| Ross | b | −0.4 ± 0.17 | d | 0.2 ± 0.06 | b | −0.03 ± 0.004 | c | 0.0010 ± 0.0004 | a | 35.2 ± 7.3 | b | 5.9 ± 1.6 | c | 714.6 ± 231.4 | 0.9936 | ||||
| Turkey | bc | −0.4 ± 0.06 | c | 0.3 ± 0.05 | b | −0.03± 0.003 | a | 0.0020 ± 0.0004 | b | 23.4 ± 2.0 | b | 5.1 ± 0.9 | c | 534.2 ± 119.9 | 0.9924 | ||||
| AFB1 monoalcohol | Duck | a | −0.3 ± 0.01 | a | 0.2 ± 0.05 | a | −0.02 ± 0.004 | bc | 0.0004 ± 0.0002 | b | 56.3 ± 11.7 | ab | 11.5± 3.9 | ab | 2071.0 ± 1004.4 | 0.9917 | |||
| RIR | c | −0.5 ± 0.09 | ab | 0.2 ± 0.03 | b | −0.03 ± 0.003 | b | 0.0005 ± 0.0001 | b | 48.6± 7.0 | bc | 9.8 ± 2.6 | bc | 1496.6 ± 523.0 | 0.9930 | ||||
| Quail | c | −0.5 ± 0.09 | bc | 0.2 ± 0.03 | a | −0.02 ± 0.003 | c | 0.0002 ± 0.0001 | a | 76.2 ± 10.3 | a | 13.4 ± 3.0 | a | 2749.9 ± 856.8 | 0.9896 | ||||
| Ross | c | −0.5 ± 0.12 | c | 0.1 ± 0.03 | b | −0.03 ± 0.005 | b | 0.0004 ± 0.0002 | b | 52.9 ± 10.2 | cd | 8.1 ± 2.9 | cd | 1213.8 ± 559.1 | 0.9934 | ||||
| Turkey | b | −0.4 ± 0.05 | bc | 0.2 ± 0.02 | c | −0.03 ± 0.002 | a | 0.0009 ± 0.0002 | c | 34.6 ± 3.3 | d | 5.5 ± 1.0 | d | 676.7 ± 145.1 | 0.9934 | ||||
Table 4.
Comparison of model parameters obtained by non-linear regression of AFBO, and AFL (Hoerl model). tmax: time to peak; Cmax: concentration at tmax; AUC: area under the curve; R2: coefficient of determination. All values are presented as the mean of 12 individuals ± standard deviation. Values with different letters are statistically significant.
| Reaction product | Poultry species | Model parameters |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | tmax (minutes) | Cmax (pM) | AUC (pmol.minute/L) | R2 | ||||||||||||
| AFBO | Duck | a | 2977.3 ± 211.8 | c | 0.3 ± 0.02 | c | 0.7 ± 0.13 | c | 0.6 ± 0.1 | a | 1032.7 ± 58.7 | a | 2059.8 ± 66.2 | 0.9889 | ||||
| RIR | b | 482.5 ± 106.6 | b | 0.6 ± 0.04 | a | 1.0 ± 0.02 | b | 2.2 ± 0.2 | b | 380.5 ± 45.7 | a | 2240.0 ± 31.3 | 0.9989 | |||||
| Quail | b | 670.4 ± 394.3 | b | 0.6 ± 0.09 | a | 1.0 ± 0.04 | b | 2.0 ± 0.5 | b | 435.9 ± 125.5 | a | 2236.0 ± 45.0 | 0.9979 | |||||
| Ross | c | 191.2 ± 84.6 | a | 0.8 ± 0.06 | b | 0.9 ± 0.06 | a | 3.8 ± 1.2 | c | 232.1 ± 83.6 | a | 2220.7 ± 1205.0 | 0.9939 | |||||
| Turkey | b | 597.1 ± 206.4 | b | 0.6 ± 0.05 | a | 1.0 ± 0.02 | b | 2.0 ± 0.3 | b | 423.5 ± 67.9 | a | 2255.9 ± 14.6 | 0.9992 | |||||
| AFL | Duck | d | 1.3 ± 1.1 | c | 0.3 ± 0.02 | bc | 1.2 ± 0.3 | c | 0.9 ± 0.2 | d | 0.4 ± 0.3 | d | 0.8 ± 0.7 | 0.992 | ||||
| RIR | a | 6.6 ± 0.8 | b | 0.6 ± 0.04 | ab | 1.3 ± 0.1 | b | 2.5 ± 0.3 | b | 6.0 ± 0.8 | b | 33.5 ± 9.6 | 0.995 | |||||
| Quail | bc | 4.5 ± 1.1 | b | 0.6 ± 0.09 | a | 1.5 ± 0.2 | b | 2.6 ± 0.6 | c | 4.0 ± 0.9 | bc | 22.3 ± 10.7 | 0.996 | |||||
| Ross | ab | 5.6 ± 1.2 | a | 0.8 ± 0.07 | c | 1.1 ± 0.1 | a | 4.0 ± 0.9 | a | 8.2 ± 2.5 | a | 85.8 ± 42.8 | 0.984 | |||||
| Turkey | cd | 3.0 ± 1.0 | b | 0.6 ± 0.05 | ab | 1.4 ± 0.1 | b | 2.4 ± 0.3 | c | 2.6 ± 1.1 | cd | 14.1 ± 6.7 | 0.996 | |||||
3.1. AFBO production
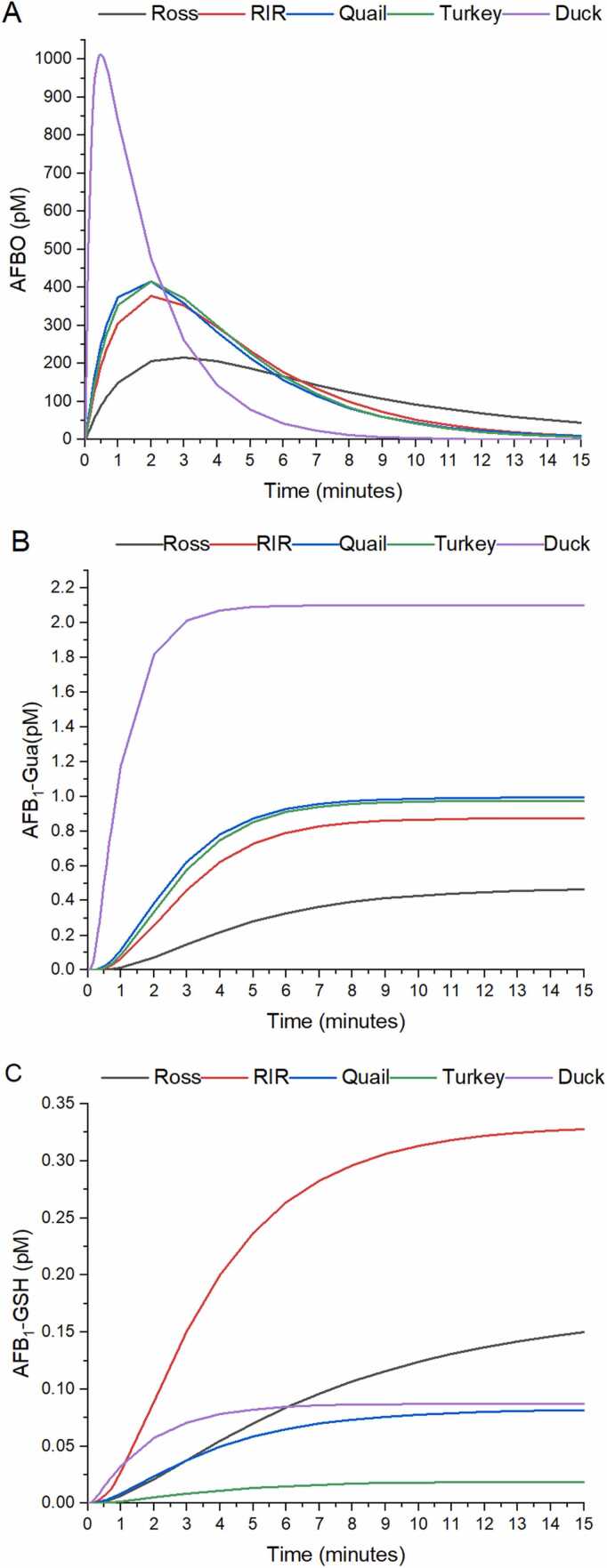
Fig. 2A shows the change in AFBO concentration (AFB1 epoxidation activity). During the first 5 minutes, an AFBO peak appears and fades out until zero after 15 minutes for all poultry species. The highest concentration reached was that of the duck (1032.6 ± 58.7 pM) and at the shortest time (0.6 ± 0.1 minutes). In contrast, the lowest peak was found for the Ross chicken breed (232.1 ± 83.6 pM; 4.5 times lower) which also occurred at a later time (3.8 ± 1.2 min; Table 4). In all cases the AUC did not present statistical differences.
Fig. 2.
Average production of AFBO (A), AFB1-Gua (B), and AFB1-GSH (C) over a lapse of 15 minutes, for 12 individuals from 5 commercial poultry species. RIR: Rhode Island Red breed.
3.2. AFB1-Gua production and AFB1-GSH production
The adduction of AFBO with DNA (AFB1-Gua production) is shown in Fig. 2B. Production of the adduct reaches a maximum of 2.1 ± 0.2 pM in the duck (value of the b model parameter), while in the Ross chicken breed it reaches a maximum at 0.5 ± 0.2 pM (3.8 times lower than the duck; Table 2). It is interesting to note that the duck reaches the maximum AFB1-Gua production much faster than the Ross breed. Regarding AFB1-GSH production (Fig. 2C), the RIR chicken breed has the highest AFB1-GSH production at 0.4 ± 0.2 pM (value of the b model parameter), after 15 minutes of simulation. In the duck, AFB1-GSH production reaches a maximum concentration of 0.1 ± 0.1 pM (3.9 times lower than the RIR breed) and in the turkey, AFB1-GSH production reaches a maximum of 0.02 pM (16.7 times lower compared to the RIR breed). AFBO and AFB1-GSH production showed statistical differences (p <0.05) among the different poultry species (see Table 2).
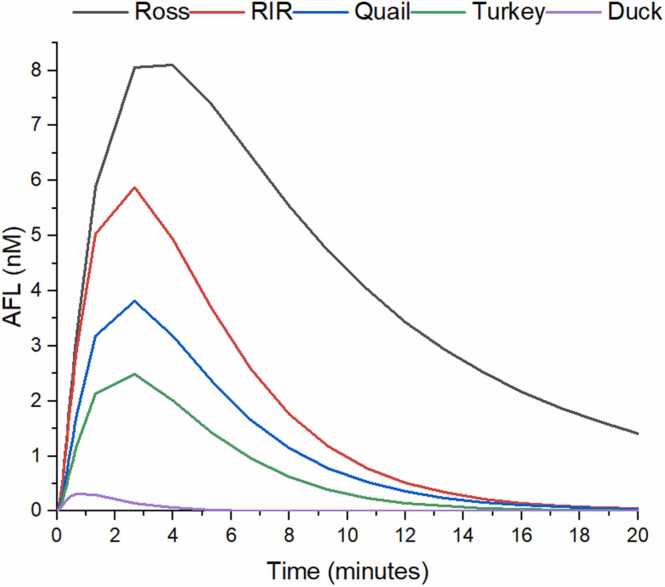
3.3. Net AFL production
AFL production encompasses two enzyme-catalyzed activities: AFB1 reductase and AFL oxidoreductase. For this reason, a change in AFL concentration (in nM) affects the net production of AFL. Fig. 3 shows the appearance of a peak that reached a maximum between 3 and 5 minutes of simulation and fades away to 1 nM between 10 and 20 minutes. Peak height ranged from 8.2 ± 2.5 pM in the Ross chicken breed to 0.4 ± 0.3 pM in duck (23.5 times lower than Ross; Table 4). Regarding the AUC, the Ross breed had the higher value by far, followed by the RIR breed > quail > turkey > duck (Table 4).
Fig. 3.
Average production of AFL over a lapse of 20 minutes, for 12 individuals from 5 commercial poultry species. RIR: Rhode Island Red breed.
3.4. AFB1 dialdehyde production and production of AFB1- Lys
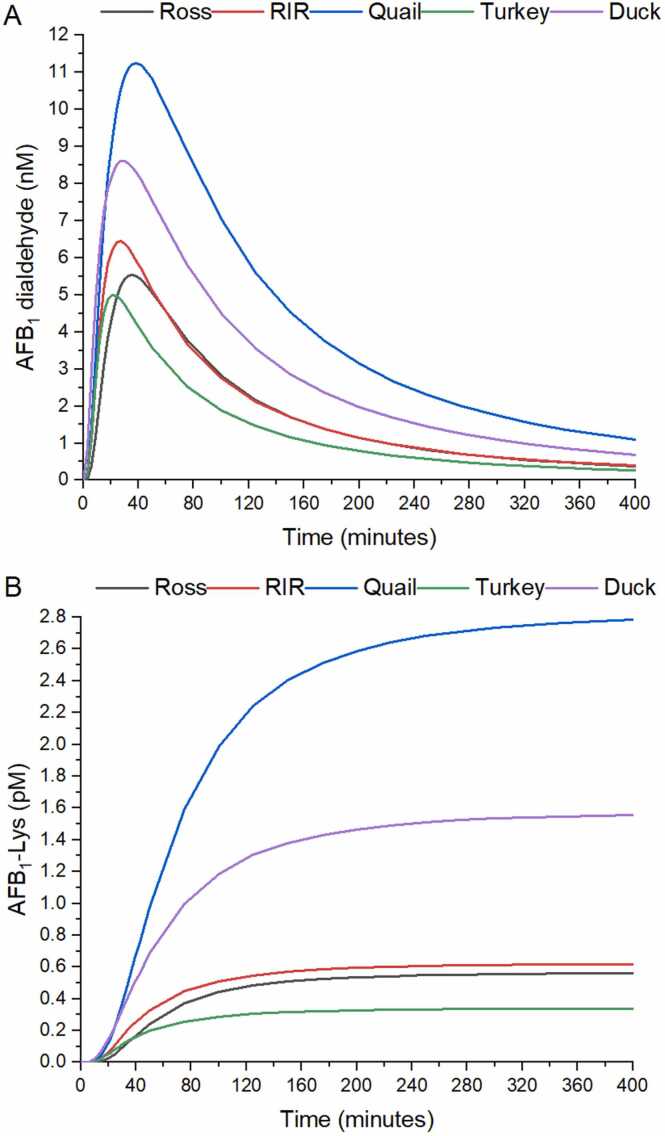
Fig. 4A presents the change in AFB1 dialdehyde concentration. Between 30 and 50 minutes of simulation AFB1 dialdehyde reaches a maximum in all species and then, fades out to less than 2 nM after 400 minutes. The quail and the duck showed the higher AFB1 dialdehyde peak (11.1 ± 2.6 and 9.0 ± 2.5 nM, respectively) and the largest AUC values (1721.1 ± 376.7 and 1201.5 ± 501.3 nmol*minute/L, respectively) with a magnitude more than two times higher in quail compared to the RIR breed (6.6 ± 0.8 pM), Ross breed (5.9 ± 1.6 pM), or Turkey (5.1 ± 0.9 pM; Table 3). Fig. 4B presents the production of AFB1-Lys adducts approaching to a plateau concentration after 400 minutes in all poultry species. Quail and duck reached the highest plateau with values of 2.8 ± 1.0 and 1.6 ± 1.2 pM, respectively. Turkey presented the lowest AFB1-Lys plateau concentration of 0.3 ± 0.1 pM (Table 2).
Fig. 4.
Average production of AFB1 dialdehyde (A), and AFB1-Lys (B) over a lapse of 400 minutes, for 12 individuals from 5 commercial poultry species. RIR: Rhode Island Red breed.
3.5. Production of AFB1 monoalcohol and AFB1 dialcohol
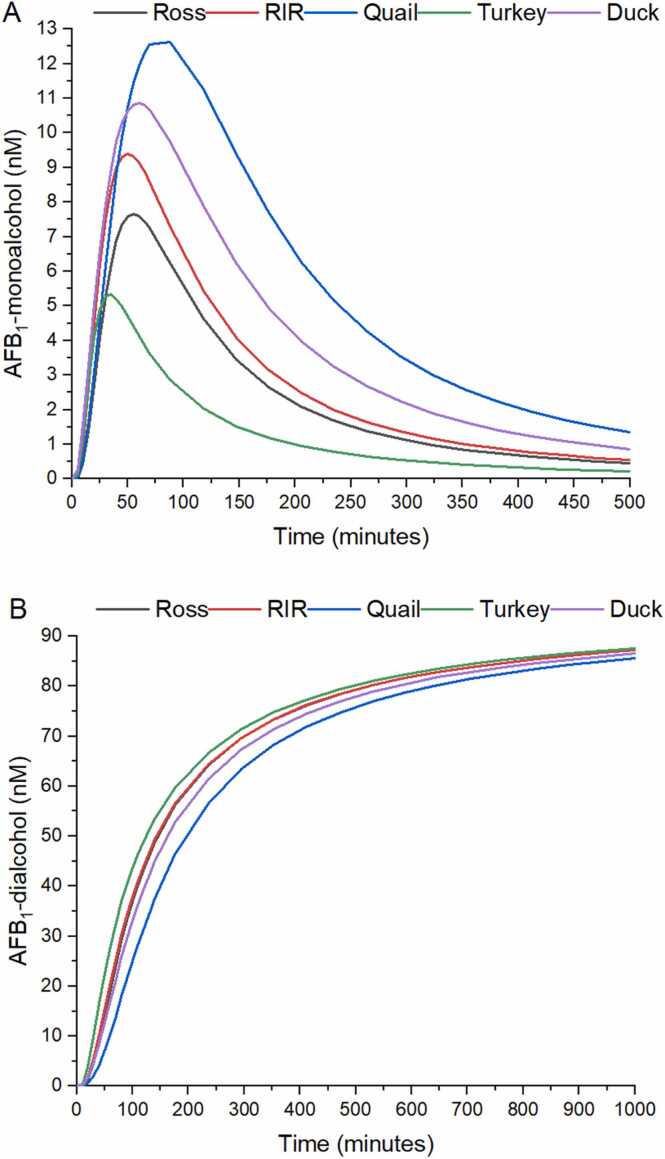
Fig. 5A shows AFB1 monoalcohol production and Fig. 5B presents the AFB1 dialcohol production. AFB1 monoalcohol production reaches a maximum before 100 minutes, and AFB1 dialcohol production increases further than 1440 minutes in all poultry species. The highest AFB1 monoalcohol maximum peak reaches a value of 13.4 ± 3.0 pM in quail, and the lower was present in turkey (5.5 ± 1.0 pM). Similarly, the largest AUC value was the one recorded for quail (2749.9 ± 856.8 pmol/minute/L) and the lowest the one of the turkey (676.7 ± 145.1 pmol/minute/L. AFB1 dialcohol plateau values ranged between 88.6 ± 0.4 (for the quail) and 92.08 ± 0.7 nM (for the turkey; Table 2).
Fig. 5.
Average production of AFB1 monoalcohol (A), and AFB1 dialcohol (B) over a lapse of 500 and 1000 minutes, respectively, for 12 individuals from 5 commercial poultry species. RIR: Rhode Island Red breed.
4. Discussion
The different toxic effects of AFB1 in poultry can be explain by clustering those reactions that produce toxic products (for example AFBO or AFB1 dialdehyde) and those reactions that inactivates these toxic products (for example AFB1-GSH or AFB1 dialcohol) into two pathways: the genotoxic and the cytotoxic pathways.
The “genotoxic pathway” starts with the comparison of AUC among the different poultry species evaluated, resulting in a statistically equal amount of AFBO produced by all species; however, there are large differences in the time to reach the peak. The Ross chicken breed is the species that reaches a maximum of AFBO in a longer time which is reflected on the maximum concentration (232.1 ± 83.6 pM). The Ross breed is followed by the RIR chicken breed, the quail, and the turkey, which present an intermediate tmax and Cmax. Finally, the duck presents the highest AFBO concentration peak (1032.7 ± 58.7 pm), which was more than 4 times higher than the Ross breed value, in a shorter time (0.6 ± 0.1 minutes), around 6 times shorter than the Ross breed. At this point, the exposure to AFBO was much higher for the duck, resulting theoretically in a higher attack of AFBO to DNA, leading to a higher amount of DNA adducts and DNA damage. Considering the AFB1-Gua production, it is important to note that the duck species present the highest AFB1-Gua peak production, which suggest a largest DNA damage. The duck produces 2.1 ± 0.2 pM of AFB1-Gua compared to Ross Breed, which only produces 0.5 ± 0.2 pM of AFB1-Gua. Fig. 2A and 2B show how the production of AFB1-Gua increases as AFBO production rises. In addition to the deleterious effects of AFB1, the effect of glutathione S-transferase (GST) activity seems to be partially related to sensitivity. The highest value of AFB1-GSH production corresponded to chicken breeds (Ross and RIR), the lower values to mid-tolerant species (quail and turkey) and an intermediate value to the most sensitive species (duck). The time needed to reach half the maximum of AFB1-GSH amount is the longest in the Ross breed and the shortest in the duck, suggesting that duck detoxification of AFB1 through GST activity tries to inactivate the AFBO produced by CYP450 enzymes. Despite this, the enzyme capacity is overwhelmed by the high AFBO-level production. In the same way, the high capacity of GST activity in chicken breeds and the low AFBO production explain why half the maximum production of AFB1-GSH in this poultry species is accomplished at longer times. Thus, the origin of the high rate of DNA adduction by AFB1 in the duck is the massive production of AFBO through CYP450 enzymes and the low GST activity. There is no evidence of the development of hepatocarcinoma due to a single dose of AFB1 in duck [58], which in turn suggest that continuous administration of AFB1 is required to develop tumors.
Regarding AFL production, chicken and quail are recognized as being resistant to the adverse effects of AFB1. Results demonstrate that there is a very large difference in AFL production between the Ross breed and the duck, being around 106 times higher in the Ross breed. In the case of the other poultry species, the RIR breed produces 42 times more AFL than duck and the quail produces 27 times more than duck. Since the 1970s, it has been proposed that AFL was merely an AFB1 reservoir or storage form in sensitive species like duck or rainbow trout and that this AFB1 reservoir could potentially lead to chronic effects because of the extension of the half-life of the toxin in the organism [3], [38], [49]. However, in a previous investigation, our research group has proposed a new role for AFL in poultry species, where it acts as a reservoir of AFB1 in pursuit of preventing AFB1 epoxidation and providing a higher tolerance to AFB1 exposure.
In the “cytotoxic pathway”, total production of AFB1 dialdehyde is the highest in quail followed by duck>RIR breed>Ross breed>turkey. The simulation for this metabolite is distributed with a peak maximum in the order quail = duck > RIR breed>Ross breed>turkey. AFB1 dialdehyde peak increases faster in the duck, the RIR breed, and the turkey and increases slower in the quail and the Ross breed. The simulation suggests that the cytotoxic effects in quail and duck are caused by a large AFB1 dialdehyde production in a short period of time. A higher concentration of AFB1 dialdehyde available in the hepatocyte increases the possibility of protein adduction by this metabolite. According to this, the amount of AFB1-Lys should be in the same way the largest in the quail and the duck, and sure enough, Fig. 4B shows that quail and duck produce the higher total amount of AFB1-Lys (2.8 ± 1.0 and 1.6 ± 1.2 pM respectively). At this point, the higher AFB1 bioactivation explains why the cytotoxic effects in quail and especially in duck are more severe than in resistant species like chicken breeds and turkey. A higher amount of available AFBO leads to a higher production of AFB1 dihydrodiol which in turn rearranges into AFB1 dialdehyde (the putative toxic metabolite of AFB1 associated to cytotoxic effects) to finally adduct to proteins like albumin [4].
The final step of AFB1 metabolism occurs when the AFAR enzyme catalyzes the reduction of AFB1 dialdehyde into AFB1 monoalcohol, and in a subsequent step, the reduction of AFB1 monoalcohol into AFB1 dialcohol. We propose to call this step for poultry species as “the elimination pathway”, which in turn can be considered as a detoxification pathway. Although the GST activity is considered a detoxification reaction because of the neutralization of AFBO, the amount of AFBO conjugated with GSH occurs only in the pM order. However, the concentrations of AFB1 dialdehyde, monoalcohol and dialcohol are found in nanomoles (a thousand times higher), leading to the elimination of a bulk quantity of AFB1 from the cell. AFB1 monoalcohol production in the poultry species studied, represented as the AUC value (Table 3), showed the order quail>duck>RIR breed>Ross breed>turkey. In contrast, maximum AFB1 dialcohol production, represented as the “b” parameter of the Hill model (Table 2) presented a reverse order (turkey>Ross and RIR breeds>duck>quail), suggesting an apparent saturation of the AFAR enzyme activity due to the overproduction of the monoalcohol in the quail but not in the turkey. Finally, the fact that all avian species reach a very close value of AFB1 dialcohol maximum production after 1000 minutes suggests that the AFB1 is eliminated in the form of dialcohol from the hepatocyte. In rats, the presence of dialcohol in urine supports this hypothesis [31]. Further, Benkerroum [5] propose that the lack of correlation between albumin adducts and AFB1 dialdehyde production is caused by the preferred route of reduction of the dialdehyde by AFAR enzyme into the AFB1 dialcohol than the adduction of AFB1 dialdehyde to lysine.
5. Concluding remarks
Information obtained from the simulation of enzyme kinetic parameters of reactions presented in Table 1 showed how the metabolism of AFB1 differs among the four poultry species evaluated and gives insight into the explanation of the resistance or sensitivity to AFB1 observed in vivo. It is important to highlight that the results from this study are limited to only hepatic metabolism and do not resemble the effect of AFB1 in extrahepatic tissues. Pursuing the explanation of tolerance and sensitivity, we focus mainly on two contrasting poultry species: the chicken and the duck. In the genotoxic pathway, it is observed in the chicken that the low production of AFBO is related to two factors: the high production of the conjugate AFB1-GSH and the high production of AFL. These two factors lead to a low production of DNA adducts. On the other hand, the duck presents severe signs of acute poisoning due to the high production of AFBO in a much shorter time than the other species. This is mainly related to two factors: low production of AFB1-GSH and low production of AFL. Therefore, the production of adducts with guanine is the highest. On the cytotoxic pathway it was observed that mid-tolerant species such as quail and turkey present extreme differences in AFB1-Lys production, associated to a low AFB1 dialdehyde elimination as AFB1 dialcohol in quail and a high value in turkey. This contrasting difference can explain why egg weight and egg production parameters in quail can be affected by the administration of 50 – 400 ppb of AFB1 [45], [46], [47], [56], meanwhile body weight in turkey is affected by the administration of 200 – 750 ppm of AFB1 [17], [22], [23], [34], [35], [50], [51], [64]. In the same way, the duck presents a higher AFB1-Lys production due to a lower AFB1 dialcohol production and a higher AFB1 dialdehyde production compared to chicken breeds.
To approach to a more precise and more comprehensive model that resembles the in vivo adverse effects of AFB1 consumption, it is necessary to investigate other parameters not considered in this study, for example, the transmembrane transport of AFB1 biotransformation products. For AFB1, it has been reported that the most probable transmembrane transport occurs by simple diffusion [41], but more recently reports has proposed the intervention of transporters of the organic anion transporters family (Organic Anion Transporter-OAT) and transporters of the organic cation transporters family (Organic Cation Transporter-OCT; [60]). Burt and Thorgeirsson [7] postulate the induction of the MDR-1 gene (canalicular efflux transporter) as the intake route of AFB1 in rats, and other studies have reported that the transport of AFB1 and AFB1-GSH is mediated by the MRP1 transporter [10], [37], [59]. The study of transmembrane transport would not only allow the evaluation of in vivo biotransformation rates within the hepatocyte, because biotransformation rates depend on the cytosolic concentration of AFB1 and its biotransformation products, but also will help to find a relationship with transmembrane transporters and AFB1 resistance or sensitivity by removing AFB1 biotransformation products such as AFB1 dialcohol from the target cell, favoring detoxification pathways [29].
In addition to in silico simulation and in vitro assays, ex vivo experiments performed with cell cultures from poultry could complement in silico findings. It has been possible to evaluate the in vitro susceptibility to AFB1 exposure in human and mouse by using hematopoietic tissue, and in mice the ex vivo effects have also been investigated [52]. Another topic to consider is the adduction of AFB1 dialdehyde to proteins belonging to the “DNA repairing system”, which would directly affects the repair of the AFB1-Gua adducts produced [54]. In addition to the impact of AFB1 consumption on the DNA repairing system, the comparison of the effect of AFB1 on the chromatin condensation patterns between species would also contribute to the discovery of new factors associated with sensitivity and hepatocarcinoma development [11]. Beyond the phase I and II biotransformation processes that occur in the hepatocyte, there are no reports of phase I biotransformation reactions carried out by the CYP enzymes located in the enterocyte (the so-called “first pass” effect). For example, AFB1-DNA adducts have been found in rat and human enterocytes, especially in mature enterocytes expressing the CYP3A4 isoform [33]. Moreover, in Cherry Valley ducks it has been proposed that the transport processes of doxycycline hydrochloride (an antibiotic) are affected by the intake of aflatoxin B1 and its possible bioactivation in enterocytes [28]. In addition to enterocytes, it has also been found that blood components, such as erythrocytes, can metabolize AFB1 to AFL and vice versa [8]. Thus, there is a potential of biotransform AFB1 in tissues different from the hepatocytes, which could affect the systemic concentration of the toxin. In addition to the biotransformation processes already described in this study and in the literature, the possible glucuronidation and/or sulfoconjugation of AFB1 dialcohol and monoalcohol has not been explored in poultry. It has been observed that dibenzo[a,l]pyrene-trans-11,12-diol is enzymatically conjugated with glucuronic acid by human liver microsomes [48] and in intestinal cells from channel catfish (Ictalurus punctatus), sulfotransferase and glucuronidase activity has been found for benzo[a]pyrene-7,8-dihydrodiol [62]. If similar compounds to AFB1 can be glucuronidated and/or sulfoconjugated, the phase II biotransformation of AFB1 dialcohol and monoalcohol appears as a potential topic to investigate.
CRediT authorship contribution statement
Gonzalo J. Diaz:Writing – review & editing, Writing – original draft. Hansen W. Murcia:Writing – review & editing, Writing – original draft, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Rubén D. Acosta:Writing – review & editing, Writing – original draft, Software, Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acnowledgements
Funding: This work was supported by Vicerrectoría de Ciencia, Tecnología e Investigación (VCTI), Universidad Antonio Nariño, Bogotá, D.C., Colombia, Convocatoria Interna 2023 “Proyectos de Ciencia, Tecnología, Innovación y Creación” [Proyecto 2023203]; Ministerio de Ciencia, Tecnología e Innovación, Bogotá D.C., Colombia [Convocatoria 647 “Doctorados Nacionales 2014”]. Finally, we want to thank to the “Fondo Editorial VCTI” Universidad Antonio Nariño.
Handling Editor: Prof. L.H. Lash.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2024.101752.
Contributor Information
Hansen W. Murcia, Email: hmurcia14@uan.edu.co.
Gonzalo Diaz, Email: gjdiazg@unal.edu.co.
Rubén Darío Acosta, Email: rdacostav@unal.edu.co.
Appendix A. Supplementary material
Supplementary material.
Data Availability
Data will be made available on request.
References
- 1.Alexander S.P.H., Fabbro D., Kelly E., et al. The concise guide to pharmacology 2023/24: enzymes. Br. J. Pharmacol. 2023;180:S289–S373. doi: 10.1111/bph.16181. [DOI] [PubMed] [Google Scholar]
- 2.Alam T., Suryanto P., Susyanto N., Kurniasih B., Basunanda P., Putra E.T.S., Kastono D., Respatie D.W., Widyawan M.H., Nurmansyah Ansari, Taryono A. Performance of 45 non-linear models for determining critical period of weed control and acceptable yield loss in Soybean agroforestry systems. Sustainability. 2022;14:7636. doi: 10.3390/su14137636. [DOI] [Google Scholar]
- 3.Bailey G.S., Loveland P.M., Pereira A.C., Pierce D., Hendricks J.D., Groopman J.D. Quantitative carcinogenesis and dosimetry in rainbow trout for aflatoxin B1 and aflatoxicol, two aflatoxins that form the same DNA adduct. Mutat. Res. 1994;313:25–38. doi: 10.1016/0165-1161(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 4.Barraud L., Douki T., Guerret S., Chevallier M., Jamard C., Trepo C., Wild C.P., Cadet J., Cova L. The role of duck hepatitis B virus and aflatoxin B1 in the induction of oxidative stress in the liver. Cancer Detect. Prev. 2001;25:192–201. [PubMed] [Google Scholar]
- 5.Benkerroum N. Retrospective and prospective look at aflatoxin research and development from a practical standpoint. Int. J. Environ. Res. Public Health. 2019;16:3633. doi: 10.3390/ijerph16193633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blount W.P., Turkey X. Dis. Turk. 1961;9:52–77. [Google Scholar]
- 7.Burt R.K., Thorgeirsson S.S. Coinduction of MDR-1 Multidrug-resistance and cytochrome P-450 genes in rat liver by xenobiotics. J. Natl. Cancer Inst. 1988;80:138–1386. doi: 10.1093/jnci/80.17.1383. [DOI] [PubMed] [Google Scholar]
- 8.Chang W., Lin J., Wu K., Hsiung K. In vitro interconversion of aflatoxin B1 and aflatoxicol by rat erythrocytes. Biochem. Pharmacol. 1985;34:2566–2569. doi: 10.1016/0006-2952(85)90546-5. [DOI] [PubMed] [Google Scholar]
- 9.Cullen J.M. and Newberne P.M. Acute Hepatotoxicity of Aflatoxins. the Toxicology of Aflatoxins. Human Health, Veterinary, and Agricultural Significance. San Diego, Academic Press, 1994.
- 10.Deeley R.G., Cole S.P.C. Substrate recognition and transport by multidrug resistant protein 1 (ABCC1) FEBS Lett. 2006;580:1103–1111. doi: 10.1016/j.febslet.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 11.Delcuve G.P., Moyer R., Bailey G., Davie J.R. Gene-specific differences in the aflatoxin B1 adduction of chicken erythrocyte chromatin. Cancer Res. 1988;48:7146–7149. [PubMed] [Google Scholar]
- 12.Diaz J.G., Murcia H.W., Cepeda S.M., Boermans H.J. The role of selected cytochrome P450 enzymes on the bioactivation of aflatoxin B1 by duck liver microsomes. Avian Pathol. 2010;39:279–285. doi: 10.1080/03079457.2010.495109. [DOI] [PubMed] [Google Scholar]
- 13.Diaz J.G., Murcia H.W., Cepeda S.M. Bioactivation of aflatoxin B1 by turkey liver microsomes: responsible cytochrome P450 enzymes. Brit. Poult. Sci. 2010;51:828–837. doi: 10.1080/00071668.2010.528752. [DOI] [PubMed] [Google Scholar]
- 14.Diaz J.G., Murcia H.W., Cepeda S.M. Cytochrome P450 enzymes involved in the metabolism of aflatoxin B1 in chickens and quail. Poult. Sci. 2010;89:2461–2469. doi: 10.3382/ps.2010-00864. [DOI] [PubMed] [Google Scholar]
- 15.Diaz G.J., Murcia H.W. An unusually high production of hepatic aflatoxin B1-dihydrodiol, the possible explanation for the high susceptibility of ducks to aflatoxin B1. Sci. Rep. 2019;9:8010. doi: 10.1038/s41598-019-44515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz G.J. and Murcia H.W. Biotransformation of Aflatoxin B1 and Its Relationship with the Differential Toxicological Response to Aflatoxin in Commercial Poultry Species. Aflatoxins: Biochemistry and Molecular Biology, Croatia: Intech Open Access, 2011. http://doi.org/10.5772/22109.
- 17.Diaz G.J., Cortés A., Botero L. Evaluation of the ability of a feed additive to ameliorate the adverse effects of aflatoxins in turkey poults. Brit. Poult. Sci. 2009;50:240–250. doi: 10.1080/00071660902774566. [DOI] [PubMed] [Google Scholar]
- 18.Esaki H., Kumagai S. Glutathione-S -transferase activity towards aflatoxin epoxide in livers of mastomys and other rodents. Toxicon. 2002;40:941–945. doi: 10.1016/S0041-0101(02)00090-9. [DOI] [PubMed] [Google Scholar]
- 19.Funahashi A., Matsuoka Y., Jouraku A., Morohashi M., Kikuchi N., Kitano H. Cell designer 3.5: a versatile modeling tool for biochemical networks. Proc. IEEE. 2008;96:1254–1265. doi: 10.1109/JPROC.2008.925458. [DOI] [Google Scholar]
- 20.Funahashi A., Tanimura N., Morohashi M., Kitano H. Cell designer: a process diagram editor for gene-regulatory and biochemical networks. Biosilico. 2003;1:159–162. doi: 10.1016/S1478-5382(03)02370-9. [DOI] [Google Scholar]
- 21.Gadagkar S.R., Call G.B. Computational tools for fitting the Hill equation to dose–response curves. J. Pharmacol. Toxicol. Methods. 2015;71:68–76. doi: 10.1016/j.vascn.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Giambrone J.J., Diener U.L., Davis N.D., Panangala V.S., Hoerr F.J. Effect of purified aflatoxin on turkeys. Poult. Sci. 1985;64:859–865. doi: 10.3382/ps.0640859. [DOI] [PubMed] [Google Scholar]
- 23.Giambrone J.J., Diener U.L., Davis N.D., Panangala V.S., Hoerr F.J. Effects of aflatoxin on young turkeys and broiler chickens. Poult. Sci. 1985;64:1678–1684. doi: 10.3382/ps.0641678. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg H.E., England M.J., Hellriegel E.T., Bjornsson T.D. Time of peak drug concentration after a single dose and a dose at steady state. J. Clin. Pharmacol. 1997;37:480–485. doi: 10.1002/j.1552-4604.1997.tb04325.x. [DOI] [PubMed] [Google Scholar]
- 25.Guengerich F.P., Arneson K.O., Williams K.M., Deng Z., Harris T.M. Reaction of aflatoxin B1 oxidation products with lysine. Chem. Res. Toxicol. 2002;15:780–792. doi: 10.1021/tx010156s. [DOI] [PubMed] [Google Scholar]
- 26.Guengerich F.P., Johnson W.W., Shimada T., Ueng Y., Yamazaki H., Langouet S. Activation and detoxication of aflatoxin B1. Mut. Res. 1998;402:121–128. doi: 10.1016/S0027-5107(97)00289-3. [DOI] [PubMed] [Google Scholar]
- 27.Hill A.V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910;40 doi: 10.1113/jphysiol.1910.sp001386. iv–vii. [DOI] [Google Scholar]
- 28.He X., Ye G., Zhao S., Yin Z., Zhao L., Lv C., Li Y. Pharmacokinetics of doxycycline hydrochloride in Cherry Valley duckling during aflatoxicosis. Int. J. Clin. Exp. Med. 2016;9:13035–13040. [Google Scholar]
- 29.Hayes J.D., Judah D., McLellan L.I., Neal G.E. Contribution of the glutathione S-transferases to the mechanisms of resistance to aflatoxin B1. Pharmacol. Ther. 1991;50:443–472. doi: 10.1016/0163-7258(91)90053-o. [DOI] [PubMed] [Google Scholar]
- 30.Hyams, D.G., CurveExpert Professional Documentation. Release 2.7.3, 〈https://www.curveexpert.net/support/documentation/〉, 2020.
- 31.Johnson D.N., Egner P.A., OBrian G., Glassbrook N., Roebuck B.D., Sutter T.R., Payne G.A., Kensler T.W., Groopman J.D. Quantification of urinary aflatoxin B1 dialdehyde metabolites formed by aflatoxin aldehyde reductase using isotope dilution tandem mass spectrometry. Chem. Res. Toxicol. 2008;21:752–760. doi: 10.1021/tx700397n. [DOI] [PubMed] [Google Scholar]
- 32.Johnson W.W., Harris T.M., Guengerich F.P. Kinetics and mechanism of hydrolysis of aflatoxin B1 exo-8,9-epoxide and rearrangement of the dihydrodiol. J. Am. Chem. Soc. 1996;118:8213–8220. doi: 10.1021/ja960525k. [DOI] [Google Scholar]
- 33.Kolars J.C., Benedict P., Schmiedlin-Ren P., Watkins B. Aflatoxin B1-adduct formation in rat and human small bowel enterocytes. Gastroenterology. 1994;106:433–436. doi: 10.1016/0016-5085(94)90602-5. [DOI] [PubMed] [Google Scholar]
- 34.Kubena L.F., Edrington T.S., Kamps-Holtzapple C., Harvey R.B., Elissalde M.H., Rottinghaus G.E. Effects of feeding fumonisin B1 present in Fusarium moniliforme culture material and aflatoxin singly and in combination to turkey poults. Poult. Sci. 1995;74:1295–1303. doi: 10.3382/ps.0741295. [DOI] [PubMed] [Google Scholar]
- 35.Kubena L.F., Huff W.E., Harvey R.B., Yersin A.G.Ellissalde M.H., Witzel D.A., Giroir L.E., Phillips T.D., Petersen H.D. Effects of a hydrated sodium calcium aluminosilicate on growing turkey poults during aflatoxicosis. Poult. Sci. 1991;70:1823–1830. doi: 10.3382/ps.0701823. [DOI] [PubMed] [Google Scholar]
- 36.Lauwers M., Croubels S., De Baer S., Sevastiyanova M., Romera E.M., Letor B., Gougoulias C., Devreese M. Supplementary materials: assessment of dried blood spots for multimycotoxin biomarker analysis in pigs and broiler chickens. Toxins. 2019;11:S1–S8. doi: 10.3390/toxins11090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leslie E.M., Deeley R.G., Cole S.P.C. Multidrug resistant proteins: role of P-glycoprotein, MRP1, MRP2 and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Loveland P., Wilcox J., Pawlowski N., Bailey G., Metabolism D.N.A. binding of aflatoxicol and aflatoxin B1in vivo and in isolated hepatocytes from rainbow trout (Salmo gairdneri) Carcinogenesis. 1987;8:1065–1070. doi: 10.1093/carcin/8.8.1065. [DOI] [PubMed] [Google Scholar]
- 39.Lozano M.C., Diaz G.J. Microsomal and cytosolic biotransformation of aflatoxin B1 in four poultry species. Brit. Poult. Sci. 2006;47:734–741. doi: 10.1080/00071660601084390. [DOI] [PubMed] [Google Scholar]
- 40.Motulsky H.M. Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting. GraphPad Software Inc. San Diego, CA. www.graphpad.com, 2003.
- 41.Müller N., Petzinger E. Hepatocellular uptake of aflatoxin B1 by non-ionic diffusion. Inhibition of bile acid transport by interference with membrane lipids. Biochim. Biophys. Acta. 1988;938:334–344. doi: 10.1016/0005-2736(88)90131-9. [DOI] [PubMed] [Google Scholar]
- 42.Murcia H.W., Diaz G.J. Protective effect of glutathione S-transferase enzyme activity against aflatoxin B1 in poultry species: relationship between glutathione S-transferase enzyme kinetic parameters, and resistance to aflatoxin B1. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murcia H.W., Diaz G.J. In vitro hepatic aflatoxicol production is related to a higher resistance to aflatoxin B1 in poultry. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-62415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murcia H.W., Diaz G.J. Dealing with aflatoxin B1 dihydrodiol acute effects: impact of aflatoxin B1-aldehyde reductase enzyme activity in poultry species tolerant to AFB1 toxic effects. PLoS One. 2020;15 doi: 10.1371/journal.pone.0235061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogido R., Oliveira C.A.F., Ledoux D.R., Rottinghaus G.E., Corrêa B., Butkeraitis P., Reis T.A., Gonçalez E., Albuquerque R. Effects of prolonged administration of aflatoxin B1 and fumonisin B1 in laying Japanese Quail. Poult. Sci. 2004;83:1953–1958. doi: 10.1093/ps/83.12.1953. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira C.A.F., Ogido R., Ledoux D.R., Rottinghaus G.E., Corrêa B., Reis T.A., Gonçalez E. The quality of eggs from Japanese Quail, Coturnix japonica, fed ration containing aflatoxin B1 and fumonisin B1. J. Poult. Sci. 2007;44:29–33. doi: 10.2141/jpsa.44.29. [DOI] [Google Scholar]
- 47.Oliveira C.A.F., Rosmaninho J.F., Butkeraitis P., Corrêa B., Reis T.A., Guerra J.L., Albuquerque R., Moro M.E.G. Effect of low levels of dietary aflatoxin B1 on laying Japanese Quail. Poult. Sci. 2002;81:976–980. doi: 10.1093/ps/81.7.976. [DOI] [PubMed] [Google Scholar]
- 48.Olson K.C., Sun D., Chen G., Sharma A.K., Amin S., Ropson I.J., Spratt T.E., Lazarus P. Characterization of dibenzo[a,l]pyrene-trans-11,12-diol (dibenzo [def,p]chrysene) glucuronidation by UDP-glucuronosyltransferases. Chem. Res. Toxicol. 2011;24:1549–1559. doi: 10.1021/tx200178v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patterson D., Roberts B. Aflatoxin metabolism in duck-liver homogenates: the relative importance of reversible cyclopentenone reduction and hemiacetal formation. Food Cosmet. Toxicol. 1972;10:501–512. doi: 10.1016/S0015-6264(72)80084-1. [DOI] [PubMed] [Google Scholar]
- 50.Quist C.F., Bounous D.I., Kilburn J.V., Nettles V.F., Wyatt R.D. The effect of dietary aflatoxin on wild turkey poults. J. Wildl. Dis. 2000;36:436–444. doi: 10.7589/0090-3558-36.3.436. [DOI] [PubMed] [Google Scholar]
- 51.Rauber R.H., Dilkin P., Giacomini L.Z.Araújo, de Almeida C.A., Mallman C.A. Performance of turkey poults fed different doses of aflatoxins in the diet. Poult. Sci. 2007;86:1620–1624. doi: 10.1093/ps/86.8.1620. [DOI] [PubMed] [Google Scholar]
- 52.Roda E., Coccini T., Acerbi D., Castoldi A.F., Manzo L. Comparative in vitro and ex-vivo myelotoxicity of aflatoxins B1 and M1 on haematopoietic progenitors (BFU-E, CFU-E, and CFU-GM): Species related susceptibility. Toxicol. Vitr. 2010;24:217–223. doi: 10.1016/j.tiv.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Rodríguez-Belenguer P., March-Vila E., Pastor M., Mangas-Sanjuan V., Soria-Olivas E. Usage of model combination in computational toxicology. Toxicol. Let. 2023;389:34–44. doi: 10.1016/j.toxlet.2023.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Sarasin A.R., Smith C.A., Hanawalt P.C. Repair of DNA in human cells after treatment with activated aflatoxin B1. Cancer Res. 1977;37:1786–1793. [PubMed] [Google Scholar]
- 55.SAS Institute Inc. Base SAS® 9.4 Procedures Guide: Statistical Procedures, Second Edition. 〈www.support.sas.com/bookstore〉, 2013.
- 56.Sawhney D.S., Vadehra D.V., Baker R.C. Aflatoxicosis in the laying Japanese Quail (Coturnix Coturnix japonica) Poult. Sci. 1973;52:465–473. doi: 10.3382/ps.0520465. [DOI] [PubMed] [Google Scholar]
- 57.Scheff J.D., Almon R.R., DuBois D.C., Jusko W.J., Androulakis I.P. 2011. Assessment of pharmacologic area under the curve when baselines are variable. Pharmacol. Res. 2011;28:1081–1089. doi: 10.1007/s11095-010-0363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Słowik J., Graczyk S., Madej J.A. The effect of a single dose of aflatoxin B1 on the value of nucleolar index of blood lymphocytes and on histological changes in the liver, bursa Fabricii, suprarenal glands and spleen in ducklings. Folia Histochem. Cytobiol. 1985;23:71–79. [PubMed] [Google Scholar]
- 59.Loe D.W., Stewart R.K., Massey T.E., Deeley R.G., Cole S.P.C. ATP-dependent transport of aflatoxin B1 and its glutathione conjugates by the product of the multidrug resistant protein (MRP) gene. Mol. Pharmacol. 1997;51:1034–1041. doi: 10.1124/mol.51.6.1034. [DOI] [PubMed] [Google Scholar]
- 60.Tachampa K., Takeda M., Khamdang S., Noshiro-Kofugi R., Tsuda M., Jariyawat S., Fukutomi T., Sophasan S., Anzai N., Endou H. Interactions of organic anion transporters and organic cation transporters with mycotoxins. J. Pharmacol. Sci. 2008;106:435–443. doi: 10.1254/jphs.fp0070911. [DOI] [PubMed] [Google Scholar]
- 61.Tulayakul P., Sakuda S., Dong K.S., Kumagai S. Comparative activities of glutathione-S-transferase and dialdehyde reductase toward aflatoxin B1 in livers of experimental and farm animals. Toxicon. 2005;46:204–209. doi: 10.1016/j.toxicon.2005.03.023. http://doi.org/0.1016/j.toxicon.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 62.Van der Hurk P., James M.O. Sulfation and glucuronidation of benzo[a]pyrene-7,8-dihydrodiol in intestinal mucosa of channel catfish (Ictalurus punctatus) Mar. Environ. Res. 2000;50:11–15. doi: 10.1016/s0141-1136(00)00067-2. [DOI] [PubMed] [Google Scholar]
- 63.Wieczerzak M., Kudlak B., Yotova G., Nedyalkova M., Tsakovski S., Simeonov S., Namieśnik J. Modeling of pharmaceuticals mixtures toxicity with deviation ratio and best-fit functions models. Sci. Total Environ. 2016;571:259–268. doi: 10.1016/j.scitotenv.2016.07.186. [DOI] [PubMed] [Google Scholar]
- 64.Witlock D.R., Wyatt R.D. Effect of dietary aflatoxin on homeostasis of young turkey poults. Poult. Sci. 1981;60:528–531. doi: 10.3382/ps.0600528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data will be made available on request.