Abstract
Background
Robot-assisted surgery is widely performed for renal cell carcinoma (RCC) with inferior vena cava (IVC) tumor thrombi. Although many chemotherapeutic options are available for the treatment of unresectable RCC, there are very few reports on robot-assisted radical nephrectomy (RARN) with inferior vena cava thrombectomy (IVCT) after presurgical treatment with immune checkpoint inhibitors and tyrosine kinase inhibitors. We believe that pre-surgical treatment can provide minimally invasive surgical benefits to high-risk patients during the perioperative period.
Case Description
A 77-year-old male with right RCC that invaded the IVC (cT3bN0M0, Mayo classification level III) underwent pembrolizumab and axitinib combination therapy because he had high surgical risk due to angina pectoris. The level of the tumor thrombus decreased from level III to II, and RARN with IVCT was then performed. Surgery was performed without complications, and the patient was discharged on postoperative day seven. The pathological diagnosis was clear cell RCC (ypT3b, G2). Adjuvant chemotherapy using pembrolizumab monotherapy is still ongoing.
Conclusions
In this report, the inferior vena cave tumor thrombus level was down staged from level III to level II by treatment with pembrolizumab and axitinib. RARN with IVCT was safely performed without complication completely under robotic assistance.
Keywords: Robot-assisted radical nephrectomy (RARN), inferior vena cava thrombectomy (IVCT), immune checkpoint inhibitor (ICI), pre-surgical treatment, case report
Introduction
Background
The frequency of tumor thrombus of the inferior vena cava (IVC) in renal cell carcinoma (RCC) is reported to be 4–10% (1). Furthermore, 5-year survival rates for RCC with an IVC tumor thrombus have been reported to range from 40–68% (1). The level of IVC thrombus does not affect disease-specific survival rates but significantly affects perioperative complications (2). The surgical approach to RCC with IVC tumor thrombus is shifting from open surgery to robot-assisted surgery in this era of robotic surgery. Preoperative tumor thrombus level reduction and robot-assisted surgery would greatly benefit patients by avoiding invasive surgery.
In Japan, robot-assisted radical nephrectomy (RARN) for RCC was recently approved to be covered by insurance and there are few accumulated cases, although RARN is widely performed worldwide. In addition, many cases of robot-assisted IVC thrombectomy have been reported.
Rationale and knowledge gap
There are only a few case reports on combination therapy with immune checkpoint inhibitor (ICI) and tyrosine kinase inhibitor (TKI) for downstaging IVC tumor thrombi, although combination therapy with ICI and TKI for unresectable RCC is widely administered. Downstaging of the tumor thrombus of RCC is expected to be widely practiced in the future, as it can reduce complications in perioperative high-risk patients by making minimally invasive surgical treatments possible. We present this case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1547/rc).
Case presentation
An incidental renal mass was observed upon closer examination of a plain computed tomography (CT) obtained for the evaluation of prostate cancer in a 77-year-old man. There was no history of abdominal surgery. No major problems were found in blood tests (serum creatinine 0.94 mg/dL, estimated glomerular filtration rate 61 mL/min). Contrast-enhanced CT showed right RCC and a tumor thrombus of the IVC extending to the confluence of the hepatic veins (Figure 1A,1B). No lymph node or distant metastasis was observed. The preoperative diagnosis was right RCC with IVC tumor thrombus [cT3bN0M0, Mayo classification level III, International Metastatic RCC Database Consortiuma (IMDC) intermediate risk]. In this case, we did not perform a tumor biopsy because we wanted to start treatment early and consider the risk of tumor dissemination. Due to his history of angina pectoris and advanced age, the risk of perioperative complications was extremely high, and we determined that radical nephrectomy was difficult at that time. Considering the ease of controlling adverse effects and efficacy, the patient was administered pembrolizumab 200 mg (every 3 weeks) and axitinib 10 mg. We decided to administer three courses of chemotherapy after consultation with the patient, although there was possibility of tumor progression. Due to the appearance of hand-foot syndrome, the dose of axitinib was reduced to 6 mg, and pembrolizumab was administered for three cycles. Repeat contrast-enhanced CT showed that the tumor embolus height had decreased by 45 mm (−53%) and was down staged from Mayo classification level III to II (Figure 2A-2C). We determined that radical resection was possible, and RARN and IVCT were performed using the da Vinci Xi Surgical System.
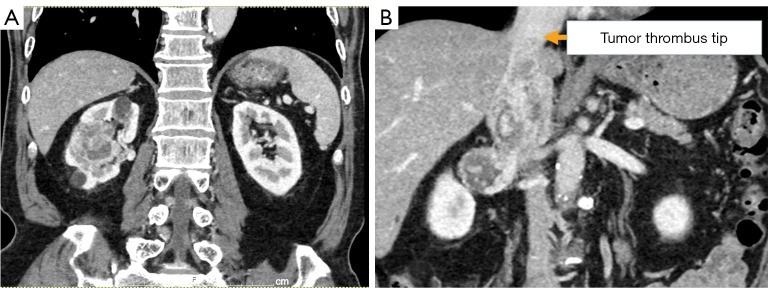
Figure 1.
Contrast-enhanced CT findings in this case. (A) Suspicious findings of clear cell renal cell carcinoma in the right kidney. (B) The tumor thrombus tip reached the confluence of the hepatic vein and IVC. CT, computed tomography; IVC, inferior vena cava.
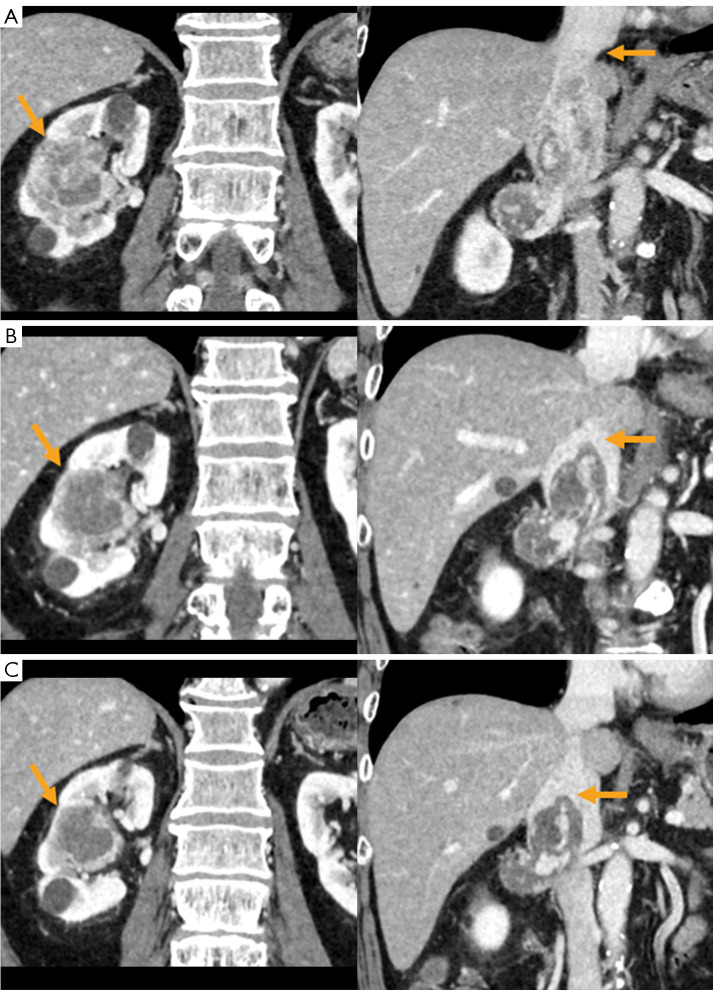
Figure 2.
Determination of effectiveness of chemotherapy confirmed by contrast-enhanced CT findings. (A) The first medical examination (yellow arrows: primary tumor and tumor thrombus tip). (B) After completion of the second course of pembrolizumab (yellow arrows: primary tumor and tumor thrombus tip). (C) After completion of the third course of pembrolizumab (yellow arrows: primary tumor and tumor thrombus tip). CT, computed tomography.
The port positions were determined using a port arrangement similar to that of robot-assisted partial nephrectomy (RAPN), as shown in Figure 3, with four 8 mm daVinci ports and two 12 mm assistant ports. The left port of the assistant was used as a wound retractor. The details of the surgery are shown in Figure 4A-4H. The ascending colon was prolapsed to expose the IVC. The right renal artery was secured between the aorta and the IVC, and clipping was performed. The right and left renal veins and the IVC were also exposed cranially. The inferior right hepatic vein was dissected using Endo GIATM (Covidien, Massachusetts, USA), and the short hepatic vein was dissected using LigaSureTM (Covidien). Once the liver was fully decompressed, the IVC tumor thrombus level was confirmed by echocardiography. The IVC cephalad to the tumor thrombus, the IVC caudal to the right renal vein, and the left renal vein were secured using vessel tape and clamped with laparoscopic bulldog forceps. An incision was made on the IVC and the tumor thrombus, right kidney, and right renal vein were removed together. There was little adhesion between the IVC wall and the tumor. The IVC was closed using a 4-0 PROLENE suture. The pathological diagnosis was clear cell RCC [ypT3b, International Society of Urological Pathology (ISUP)/World Health Organization (WHO) G2, v1, ly0, fc1, rc-inf1, rp-inf1, s-inf0] with few viable tumor cells inside the kidney; however, the tumor thrombus was necrotic (Figure 5). When programmed cell death ligand 1 (PD-L1) staining was performed, the tumor proportion score was 10 and the combined positive score was 50. PD-L1-positive immune cells were expressed at the tumor margins and a large number of CD68-positive macrophages were found in the necrotic tissue (Figure 6). According to our pathologist, this patient had benefited more from the therapeutic effect of the TKI than that of the ICI. The operating time was 6 hours 2 minutes and the console time was 4 hours 48 minutes. The IVC clamping time was 47 minutes. Blood loss was 330 mL. The postoperative course was satisfactory, and the patient was discharged after 7 days without complications. Therapy with pembrolizumab 200 mg (every 3 weeks) is ongoing with no recurrence. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
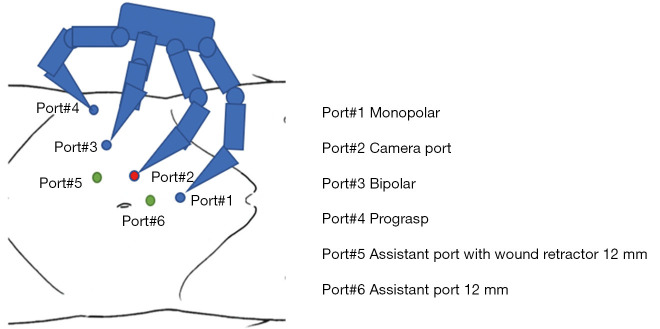
Figure 3.
The port positions are set in a similar manner to those of robot-assisted partial nephrectomy. The assistant’s left-hand port uses a wound retractor.
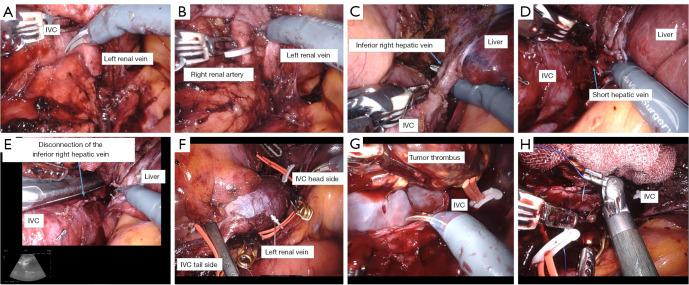
Figure 4.
The details of the surgery. (A) The left renal vein and medial IVC is dissected and exposed. (B) The right renal artery is secured caudal to the left renal vein, clipped, and taped. (C) The inferior right hepatic vein is dissected using Endo GIATM at of its confluence with the IVC. (D) The short hepatic vein is identified cephalad to the inferior right hepatic vein and is dissected using a LigasureTM. (E) Intraoperatively, an echo confirms that the tumor tip was slightly cephalic to the inferior right hepatic vein. (F) The IVC and left renal vein are clamped cephalad and caudally using laparoscopic bulldog forceps to enclose the tumor. (G) An incision is made on the IVC and the tumor thrombus is removed. Adhesion to the IVC wall is mild. (H) The IVC wall is sutured using 4-0 PROLENE sutures. IVC, inferior vena cava.
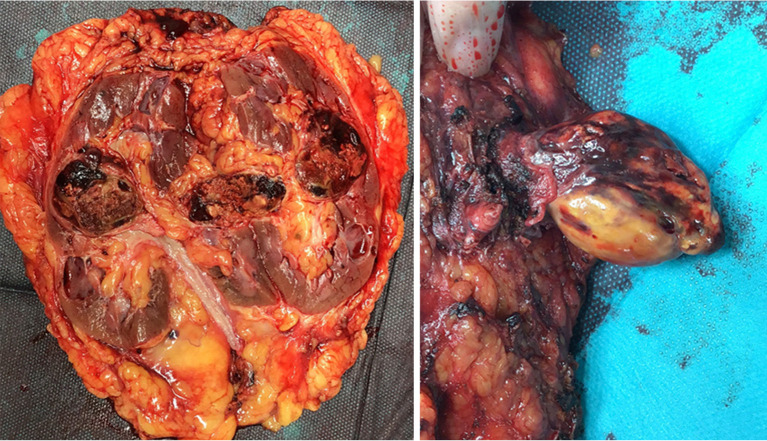
Figure 5.
The tumor thrombus is necrotic, and a few viable cells are seen in the primary tumor.
Figure 6.
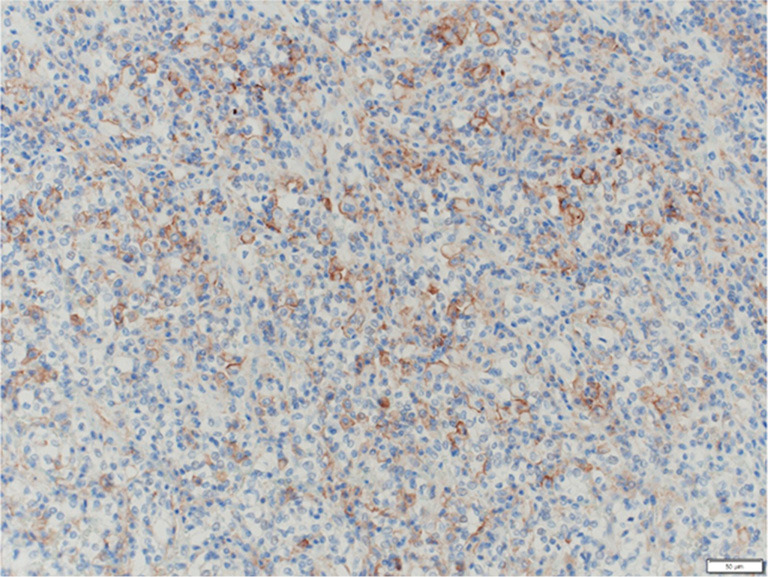
PD-L1 staining. PD-L1-positive immune cells are expressed at the tumor margins. Scale bar: 50 µm. PD-L1, programmed cell death ligand 1.
Discussion
Key findings
Usefulness and safety of RARN and IVCT in RCC. Efficacy of ICI + TKI in patients RCC with IVC tumor thrombi.
Strengths and limitations
Long-term results are unknown.
Comparison with similar researches
There are many reports on RARN for renal cancer with IVC tumor thrombus. Garg et al. reported the safety and usefulness of RARN and IVCT in 329 patients with level zero to four embolized renal cancer, with an intraoperative complication rate of 1.8%, conversion rate to open surgery rate of 1.2%, and positive margin rate of 0.01%. They also reported significantly lower blood transfusion rates, perioperative complication rates, and length of hospital stay compared with open surgery, and no significant differences in grade three or higher complication rates, 30-day postoperative mortality, operation time, or progression-free survival (PFS), making it an alternative to open surgery (3). However, there are still only a few reports on cases in which RARN was performed after downstaging the tumor thrombus with a combination therapy of an ICI and a TKI. There is no standard period for pre-surgical chemotherapy, and it is determined individually by each institution. RARN has the advantage of making minimally invasive surgical treatment possible in cases where open surgery is a high-risk procedure perioperatively. Hara et al. reported that presurgical avelumab + axitinib administration resulted in a pathological complete response (pCR) after surgical resection (4). Otsuka et al. reported using ipilimumab/nivolumab combination therapy to down stage a tumor from level four to level one in a patient with metastatic renal cell carcinoma (mRCC); this patient underwent radical nephrectomy and tumor thrombectomy, resulting in pCR (5). When chemotherapy is administered with a view to curative surgery, our policy is to use pembrolizumab plus axitinib combination therapy to treat unresectable renal cancer. This choice was based on a balance between ease of perioperative control due to axitinib’s short half-life, side effect profile, and efficacy. In a report on ICI/ICI or ICI/TKI in five cases of mRCC or non-metastatic renal cell carcinoma (nmRCC) with IVC thrombus, ipilimumab + nivolumab was selected in three cases and pembrolizumab + axitinib in two cases. All patients had reduced tumor thrombus levels, and three underwent radical nephrectomy and IVC thrombectomy. The tumor thrombus height was reduced by an average of 31 mm (average −40%) (6). Thus, there are many reports of ICI being effective in cases of tumor thrombus; however, these are only case reports. The difference in the efficacy of ICI between the primary tumor and the tumor thrombus in cases of IVC thrombus is due to the heterogeneity of the tumor, suggesting that ICI may be very effective in cases of tumor thrombus (7). In RCC, the usefulness of ICI in preoperative chemotherapy is uncertain, but several phase III clinical trials are currently ongoing, and their reports are awaited.
Additionally, a report by Klatte et al. mentions the effectiveness of neoadjuvant chemotherapy using TKI monotherapy. It has been reported that the length of tumor thrombus was decrease in 73% of cases. However, what should be considered about TKI toxicity and discontinuation of surgery due to treatment failure.
In this report, it was reported that the length of tumor thrombus was increased in 10% of cases.
It seems necessary to select cases carefully (8).
Explanations of findings
Three important points should be noted from this case. The first is appropriate patient selection and treatment choice. Robot-assisted surgery may be beneficial in cases in which open surgery is difficult; however, progressive cases may also exist, and age, symptoms, and patient background should be considered in making a choice. The choice of chemotherapy should be determined by each institution. We developed a treatment selection protocol based on the IMDC risk classification, presence or absence of impending symptoms, bone metastases, age, and whether to aim for a surgical cure. The second issue is the surgical technique. Multiple factors increase the difficulty of surgery, such as liver dehiscence, degree of adhesion between the IVC wall and the tumor, and method of clamping the IVC. Future surgical techniques need to be established. In this case, the Pringle maneuver was unnecessary, and the adhesion between the tumor and the IVC wall was negligible; therefore, the surgery could be performed smoothly. The third factor is the importance of communicating with liver surgeons. In this case, surgery was performed solely by the urology department while seeking the opinion of the liver surgeon during the procedure. We held in-depth preoperative conferences with the liver surgeon that enabled us to understand the anatomy of the liver (for example, the course of the inferior right hepatic vein) and discuss the surgical technique.
Implications and actions needed
This is one of the few cases in which RARN and IVC thrombectomy were performed after downstaging the tumor using immuno-oncology (IO)/TKI therapy.
Further study to determine the surgical technique and chemotherapy of choice is required.
Conclusions
We safely performed RARN after downstaging the tumor with pre-surgical IO/TKI combination therapy without complications and achieved surgical complete response (CR). The number of cases is few, and the efficacy of pre-surgical treatment needs to be investigated.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors would like to thank Editage (www.editage.jp) for English language editing.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Takuya Koie) for the series “Current Status of Robotic Surgery for Genitourinary Diseases in Japan” published in Translational Cancer Research. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1547/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-1547/coif). The series “Current Status of Robotic Surgery for Genitourinary Diseases in Japan” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Ciancio G, Manoharan M, Katkoori D, et al. Long-term survival in patients undergoing radical nephrectomy and inferior vena cava thrombectomy: single-center experience. Eur Urol 2010;57:667-72. 10.1016/j.eururo.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 2.Blute ML, Leibovich BC, Lohse CM, et al. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int 2004;94:33-41. 10.1111/j.1464-410X.2004.04897.x [DOI] [PubMed] [Google Scholar]
- 3.Garg H, Psutka SP, Hakimi AA, et al. A Decade of Robotic-Assisted Radical Nephrectomy with Inferior Vena Cava Thrombectomy: A Systematic Review and Meta-Analysis of Perioperative Outcomes. J Urol 2022;208:542-60. 10.1097/JU.0000000000002829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hara T, Terakawa T, Hyodo T, et al. Pathological complete response of renal cell carcinoma with vena cava tumor thrombus to neoadjuvant TKI/IO combination therapy. Urol Case Rep 2021;39:101800. 10.1016/j.eucr.2021.101800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otsuka H, Masui K, Hosomi T, et al. Preoperative ipilimumab/nivolumab combination therapy reduced operation risk by downstaging the inferior vena cava tumor thrombus extending to the right atrium in a metastatic renal cell carcinoma: A case report. Urol Case Rep 2021;40:101912. 10.1016/j.eucr.2021.101912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida K, Hata K, Iizuka J, et al. Immune Checkpoint Inhibitor Combination Therapy for Renal Cell Carcinomas With Concomitant Inferior Vena Cava Thrombi. In Vivo 2022;36:1030-4. 10.21873/invivo.12798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labbate C, Hatogai K, Werntz R, et al. Complete response of renal cell carcinoma vena cava tumor thrombus to neoadjuvant immunotherapy. J Immunother Cancer 2019;7:66. 10.1186/s40425-019-0546-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klatte T, Welsh SJ, Riddick ACP, et al. Tyrosine kinase inhibitor treatment for renal cell carcinoma with inferior vena cava tumour thrombus: a quantitative summary. BJU Int 2023;131:566-8. 10.1111/bju.15966 [DOI] [PubMed] [Google Scholar]