Abstract
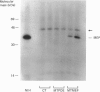
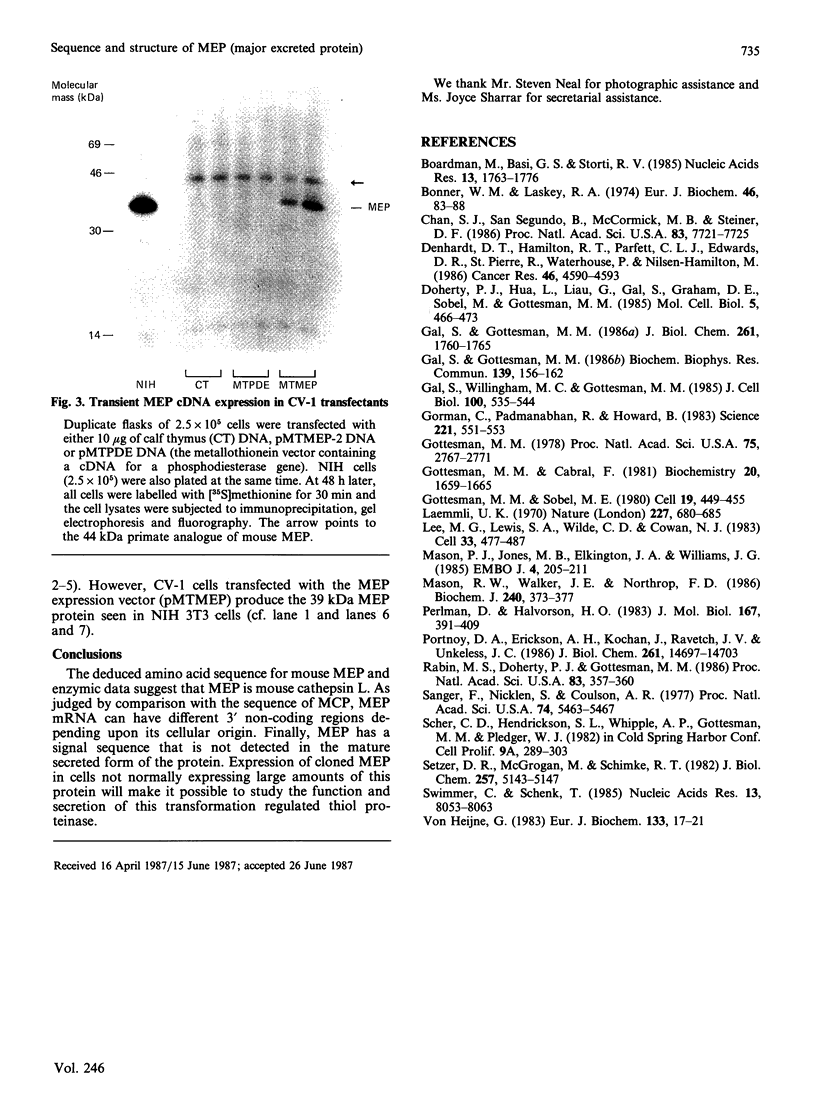
The major excreted protein (MEP) of malignantly transformed mouse fibroblasts is a secreted thiol proteinase. Sequencing of the MEP cDNA shows the coding region for the protein to be identical with the sequence for a mouse cysteine proteinase isolated from macrophages, but the MEP cDNA is polyadenylated at a different site in the 3' non-coding region. Strong homology of MEP with human cathepsin L suggests that MEP is the mouse analogue of cathepsin L. Amino acid sequencing of the N-terminus of the secreted form of MEP indicates that, during secretion, the polypeptide is cleaved between amino acids 17 and 18. We have placed the MEP cDNA in a eukaryotic expression vector and demonstrated the production of the 39 kDa polypeptide form of mouse MEP in monkey CV-1 cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boardman M., Basi G. S., Storti R. V. Multiple polyadenylation sites in a Drosophila tropomyosin gene are used to generate functional mRNAs. Nucleic Acids Res. 1985 Mar 11;13(5):1763–1776. doi: 10.1093/nar/13.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chan S. J., San Segundo B., McCormick M. B., Steiner D. F. Nucleotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7721–7725. doi: 10.1073/pnas.83.20.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Hamilton R. T., Parfett C. L., Edwards D. R., St Pierre R., Waterhouse P., Nilsen-Hamilton M. Close relationship of the major excreted protein of transformed murine fibroblasts to thiol-dependent cathepsins. Cancer Res. 1986 Sep;46(9):4590–4593. [PubMed] [Google Scholar]
- Doherty P. J., Hua L., Liau G., Gal S., Graham D. E., Sobel M., Gottesman M. M. Malignant transformation and tumor promoter treatment increase levels of a transcript for a secreted glycoprotein. Mol Cell Biol. 1985 Mar;5(3):466–473. doi: 10.1128/mcb.5.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal S., Gottesman M. M. The major excreted protein (MEP) of transformed mouse cells and cathepsin L have similar protease specificity. Biochem Biophys Res Commun. 1986 Aug 29;139(1):156–162. doi: 10.1016/s0006-291x(86)80093-6. [DOI] [PubMed] [Google Scholar]
- Gal S., Gottesman M. M. The major excreted protein of transformed fibroblasts is an activable acid-protease. J Biol Chem. 1986 Feb 5;261(4):1760–1765. [PubMed] [Google Scholar]
- Gal S., Willingham M. C., Gottesman M. M. Processing and lysosomal localization of a glycoprotein whose secretion is transformation stimulated. J Cell Biol. 1985 Feb;100(2):535–544. doi: 10.1083/jcb.100.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Cabral F. Purification and characterization of a transformation-dependent protein secreted by cultured murine fibroblasts. Biochemistry. 1981 Mar 17;20(6):1659–1665. doi: 10.1021/bi00509a039. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Sobel M. E. Tumor promoters and Kirsten sarcoma virus increase synthesis of a secreted glycoprotein by regulating levels of translatable mRNA. Cell. 1980 Feb;19(2):449–455. doi: 10.1016/0092-8674(80)90519-x. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M. Transformation-dependent secretion of a low molecular weight protein by murine fibroblasts. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2767–2771. doi: 10.1073/pnas.75.6.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Mason P. J., Jones M. B., Elkington J. A., Williams J. G. Polyadenylation of the Xenopus beta 1 globin mRNA at a downstream minor site in the absence of the major site and utilization of an AAUACA polyadenylation signal. EMBO J. 1985 Jan;4(1):205–211. doi: 10.1002/j.1460-2075.1985.tb02337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Walker J. E., Northrop F. D. The N-terminal amino acid sequences of the heavy and light chains of human cathepsin L. Relationship to a cDNA clone for a major cysteine proteinase from a mouse macrophage cell line. Biochem J. 1986 Dec 1;240(2):373–377. doi: 10.1042/bj2400373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Erickson A. H., Kochan J., Ravetch J. V., Unkeless J. C. Cloning and characterization of a mouse cysteine proteinase. J Biol Chem. 1986 Nov 5;261(31):14697–14703. [PubMed] [Google Scholar]
- Rabin M. S., Doherty P. J., Gottesman M. M. The tumor promoter phorbol 12-myristate 13-acetate induces a program of altered gene expression similar to that induced by platelet-derived growth factor and transforming oncogenes. Proc Natl Acad Sci U S A. 1986 Jan;83(2):357–360. doi: 10.1073/pnas.83.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Schimke R. T. Nucleotide sequence surrounding multiple polyadenylation sites in the mouse dihydrofolate reductase gene. J Biol Chem. 1982 May 10;257(9):5143–5147. [PubMed] [Google Scholar]
- Swimmer C., Shenk T. Selection of sequence elements that substitute for the standard AATAAA motif which signals 3' processing and polyadenylation of late simian virus 40 mRNAs. Nucleic Acids Res. 1985 Nov 25;13(22):8053–8063. doi: 10.1093/nar/13.22.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]