Abstract
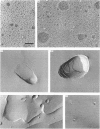
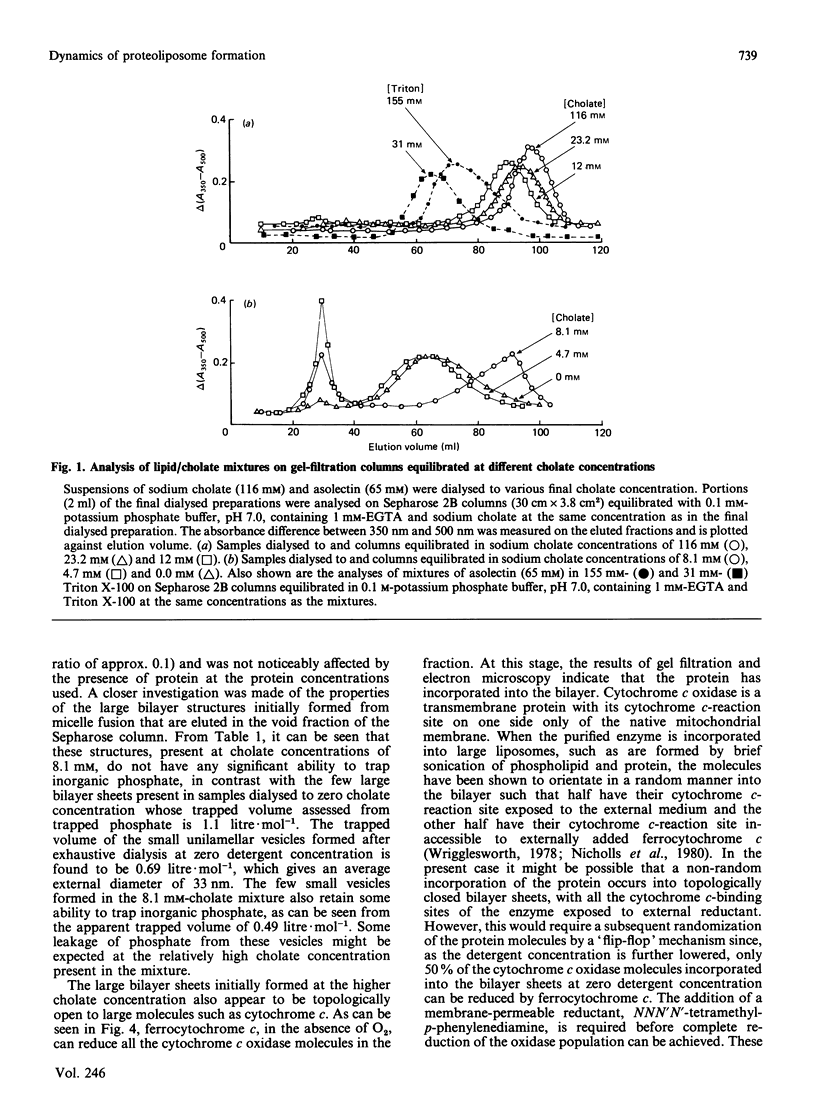
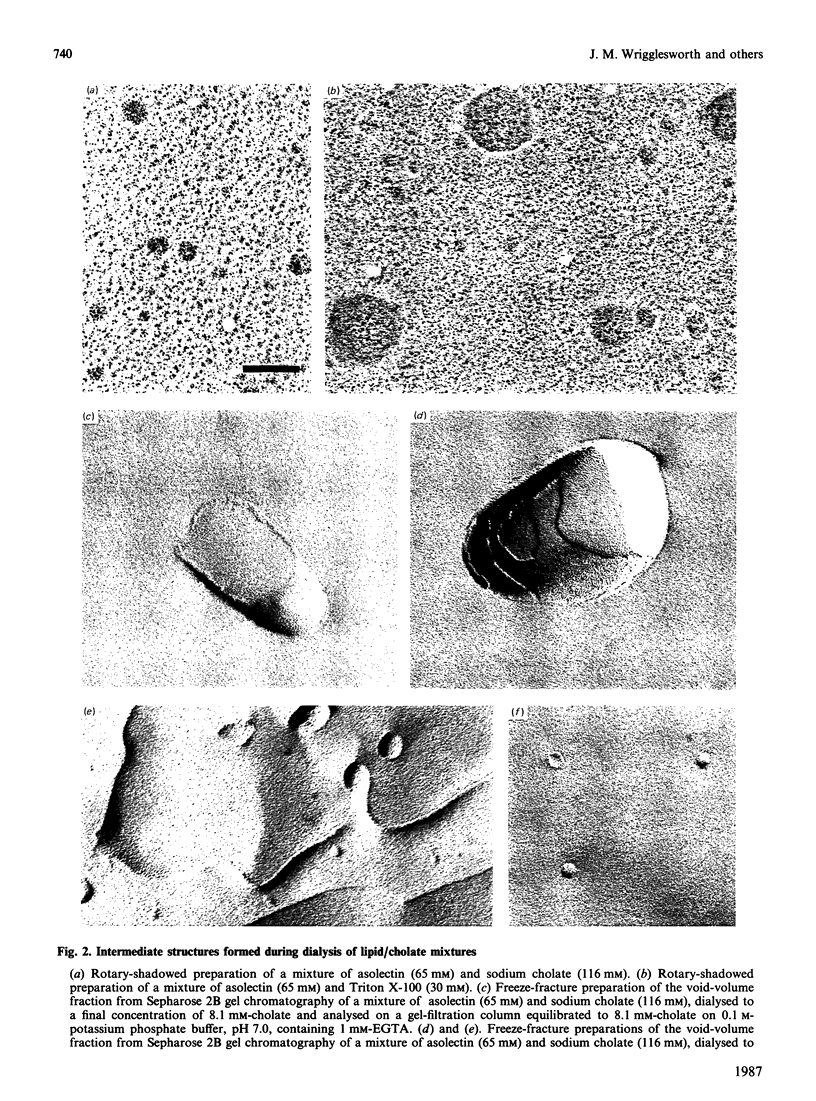
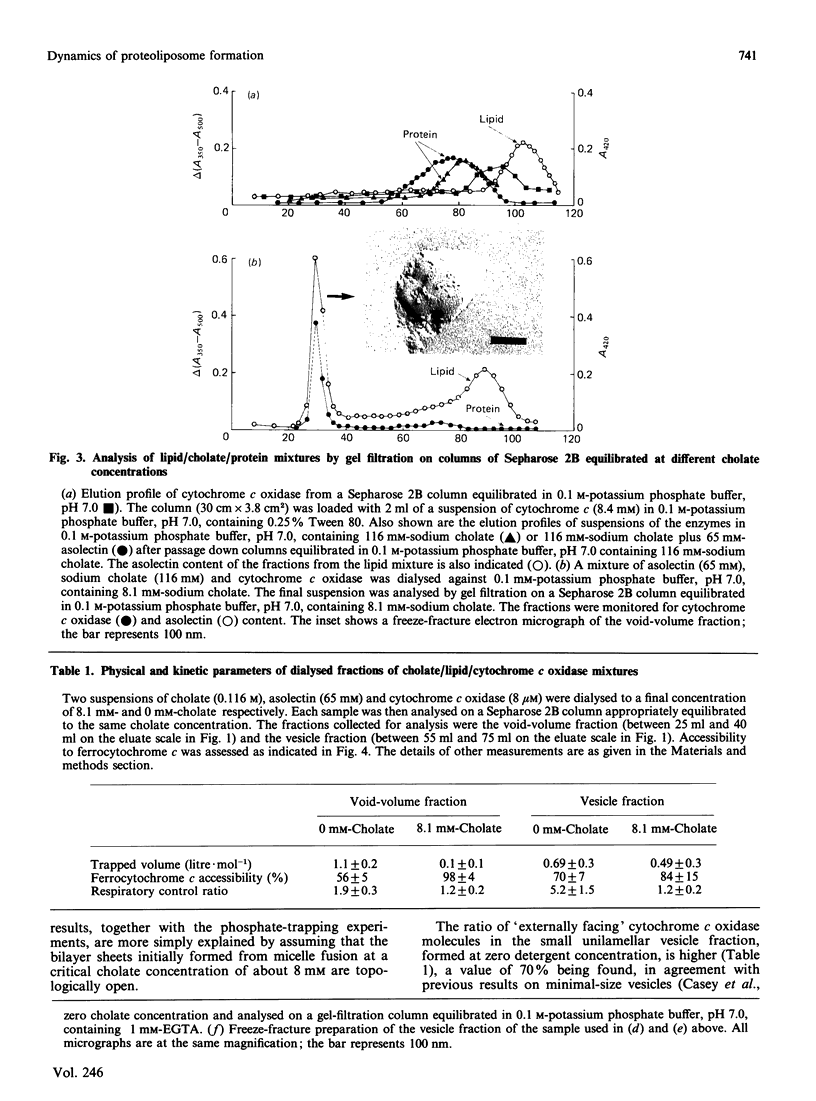
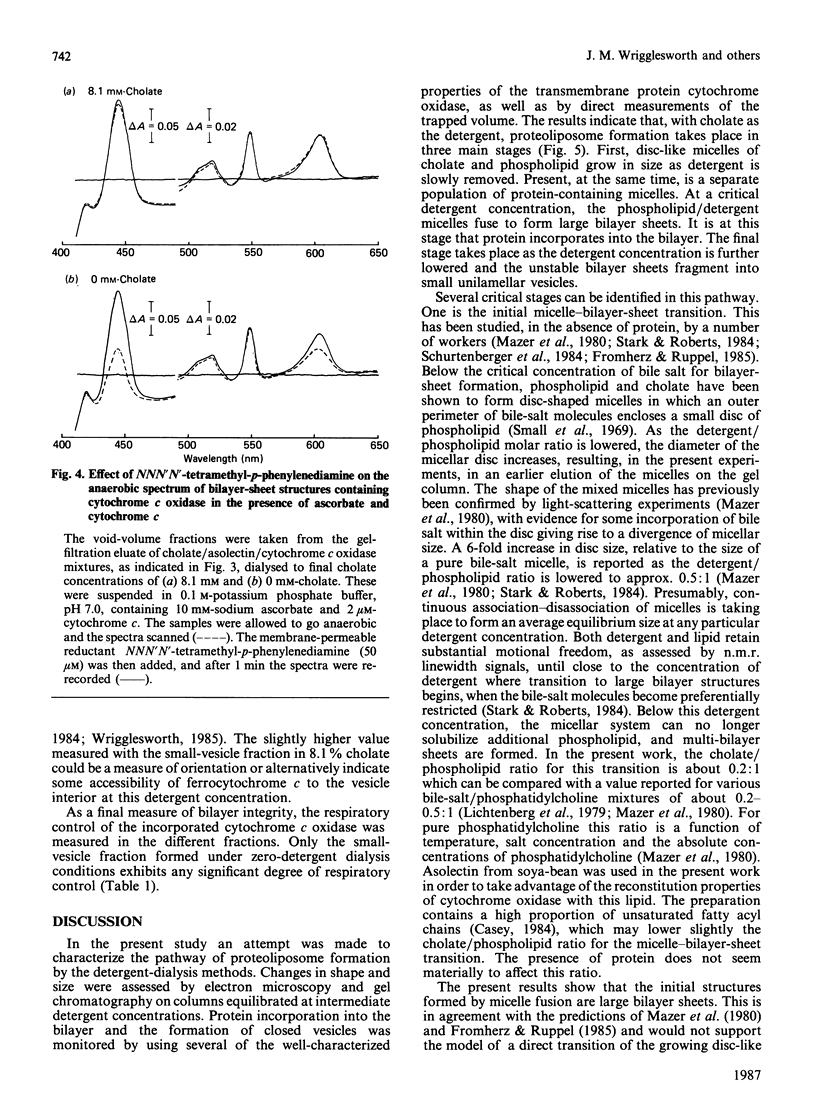
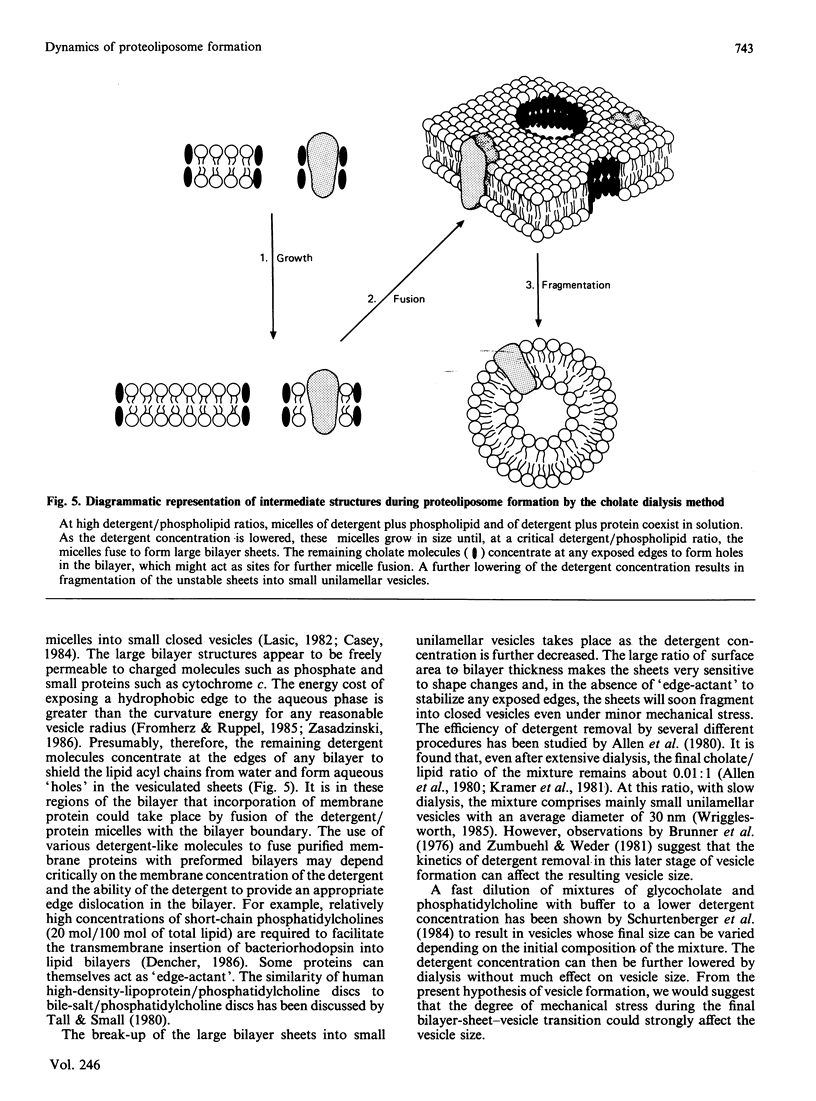
1. The intermediate structures formed during dialysis of mixtures of cholate, phospholipid and cytochrome c oxidase were analysed by gel chromatography and electron microscopy. Measurements of trapped phosphate and the degree of respiratory control were used to assess the integrity of the vesicular structures formed. Protein orientation in the bilayer was monitored by the accessibility of cytochrome c to cytochrome c oxidase. 2. The results indicate that proteoliposome formation by the detergent-dialysis procedure takes place in three distinct stages. In the first stage, cholate/phospholipid and cholate/phospholipid/protein micelles coexist in solution and grow in size as the detergent is slowly removed. At a detergent/phospholipid molar ratio of about 0.2, micelle fusion results in the formation of large bilayer aggregates permeable to both phosphate and cytochrome c. It is at this stage that cytochrome c oxidase is incorporated into the bilayer. In the final stage of dialysis the bilayer sheets fragment into small unilamellar vesicles. 3. The orientation of membrane protein in the final vesicles appears to be determined by the effect of protein conformation on the initial curvature of the bilayer sheets during the fragmentation process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. M., Romans A. Y., Kercret H., Segrest J. P. Detergent removal during membrane reconstitution. Biochim Biophys Acta. 1980 Sep 18;601(2):328–342. doi: 10.1016/0005-2736(80)90537-4. [DOI] [PubMed] [Google Scholar]
- Brunner J., Skrabal P., Hauser H. Single bilayer vesicles prepared without sonication. Physico-chemical properties. Biochim Biophys Acta. 1976 Dec 2;455(2):322–331. doi: 10.1016/0005-2736(76)90308-4. [DOI] [PubMed] [Google Scholar]
- Casey R. P. Membrane reconstitution of the energy-conserving enzymes of oxidative phosphorylation. Biochim Biophys Acta. 1984 Dec 17;768(3-4):319–347. doi: 10.1016/0304-4173(84)90021-1. [DOI] [PubMed] [Google Scholar]
- Casey R. P., O'Shea P. S., Chappell J. B., Azzi A. A quantitative characterisation of H+ translocation by cytochrome c oxidase vesicles. Biochim Biophys Acta. 1984 Apr 26;765(1):30–37. doi: 10.1016/0005-2728(84)90153-1. [DOI] [PubMed] [Google Scholar]
- Dencher N. A. Spontaneous transmembrane insertion of membrane proteins into lipid vesicles facilitated by short-chain lecithins. Biochemistry. 1986 Mar 11;25(5):1195–1200. doi: 10.1021/bi00353a038. [DOI] [PubMed] [Google Scholar]
- Eytan G. D. Use of liposomes for reconstitution of biological functions. Biochim Biophys Acta. 1982 Oct 20;694(2):185–202. doi: 10.1016/0304-4157(82)90024-7. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G. Old drugs in new clothing. Nature. 1984 Jul 19;310(5974):186–187. doi: 10.1038/310186b0. [DOI] [PubMed] [Google Scholar]
- Kagawa Y. Reconstitution of the energy transformer, gate and channel subunit reassembly, crystalline ATPase and ATP synthesis. Biochim Biophys Acta. 1978 Sep 21;505(1):45–93. doi: 10.1016/0304-4173(78)90008-3. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Hasselbach H. J., Semenza G. Rapid transmembrane movement of phosphatidylcholine in small unilamellar lipid vesicles formed by detergent removal. Biochim Biophys Acta. 1981 Apr 22;643(1):233–242. doi: 10.1016/0005-2736(81)90236-4. [DOI] [PubMed] [Google Scholar]
- Kuboyama M., Yong F. C., King T. E. Studies on cytochrome oxidase. 8. Preparation and some properties of cardiac cytochrome oxidase. J Biol Chem. 1972 Oct 25;247(20):6375–6383. [PubMed] [Google Scholar]
- Lasic D. D. A molecular model for vesicle formation. Biochim Biophys Acta. 1982 Nov 22;692(3):501–502. doi: 10.1016/0005-2736(82)90404-7. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D., Zilberman Y., Greenzaid P., Zamir S. Structural and kinetic studies on the solubilization of lecithin by sodium deoxycholate. Biochemistry. 1979 Aug 7;18(16):3517–3525. doi: 10.1021/bi00583a013. [DOI] [PubMed] [Google Scholar]
- Mazer N. A., Benedek G. B., Carey M. C. Quasielastic light-scattering studies of aqueous biliary lipid systems. Mixed micelle formation in bile salt-lecithin solutions. Biochemistry. 1980 Feb 19;19(4):601–615. doi: 10.1021/bi00545a001. [DOI] [PubMed] [Google Scholar]
- Nicholls P., Hildebrandt V., Wrigglesworth J. M. Orientation and reactivity of cytochrome aa3 heme groups in proteoliposomes. Arch Biochem Biophys. 1980 Oct 15;204(2):533–543. doi: 10.1016/0003-9861(80)90065-x. [DOI] [PubMed] [Google Scholar]
- Racker E. Reconstitution of cytochrome oxidase vesicles and conferral of sensitivity to energy transfer inhibitors. J Membr Biol. 1972 Dec 29;10(3):221–235. doi: 10.1007/BF01867856. [DOI] [PubMed] [Google Scholar]
- Rögner M., Ohno K., Hamamoto T., Sone N., Kagawa Y. Net ATP synthesis in H+ -atpase macroliposomes by an external electric field. Biochem Biophys Res Commun. 1979 Nov 14;91(1):362–367. doi: 10.1016/0006-291x(79)90627-2. [DOI] [PubMed] [Google Scholar]
- Schurtenberger P., Mazer N., Waldvogel S., Känzig W. Preparation of monodisperse vesicles with variable size by dilution of mixed micellar solutions of bile salt and phosphatidylcholine. Biochim Biophys Acta. 1984 Aug 8;775(1):111–114. doi: 10.1016/0005-2736(84)90241-4. [DOI] [PubMed] [Google Scholar]
- Seckler R., Wright J. K. Sidedness of native membrane vesicles of Escherichia coli and orientation of the reconstituted lactose: H+ carrier. Eur J Biochem. 1984 Jul 16;142(2):269–279. doi: 10.1111/j.1432-1033.1984.tb08281.x. [DOI] [PubMed] [Google Scholar]
- Small D. M., Penkett S. A., Chapman D. Studies on simple and mixed bile salt micelles by nuclear magnetic resonance spectroscopy. Biochim Biophys Acta. 1969 Jan 21;176(1):178–189. doi: 10.1016/0005-2760(69)90086-1. [DOI] [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Kagawa Y. Reconstitution of vesicles capable of energy transformation from phospholipids and adenosine triphosphatase of a thermophilic bacterium. J Biochem. 1977 Feb;81(2):519–528. doi: 10.1093/oxfordjournals.jbchem.a131485. [DOI] [PubMed] [Google Scholar]
- Tall A. R., Small D. M. Body cholesterol removal: role of plasma high-density lipoproteins. Adv Lipid Res. 1980;17:1–51. [PubMed] [Google Scholar]
- Van Der Werf P., Koshland D. E., Jr Identification of a gamma-glutamyl methyl ester in bacterial membrane protein involved in chemotaxis. J Biol Chem. 1977 Apr 25;252(8):2793–2795. [PubMed] [Google Scholar]
- Wrigglesworth J. M. Quantization of membrane potential generation by cytochrome c oxidase in small vesicles. J Inorg Biochem. 1985 Mar-Apr;23(3-4):311–316. doi: 10.1016/0162-0134(85)85040-6. [DOI] [PubMed] [Google Scholar]
- Zasadzinski J. A. Transmission electron microscopy observations of sonication-induced changes in liposome structure. Biophys J. 1986 Jun;49(6):1119–1130. doi: 10.1016/S0006-3495(86)83741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbuehl O., Weder H. G. Liposomes of controllable size in the range of 40 to 180 nm by defined dialysis of lipid/detergent mixed micelles. Biochim Biophys Acta. 1981 Jan 8;640(1):252–262. doi: 10.1016/0005-2736(81)90550-2. [DOI] [PubMed] [Google Scholar]