Abstract
Sodium-ion batteries (SIBs) are a more sustainable alternative to lithium-ion batteries (LIBs) considering the abundance, global distribution, and low cost of sodium. However, their economic impact remains small compared to LIBs, owing in part to the lag in materials development where significant improvements in energy density and safety remain to be realized. Deep eutectic solvents (DESs) show promise as alternatives to conventional electrolytes in SIBs because of their nonflammable nature. However, their practical application has thus far been hindered by their limited electrochemical stability window. In particular, DESs based on N-methylacetamide have thus far been reported not to be stable with sodium metal. In contrast, this work reports a superconcentration strategy where sodium-ion conducting DESs, based on the dissolution of NaFSI in N-methylacetamide, are simultaneously stable with sodium metal and Prussian blue as state-of-the-art positive electrode material. At 60 °C, the nonflammable DES outperforms a conventional liquid electrolyte in terms of rate performance and capacity retention. Therefore, these novel DES compositions pave the way for the use of DESs in practical applications with an improved safety and sustainability.
1. Introduction
Since their initial commercialization in 1991, rechargeable lithium-ion batteries (LIBs) have evolved into successful energy storage systems. However, lithium is not considered an abundant element because its relative global abundance is only 0.01%.1 Furthermore, lithium is unevenly distributed across the globe. Based on the larger abundance (2.83%) and more even global distribution of sodium, along with its low cost and low electrochemical potential (−2.71 V vs standard hydrogen electrode (SHE)), close to that of lithium (−3.04 V vs SHE), sodium-ion batteries (SIBs) are a promising alternative to LIBs.2−5
The economic impact of SIBs remains small compared to that of LIBs due to the lack of performant electrode materials and electrolytes.6−9 While numerous efforts are being undertaken to discover new electrode materials for SIBs, literature reports on the electrolyte remain relatively scarce. The most frequently used SIB electrolyte at the moment is a solution of NaPF6 (∼1 M) in a mixture of alkyl carbonate-based solvents, such as dimethyl carbonate (DMC), diethyl carbonate, ethyl carbonate (EC), or propylene carbonate (PC).10 An ideal electrolyte should possess the following qualities: a high ionic conductivity, a large thermal stability window, no undesired reactivity toward the electrodes (in a wide temperature range), and a wide electrochemical stability window. Finally, it should be inherently safe and nontoxic. All of these properties are very sensitive to the composition of salt and solvent(s), and to the use of additives in the electrolyte.11 Additives can also be used to decrease the flammability of conventional electrolytes.12,13
Deep eutectic solvents (DESs) have emerged as a potential electrolyte class in energy storage devices due to their low cost, nontoxic nature, and low vapor pressure.14,15 DESs and ionic liquids are similar in many ways, including their nonflammability and low vapor pressure. Other benefits of DESs include their low component costs and simplicity of the synthesis process.16,17 Still, reports on the use of DES electrolytes in SIBs remain scarce. Further, it is challenging to find a DES electrolyte which is simultaneously compatible with both sodium metal and high energy positive electrode materials, such as polyanion compounds, layered oxides, and Prussian blue (PB) analogues.18−20 De Sloovere et al.21 reported a series of DESs consisting of sodium bis(trifluoromethanesulfonyl)imide (NaTFSI) in N-methylacetamide (MAc). Although an increase in salt concentration significantly increased their anodic stability up to ≈4.65 V vs Na+/Na, even the most concentrated formulations were not compatible with sodium metal. In another report, Xiong et al.22 dissolved sodium bis(fluorosulfonyl)imide (NaFSI) in 1,2-dimethylimidazole to obtain a DES with excellent compatibility toward sodium metal. However, its electrochemical stability window remained limited to 3.2 V, as determined from LSV measurements at 100 mV s–1. The DES reported by Chen et al.23 consists of NaClO4, 1,3,2-dioxathiolane-2,2-dioxide (DTD) and succinonitrile, has a room temperature conductivity of 2.86 mS cm–1 and has excellent stability toward Na3V2(PO4)3, hard carbon, and sodium metal. Here, the formation of hydrogen bonds between DTD and succinonitrile enables the latter’s compatibility with sodium metal. However, the high cost of DTD may limit this DES’ commercial viability. In a similar approach, the electrochemical stability window of water was extended to 3.41 V by the dissolution of NaClO4 and succinonitrile, essentially creating a water-locked eutectic electrolyte.24
This work reports a new series of sodium-based DES electrolytes, where a superconcentration strategy is used for the dissolution of NaFSI in MAc. The coordination structure of these DESs was studied by FTIR, Raman, and NMR spectroscopy, revealing the importance of ionic interactions on the functional properties of these DESs. In particular, the coordination structure has a pronounced effect on the ionic conductivity, anodic stability limit, and compatibility toward sodium metal. In PB/sodium cells, the most concentrated DES outperformed a conventional electrolyte in terms of rate performance and electrochemical stability. Compared to previously reported DESs, the compositions reported in this work offer an improved electrochemical stability without the need for expensive hydrogen bond donors/acceptors. As such, this work brings the use of DESs in safe and sustainable applications a step closer.
2. Methods
2.1. Chemicals
Sodium bis(fluorosulfonyl)imide (NaFSI, 99.9%, Solvionic) was utilized as received. After purchasing N-methylacetamide (MAc, ≥ 99%, Sigma-Aldrich), it was dried for 72 h at 45 °C using molecular sieves (3 Å, 4–8 mesh, Acros). The chemicals were stored in a glovebox filled with argon (Sylatech, H2O < 0.1 ppm, O2 < 0.1 ppm).
2.2. Preparation of the DESs
Appropriate amounts of NaFSI and MAc were mixed at 50 °C in an argon-filled glovebox until a transparent mixture was obtained.
2.3. Physicochemical Characterization
Fourier transform infrared spectroscopy (FTIR, PerkinElmer Frontier, 16 scans, 4000–400 cm–1 scan range, 4 cm–1 resolution) was used to examine the DES coordination structure. The measurements were carried out using the MIRacle single reflection ATR. Thermogravimetric analysis (TGA, TA Instruments Q600) was used to study the thermal decomposition of the electrolytes by heating 2–8 mg of the samples (10 °C min–1 to 600 °C, N2 flow). A differential scanning calorimeter (DSC, TA Instruments Q200) was used for the calorimetric studies. The samples were heated from −90 to 60 °C at 10 °C min–1 while being sealed in aluminum hermetic pans and subjected to a 50 mL min–1 nitrogen flow. Using an Anton Paar MCR102 rheometer, the viscosity of the DES was determined at a shear rate of 100 s–1. On a Jeol ECZ400R spectrometer, NMR spectra were obtained at 399.8, 376.2, and 105.7 MHz for 1H, 19F, and 23Na spectra, respectively, using a 5 mm Royal HFX probe at RT. Acquisition parameters used were (i) for 1H NMR: a spectral width of 6 kHz (15 ppm), a 90° pulse length of 6.8 μs, an acquisition time of 2.2 s, a recycle delay time of 12 s and 64 accumulations; (ii) for 19F NMR: a spectral width of 151.5 kHz (400 ppm), a 90° pulse length of 7.9 μs, an acquisition time of 0.7 s, a recycle delay time of 10 s and 64 accumulations; and (iii) for 23Na-NMR: a spectral width of 64.1 kHz (600 ppm), a 90° pulse length of 22 μs, an acquisition of 0.2 s, a recycle delay time of 10 s and 200 accumulations. Raman spectra were collected using a Renishaw InVia Qontor Confocal Raman Microscope with a 10× objective and a 785 nm excitation wavelength to give a laser spot volume of approximately 400 μm3. The measurements were performed at room temperature in continuous scan mode (SynchroScan) with an exposure time of 10 s, 3 accumulations, 100% laser power, and a 1200 L/mm grating. The obtained spectra were analyzed using Renishaw WIRE 5.6 software and cosmic rays were removed when present.
2.4. Electrochemical Characterization
A Mettler Toledo FiveEasy Plus conductometer was used to measure the ionic conductivity of the DESs while the samples were submerged in a thermostatic bath that ranged in temperature from −10 to 60 °C. Using a three-electrode setup consisting of a stainless steel working electrode, a sodium metal counter electrode, and a sodium metal reference electrode, linear sweep voltammetry (LSV, Bio-Logic, VMP3) was used to assess the electrochemical stability window of the DESs. A glass fiber disk (18 mm in diameter and 1.55 mm in thickness, EL-CELL) soaked with the DES under investigation served as a separator between the electrodes. The working electrode potential was swept from the open-circuit potential (OCP) to 5 V vs Na+/Na in the anodic scan, and to −1 V vs Na+/Na in the cathodic scan, with a scan rate of 1 mV s–1. The electrochemical compatibility of the DESs with sodium metal was tested by electrochemical impedance spectroscopy (EIS) in a symmetric Na/DES/Na cell. To execute EIS (Bio-Logic, SP-300), the OCP was perturbed using an alternating sinusoidal potential with an amplitude of 10 mV over a frequency range of 10 kHz to 100 mHz. The long-term stability between the DES electrolyte and Na metal was assessed by a stripping/plating test in a symmetric Na/DES/Na cell, where the DES was impregnated in a glass fiber separator (19 mm diameter, 0.26 mm thickness, purchased from EL-CELL). Here, a current of 0.05 mA cm–2 was applied in alternating directions (30 min per half cycle, BioLogic SP-300).
Electrodes were formulated with 85 wt % PB (Na0.61Fe[Fe(CN)6]0.94, NEI corporation), 10 wt % carbon black (C65, Imerys), and 5 wt % PVDF (1.35 wt % in N-methyl pyrrolidone, Roth Chemicals), using a ball mill (Retsch Emax, 500 rpm, 30 min, 5 × 1 cm zirconia balls). Next, the resulting slurry was tape casted on aluminum foil with a blade height of 100 μm, dried overnight in air at 60 °C, followed by drying under vacuum in a Büchi glass oven B-585 at 110 °C for 15 h. PB/Na cells were assembled in an argon-filled glovebox. A thin glass fiber (EL-CELL, 19 mm diameter, 0.26 mm thickness) soaked with the DES electrolyte (120 μL) was used as separator. Prior to galvanostatic cycling (2.2–4 V vs Na+/Na), all coin cells underwent a 16 h OCP period.
3. Results and Discussion
Seven NaFSI/MAc-based DES electrolytes with different NaFSI concentrations were prepared. Table 1 summarizes their exact compositions, their ionic conductivity at 0, 20, and 60 °C, and their anodic stability limit. Further details are given in Supporting Information. As both DES-1 and DES-2 contain a higher mass of NaFSI than MAc, we consider these to be superconcentrated DESs.
Table 1. Molality (molar Amount of NaFSI Divided by the Weight of MAc), Molar Ratio, Anodic Stability Limit, and Ionic Conductivity at 0, 20, and 60 °C of the DESsa.
| sample | molality NaFSI (m) | [NaFSI]/[MAc] molar ratio | anodic stability limit vs Na+/Na (V) | ionic
conductivity (mS cm–1) |
||
|---|---|---|---|---|---|---|
| 0 °C | 20 °C | 60 °C | ||||
| DES-1 | 13.7 | 1:1 | ≈4.0 | 0.00343 | 0.0561 | 3.65 |
| DES-2 | 6.84 | 1:2 | ≈3.7 | 0.141 | 0.820 | 8.73 |
| DES-3 | 4.56 | 1:3 | 0.319 | 3.35 | 15.6 | |
| DES-4 | 3.42 | 1:4 | 0.754 | 4.46 | 18.0 | |
| DES-5 | 2.74 | 1:5 | 0.901 | 5.31 | 17.1 | |
| DES-6 | 1.95 | 1:7 | 1.23 | 6.50 | 18.0 | |
| DES-7 | 1.52 | 1:9 | 1.25 | 6.58 | 17.7 | |
The anodic stability limit of less concentrated DESs could not be measured due to their reactivity with Na metal (indicated by a dash).
3.1. Coordination Structure
A previous study established the importance of the solution coordination structure for the electrochemical properties of DES electrolytes consisting of NaTFSI and MAc.21 At high salt concentrations, MAc–MAc hydrogen bonds are broken in favor of strong ionic interactions between MAc, Na+, and TFSI–, giving rise to a shift in the energy levels of the molecular orbitals, and ultimately to an increased anodic stability. Given the critical role of the solution coordination structure, the chemical interactions in the NaFSI-based DESs presented in this paper were thoroughly characterized using FTIR, Raman, and NMR spectroscopy.
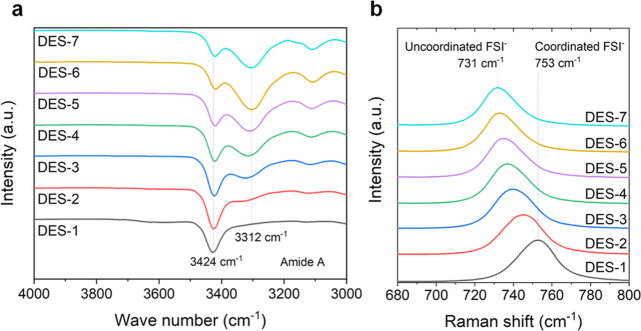
The MAc coordination environment can be readily studied using FTIR spectroscopy (Figure 1a). When its N–H moiety is involved in hydrogen bonds, the amide A band is located around 3312 cm–1. The amide A band will shift toward higher wave numbers as the hydrogen bonds are replaced by ionic interactions. In our set of DESs, the amide A band at 3424 cm–1 becomes more intense compared to the band at 3312 cm–1 as the salt concentration increases. This suggests that in highly concentrated DESs, ionic interactions replace MAc–MAc hydrogen bonds.25 The complete FT-IR spectra are given in Figure S1.26−28 Further information on the solution coordination structure was gathered with Raman spectroscopy (Figures 1b, S2). This technique is particularly useful for assessing the coordination environment of the FSI– anion, as the bands in the 700–770 cm–1 range are characteristic for its S–N stretching modes. The most relevant Raman peaks are described in Table S2. In the Raman spectra of DES-7 (which has the lowest salt content), the band at 731 cm–1 corresponds to uncoordinated FSI– anions. With increasing NaFSI concentration, the FSI– bands shift to higher Raman shifts, indicating that the FSI– anions experience more pronounced ionic interactions.29
Figure 1.
MAc—MAc hydrogen bonds are replaced by ionic interactions when the salt concentration increases. (a) FTIR and (b) Raman spectra of the investigated set of DESs with normalized peak intensities.
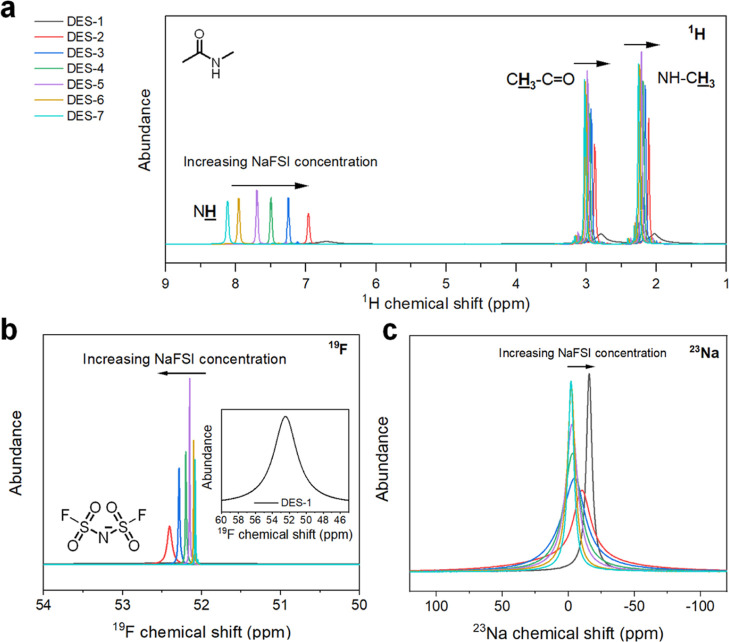
The nature of the ionic interactions in the DESs was further investigated with NMR spectroscopy (Figure 2). The 1H NMR spectra (Figure 2a) indicate that as the concentration of NaFSI increases, there is a significant upfield shift of the MAc NH signal from 8.2 to 6.7 ppm. Additionally, there is an upfield shift for both the MAc CH3–C=O methyl group (from 3.1 to 2.9 ppm) and NH–CH3 methyl group (from 2.3 to 2.15 ppm). As intermolecular hydrogen bonding causes a downfield shift of the NMR signal, the upfield shift of the signals indicates an increase in ionic interaction. Moreover, a significant increase in signal broadening is observed for DES-2 and DES-1, indicating a reduced freedom of MAc mobility. The signals in the 19F NMR spectra (Figure 2b) show a downfield shift as the concentration of NaFSI increases, suggesting that the FSI– ions take part in the ionic interactions. Also here, severe signal broadening is observed for DES-2 and DES-1, indicating a reduced freedom of mobility for the FSI– ions. The 23Na NMR spectra (Figure 2c) show that an increased NaFSI concentration leads to an upfield shift and signal broadening, suggesting that the Na+ ions are actively involved in the ionic interactions as well. The signal broadening indicates a decreased degree of Na+ ion mobility. Whereas the most pronounced upfield shift is observed for DES-1, the signal is significantly less broad than is the case for less concentrated DESs. Whereas this may suggest an increased Na+ ion mobility in this sample (and therefore, an increased sodium ion transference number), one should note that the line width is also determined by the exchange rate between chemical sites.30 In any case, the narrow signal suggests that the Na+-TFSI–-MAc complexes formed in this DES are significantly different from those formed in less concentrated DESs. The presence of such complexes may allow to achieve a combination of high ionic conductivity and broad electrochemical stability which is not possible for less concentrated DESs, where there is always a trade-off between both factors.
Figure 2.
(a) 1H, (b) 19F, and (c) 23Na NMR spectra of the set of DES electrolytes. Increasing the NaFSI concentration leads to upfield shifts of the 1H and 23Na NMR signals, and to a downfield shift of the 19F NMR signals. In the 19F spectra, DES-1 is placed as a zoomed insert.
The spectroscopy results presented above indicate that an increase in NaFSI concentration leads to the breakage of MAc–MAc hydrogen bonds. Instead, strong ionic interactions lead to the formation of Na+-TFSI–-MAc complexes. As shown in our previous work,21 this may cause a significant shift in the energy levels of the molecular orbitals, which ultimately affects the electrochemical stability window of the DES electrolytes. As such, an increase in salt concentration may allow to extend the anodic stability to higher potentials, while simultaneously allowing compatibility with sodium metal. This will be studied in detail in the following sections.
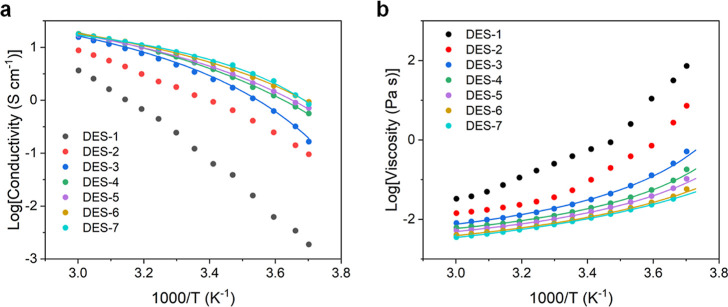
3.2. Ionic Conductivity and Viscosity
The ionic conductivity and viscosity values of the investigated DESs were measured in a broad temperature range (between −3 and 60 °C, Figure 3). Overall, the ionic conductivity decreases and the viscosity increases with increasing salt concentration, in line with the NMR spectroscopy results. For the less concentrated DESs, i.e. DES-3 to DES-7, the variation of ionic conductivity and viscosity can be described by the Vogel–Tammann–Fulcher (VTF) equations
| 1 |
| 2 |
In eqs 1 and 2, the conductivity and viscosity at infinite temperature are represented by the pre-exponential components σ0 and η0, respectively. B, B′, and T0 describe the temperature dependence. For DES-3 to DES-7, these parameters were fitted to the VTF eq (Table 2). However, the ionic conductivity and viscosity of DES-1 and DES-2 could not be reliably fitted to these equations, meaning that they cannot be accurately described by the VTF equations in the investigated temperature range. Particularly for DES-1, the variation with temperature is more pronounced than for the other DESs, which may be related to the decreased peak width observed in 23Na NMR (Figure 2c). At 20 °C, DES-1 has an ionic conductivity of 0.0561 mS cm–1, which increases to 3.65 mS cm–1 at 60 °C. Therefore, this DES is more suitable for operation at higher temperatures. In contrast, DES-2 has an ionic conductivity of 0.820 mS cm–1 at 20 °C, implying that this DES may be more suited for room temperature operation.
Figure 3.
Plot of the (a) ionic conductivity (b) viscosity of the DES electrolytes as a function of temperature. The dots represent the data points, and the full lines represent the fits to the VTF equations. For DES-1 and DES-2, neither the ionic conductivity nor the viscosity could be reliably fitted.
Table 2. Ionic Conductivity for the DES Electrolytes Combined with VTF Model Parameters.
| sample | conductivity |
viscosity |
||||
|---|---|---|---|---|---|---|
| σ0 (mS cm–1) | B (K) | T0 (K) | η0 (Pa s) | B’ (K) | T0 (K) | |
| DES-1 | ||||||
| DES-2 | ||||||
| DES-3 | 278 | 127 | 230 | 0.0493 | 85 | 237 |
| DES-4 | 460 | 167 | 213 | 0.0519 | 7 | 236 |
| DES-5 | 270 | 137 | 217 | 0.0467 | 77 | 231 |
| DES-6 | 185 | 113 | 22 | 0.0377 | 98 | 220 |
| DES-7 | 81 | 65 | 237 | 0.0323 | 11 | 213 |
3.3. Thermal Stability
The thermal stability of the set of DESs was studied with thermogravimetric analysis (TGA, Figure S3). The onset temperature for DES degradation increases with increasing NaFSI concentration, up to ≈150 °C for DES-1, as compared to ≈50 °C for DES-7, most probably due to the stronger ionic interactions in more concentrated DESs. As such, the thermal stability of the more concentrated DESs (i.e., DES-1 to DES-4) exceeds that of a conventional liquid electrolyte (i.e., 1 M NaClO4 in EC/PC). After an initial degradation step, the mass loss profiles of all DESs reach a plateau. A comparison of the experimental residual masses with the theoretical values reveals that this plateau corresponds to the composition where all MAc has evaporated. At higher temperatures, the salt is degraded, similarly to previously investigated DES compositions.21,31 The glass transition temperatures of the DESs were investigated with DSC (Figure S4).
The flammability of the most concentrated DESs was assessed by means of a combustion test where a drop of DES was exposed to a torch. Neither DES-1 nor DES-2 could be ignited after 5–6 s of flame contact, even after repeated flame contact (Supporting Information). We presume that the strong ionic interactions in these samples cause a low vapor pressure, which implies that no combustible vapor can be formed. The nonflammable nature of these DESs means that they facilitate the safety of battery operation.
3.4. Compatibility with Sodium Metal
A wide electrochemical stability window is a crucial functional property of any electrolyte. It was previously reported that sodium metal reacts quickly with MAc, but that this reactivity diminishes as more salt is dissolved into the DES.21 As such, the reactivity of MAc molecules depends on their coordination environment: when involved in an ionic bond, the reactivity is much lower than when involved in a hydrogen bond. The spectroscopy results in this paper also indicate a changing MAc coordination environment with increasing salt concentration. Therefore, we expect a similar behavior for the DESs reported in the paper at hand. A piece of sodium (∼20 mg) was added to each DES composition (1 mL), and the sodium dissolution (and corresponding formation of precipitates) was visually observed throughout time (Figure S5). The sodium metal quickly dissolved in the less concentrated DES compositions: after 5 days, it was totally dissolved in DES-5, DES-6, and DES-7, and partially dissolved in DES-4. The sodium metal was noticeably less soluble in the more concentrated DESs (DES-1, DES-2, and DES-3). Therefore, the stability of these DESs was more thoroughly investigated in Na/DES/Na symmetrical cells at room temperature, which were subjected to EIS over regular time intervals (Figure S6). The Nyquist plots show a lower charge transfer resistance (Rct) with increasing NaFSI concentration, implying that less resistive degradation layers are formed on the sodium metal surface. As implied by the stability of the Rct over time, these layers are most robust for DES-1 and DES-2. The continuously increasing Rct of the Na/DES-3/Na cells implies that this DES is continuously degraded, indicating a poor compatibility between DES-3 and sodium metal. Therefore, this DES was discarded from further investigation.
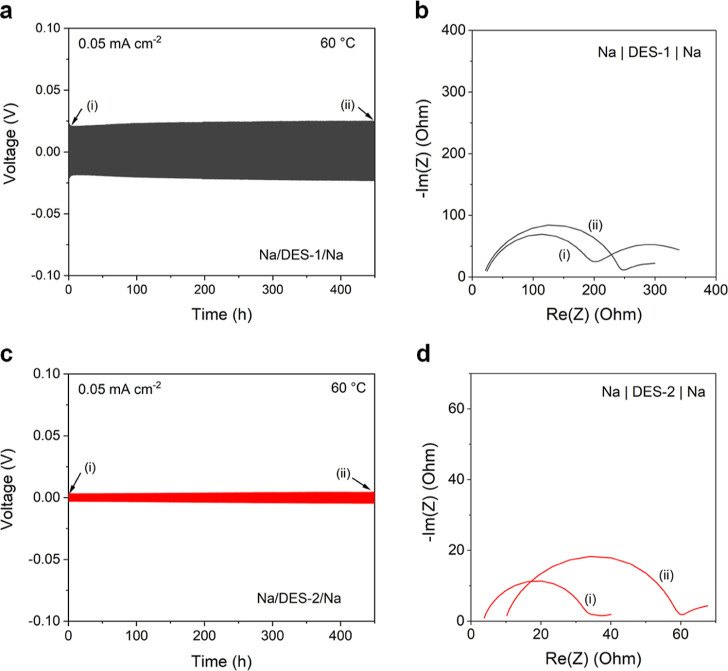
The previous results indicate that DES-1 and DES-2 are compatible with sodium metal at room temperature. As DES-1 may be more suitable for operation at higher temperatures, the stability of symmetrical Na/DES/Na cells was also investigated at 60 °C by means of galvanostatic plating/stripping tests. In these tests, a current of 0.05 mA cm–2 was applied with a half cycle capacity of 0.025 mA cm–2 (i.e., 30 min per half cycle). This process was repeated for 450 h, with a stable voltage profile without short circuit (Figure 4). A slight increase of polarization is observed over time. EIS measurements before and after the stripping/plating tests show an increasing Rct, indicating the formation of a resistive degradation film. Still, these results indicate that these two DES electrolytes have a promising compatibility with sodium metal, in contrast with previously reported MAc-based DESs.21
Figure 4.
(a) Galvanostatic stripping/plating curves of Na/DES-1/Na cells at 60 °C. A current of 0.05 mA cm–2 was applied for 30 min until the polarity was reversed. (b) Corresponding Nyquist plot representation of the impedance spectra of the Na/DES-1/Na cells before and after the stripping/plating test. (c) Galvanostatic stripping/plating curves of Na/DES-2/Na cells at 60 °C. A current of 0.05 mA cm–2 was applied for 30 min until the polarity was reversed. (d) Corresponding Nyquist plot representation of the impedance spectra of the Na/DES-2/Na cells before and after the stripping/plating test.
3.5. Anodic Stability
Using a three-electrode setup consisting of a stainless steel working electrode, a sodium metal counter electrode, and a sodium metal reference electrode, the anodic stability limits of DES-1 and DES-2 were evaluated with linear sweep voltammetry (LSV). DES-1, with its higher salt concentration, has a slightly higher anodic stability limit than DES-2 (Figure 5). This is in line with the observation that increasing salt concentrations lead to the replacement of MAc–MAc hydrogen bonds with strong ionic interactions between Na+, FSI– and MAc. The related shift in molecular orbital energy levels shifts the anodic stability of the electrolytes. Both DESs are potentially compatible with positive electrode materials operating up to 4 V vs Na+/Na. The cathodic LSVs of similar three-electrode cells indicate that both DES-1 and DES-2 are reduced at low voltages (Figure S7).
Figure 5.
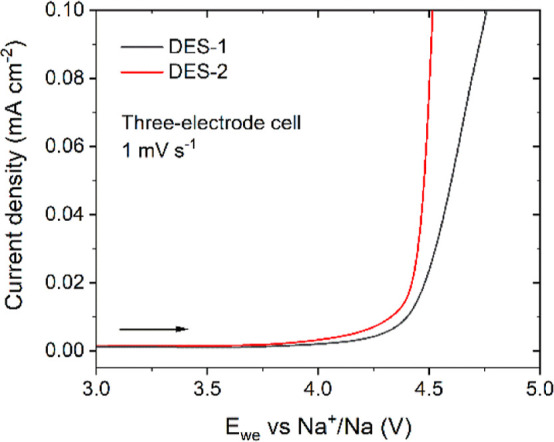
With an increase in NaFSI concentration, the anodic stability limit of the DES compositions increases. LSV of DES-1 and DES-2 impregnated in a glass fiber separator and placed between a stainless steel working electrode, a sodium metal reference electrode, and sodium metal counter electrode in a three-electrode setup. The potential at the working electrode was swept from the OCP to 5 V vs Na+/Na at a scan rate of 1 mV s–1 at room temperature.
3.6. Application in Supercapacitors and in SIBs
The functional characterization presented above suggests that the set of DESs may be used as electrolyte for supercapacitors. This is further elaborated on in the (Figures S8–S12).32−39 Additionally, both DES-1 and DES-2 are electrochemically compatible with both sodium metal and with positive electrode materials operating at up to ∼4 V vs Na+/Na. PB, a transition metal hexacyanoferrate with an open framework structure, is an excellent example of the latter. Operating between 2.2 and 4 V vs Na+/Na, it has a theoretical capacity of ∼170 mAh g–1.40 As stated above, the limited ionic conductivity of DES-1 at room temperature renders it more suitable for use at higher temperatures, whereas DES-2’s higher conductivity may enable its use at room temperature. Therefore, both PB/DES-1/Na and PB/DES-2/Na cells were constructed, where the former were tested at 60 °C and the latter were tested at room temperature. For comparison, similar cells with a conventional electrolyte were also constructed.
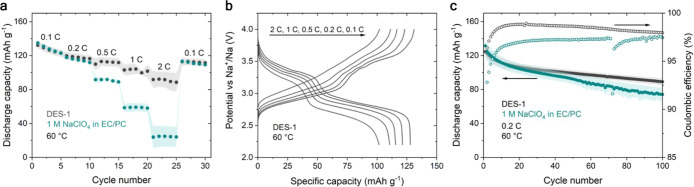
At room temperature, the use of DES-2 as electrolyte does not lead to an improved electrochemical performance compared to the conventional electrolyte (Figure S13).11 However, at 60 °C, PB/DES-1/Na cells perform significantly better than conventional cells. At rates of 0.1 and 0.2 C, both types of cell deliver a similar discharge capacity (Figure 6a). The cells containing DES-1 deliver 112, 102, and 89 mAh g–1 at 0.5, 1, and 2 C, respectively. This is considerably higher than the capacities of 89, 58, and 24 mAh g–1 delivered by the conventional cell at the same rates. The low polarization during galvanostatic cycling is evident from the galvanostatic charge/discharge curves (Figure 6b). After 100 cycles at 0.2 C, the DES-based cells retain 72% of their initial capacity of 125 mAh g–1, as compared to 56% and 131 mAh g–1 for the conventional cells (Figure 6c). The improved capacity retention of the DES-based cells is also related to an increased Coulombic efficiency throughout the entire cycling process. We attribute the superior electrochemical performance of DES-1 at 60 °C to its combination of sufficient ionic conductivity with high electrochemical stability. Using conventional, carbonate-based electrolytes without additives leads to a continuously increasing resistance during cell operation.41 This is also evident from the low Coulombic efficiency observed for 1 M NaClO4 in EC/PC in our case. We surmise that the use of DES-1 as electrolyte leads to the formation of most robust, conductive degradation layers during cell operation, thereby allowing an improved electrochemical operation in terms of rate performance and cycle stability. In lithium-based DESs, higher temperatures during cell operation can lead to a higher fluoride content in the SEI.42 A similar mechanism may be at play in the case at hand. As such, this type of DES holds promise as functional electrolyte, albeit only at higher operating temperatures. This is relevant for large-scale energy storage, where batteries can be subjected to high temperatures because of the climate, and because of the heat generated during high power operation.
Figure 6.
(a) Rate performance and (b) corresponding charge/discharge curves of PB/Na cells containing DES-1 or 1 M NaClO4 in EC/PC as liquid electrolyte. (c) Cycle stability test of PB/Na cells performed at 0.2 C. All electrochemical tests were performed in the voltage window of 2.2–4 V vs Na+/Na and at 60 °C. The average active mass loading of the PB electrode was 1.2 mg cm–2, which corresponds to 0.204 mAh cm–2 assuming a theoretical capacity of 170 mAh g–1. The shaded areas indicate the standard deviation according to three separate measurements.
4. Conclusions
Nonflammable DESs are promising alternatives for highly flammable conventional electrolytes in applications where the primary consideration is safety, such as SIBs in stationary applications. However, the number of reported DES electrolytes for SIBs has remained limited thus far, and the reported compositions have limited stability or rely on the use of expensive hydrogen bond donors/acceptors. In contrast, this work reports a series of stable DESs, based on the dissolution of NaFSI in MAc, with an electrochemical performance exceeding that of a conventional carbonate-based electrolyte at 60 °C. PB/sodium cells containing a DES electrolyte deliver 89 mAh g–1 at a rate of 2 C, whereas this value drops to 24 mAh g–1 for a conventional electrolyte. The former cells retain 72% of their initial capacity of 125 mAh g–1 after 100 cycles at 0.2 C (as compared to 56% and 131 mAh g–1 for the conventional electrolyte). The superior DES compatibility with both PB and sodium metal is achieved by a superconcentration strategy, where intermolecular hydrogen bonds are replaced by ionic interactions. These strong interactions further result in an improved thermal stability and lower flammability as compared to conventional carbonate-based electrolytes. The improved stability of this set of DESs is a step forward for the practical use of DESs as electrolytes in safe and sustainable SIBs.
Acknowledgments
The authors acknowledge the Research Foundation Flanders (FWO Vlaanderen) for financial support under the project number G053519N. Research Foundation Flanders (FWO Vlaanderen) and Hasselt University are acknowledged for the financial support of this research via the Hercules project AUHL/15/2-GOH3816N. The authors thank Gunter Reekmans for his help with the NMR measurements and the interpretation of the data. Further, the authors thank Younas Verhille for electrode preparation.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c02896.
Author Contributions
An-Sofie Kelchtermans: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review and editing, Visualization. Dries De Sloovere: Conceptualization, Methodology, Validation, Investigation, Writing - original draft, Writing - review and editing, Supervision. Jonas Mercken: Methodology (DSC, electrode formulation), Validation, Investigation, Writing - original draft, Writing - review and editing. Thomas Vranken: Methodology (EIS, Rheology), Validation, Writing - original draft, Writing - review and editing. Gianfabio Mangione: Methodology (Raman), Validation, Investigation, Writing - original draft, Writing - review and editing. Bjorn Joos: Conceptualization, Methodology, Validation, Writing - original draft, Writing - review and editing, Supervision, Funding acquisition. Willem Vercruysse: Methodology (supercapacitors), Validation, Writing - original draft, Writing - review and editing. Dries Vandamme: Methodology (supercapacitors), Writing - original draft, Writing - review and editing. Hamid Hamed: Methodology (supercapacitors and battery testing), Validation, Writing - original draft, Writing - review and editing. Mohammadhosein Safari: Methodology (supercapacitors and battery testing), Validation, Writing - original draft, Writing - review and editing. Elien Derveaux: Methodology (NMR), Validation, Investigation, Writing - original draft, Writing - review and editing. Peter Adriaensens: Methodology (NMR), Validation, Investigation, Writing - original draft, Writing - review and editing. Marlies K. Van Bael: Resources, Writing - original draft, Writing - review and editing, Supervision, Funding acquisition, Project administration. An Hardy: Conceptualization, Resources, Writing - original draft, Writing - review and editing, Supervision, Funding acquisition, Project administration.
The authors declare no competing financial interest.
Supplementary Material
References
- Pan H.; Hu Y.-S.; Chen L. Room-Temperature Stationary Sodium-Ion Batteries for Large-Scale Electric Energy Storage †. Energy Environ. Sci. 2013, 6, 2338. 10.1039/c3ee40847g. [DOI] [Google Scholar]
- Li F.; Wei Z.; Manthiram A.; Feng Y.; Ma J.; Mai L. Sodium-Based Batteries: From Critical Materials to Battery Systems. J. Mater. Chem. A Mater. 2019, 7 (16), 9406–9431. 10.1039/C8TA11999F. [DOI] [Google Scholar]
- Chayambuka K.; Mulder G.; Danilov D. L.; Notten P. H. L.; Chayambuka K.; Mulder Vito G.; Danilov D. L.; L Notten P. H. Sodium-Ion Battery Materials and Electrochemical Properties Reviewed. Adv. Energy Mater. 2018, 8 (16), 1800079. 10.1002/AENM.201800079. [DOI] [Google Scholar]
- Slater M. D.; Kim D.; Lee E.; Johnson C. S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23 (8), 947–958. 10.1002/adfm.201200691. [DOI] [Google Scholar]
- Yabuuchi N.; Kubota K.; Dahbi M.; Komaba S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114 (23), 11636–11682. 10.1021/cr500192f. [DOI] [PubMed] [Google Scholar]
- Kubota K.; Komaba S. Review-Practical Issues and Future Perspective for Na-Ion Batteries. J. Electrochem. Soc. 2015, 162 (14), A2538–A2550. 10.1149/2.0151514jes. [DOI] [Google Scholar]
- Li L.; Zheng Y.; Zhang S.; Yang J.; Shao Z.; Guo Z. Recent Progress on Sodium Ion Batteries: Potential High-Performance Anodes. Energy Environ. Sci. 2018, 11 (9), 2310–2340. 10.1039/C8EE01023D. [DOI] [Google Scholar]
- Eshetu G. G.; Elia G. A.; Armand M.; Forsyth M.; Komaba S.; Rojo T.; Passerini S. Electrolytes and Interphases in Sodium-Based Rechargeable Batteries: Recent Advances and Perspectives. Adv. Energy Mater. 2020, 10 (20), 2000093. 10.1002/aenm.202000093. [DOI] [Google Scholar]
- Yan G.; Mariyappan S.; Rousse G.; Jacquet Q.; Deschamps M.; David R.; Mirvaux B.; Freeland J. W.; Tarascon J.-M. Higher Energy and Safer Sodium Ion Batteries via an Electrochemically Made Disordered Na3V2(PO4)2F3Material. Nat. Commun. 2019, 10 (1), 585. 10.1038/s41467-019-08359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti D.; Jó E.; Boschin A.; Palací M. R.; Ponrouch A.; Johansson P. Towards Standard Electrolytes for Sodium-Ion Batteries: Physical Properties, Ion Solvation and Ion-Pairing in Alkyl Carbonate Solvents. Phys. Chem. Chem. Phys. 2276, 22, 22768. 10.1039/d0cp03639k. [DOI] [PubMed] [Google Scholar]
- Ponrouch A.; Marchante E.; Courty M.; Tarascon J. M.; Palacín M. R. In Search of an Optimized Electrolyte for Na-Ion Batteries. Energy Environ. Sci. 2012, 5 (9), 8572–8583. 10.1039/c2ee22258b.1039/c2ee22258b. [DOI] [Google Scholar]
- Zhu C.; Wu D.; Wang C.; Ma J. Flame-Retardant, Self-Purging, High-Voltage Electrolyte for Safe and Long-Cycling Sodium Metal Batteries. Adv. Funct. Mater. 2024, 2406764. 10.1002/adfm.202406764. [DOI] [Google Scholar]
- Liang H. J.; Liu H. H.; Guo J. Z.; Zhao X. X.; Gu Z. Y.; Yang J. L.; Zhang X. Y.; Liu Z. M.; Li W. L.; Wu X. L. Self-purification and silicon-rich interphase achieves high-temperature (70°C) sodium-ion batteries with nonflammable electrolyte. Energy Storage Mater. 2024, 66, 103230. 10.1016/j.ensm.2024.103230. [DOI] [Google Scholar]
- Uhl M.; Geng T.; Schuster P. A.; Schick B. W.; Kruck M.; Fuoss A.; Kuehne A. J. C.; Jacob T. Combining Deep Eutectic Solvents with TEMPO-Based Polymer Electrodes: Influence of Molar Ratio on Electrode Performance. Angew. Chem., Int. Ed. 2023, 62 (2), e202214927 10.1002/anie.202214927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl M.; Sadeeda S.; Penert P.; Schuster P. A.; Schick B. W.; Muench S.; Farkas A.; Schubert U. S.; Esser B.; Kuehne A. J. C.; Jacob T.; Uhl M.; Schick B. W.; Farkas A.; Jacob T.; Penert P.; Esser B.; Schuster P.; C Kuehne A. J.; Muench S.; Schubert U. S. All-Organic Battery Based on Deep Eutectic Solvent and Redox-Active Polymers. ChemSusChem 2023, 17 (1), e202301057 10.1002/CSSC.202301057. [DOI] [PubMed] [Google Scholar]
- Liu K.; Wang Z.; Shi L.; Jungsuttiwong S.; Yuan S. Ionic Liquids for High Performance Lithium Metal Batteries. J. Energy Chem. 2021, 59, 320–333. 10.1016/j.jechem.2020.11.017. [DOI] [Google Scholar]
- Chatterjee K.; Pathak A. D.; Lakma A.; Sharma C. S.; Sahu K. K.; Singh A. K. Synthesis, Characterization and Application of a Non-Flammable Dicationic Ionic Liquid in Lithium-Ion Battery as Electrolyte Additive. Sci. Rep. 2020, 10 (1), 9606. 10.1038/s41598-020-66341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J. Z.; Gu Z. Y.; Du M.; Zhao X. X.; Wang X. T.; Wu X. L. Emerging Characterization Techniques for Delving Polyanion-Type Cathode Materials of Sodium-Ion Batteries. Mater. Today 2023, 66, 221–244. 10.1016/j.mattod.2023.03.020. [DOI] [Google Scholar]
- Huang Z. X.; Gu Z. Y.; Heng Y. L.; Huixiang Ang E.; Geng H. B.; Wu X. L. Advanced Layered Oxide Cathodes for Sodium/Potassium-Ion Batteries: Development, Challenges and Prospects. Chem. Eng. J. 2023, 452, 139438. 10.1016/j.cej.2022.139438. [DOI] [Google Scholar]
- Gu Z. Y.; Wang X. T.; Heng Y. L.; Zhang K. Y.; Liang H. J.; Yang J. L.; Ang E. H.; Wang P. F.; You Y.; Du F.; Wu X. L. Prospects and Perspectives on Advanced Materials for Sodium-Ion Batteries. Sci. Bull. 2023, 68, 2302–2306. 10.1016/j.scib.2023.08.038. [DOI] [PubMed] [Google Scholar]
- De Sloovere D.; Vanpoucke D. E. P.; Paulus A.; Joos B.; Calvi L.; Vranken T.; Reekmans G.; Adriaensens P.; Eshraghi N.; Mahmoud A.; Boschini F.; Safari M.; Van Bael M. K.; Hardy A. Deep Eutectic Solvents as Nonflammable Electrolytes for Durable Sodium-Ion Batteries. Adv. Energy Sustainability Res. 2022, 3 (3), 2100159. 10.1002/aesr.202100159. [DOI] [Google Scholar]
- Xiong W.; Zhang X.; Tu Z.; Hu X.; Wu Y. Novel Deep Eutectic Electrolyte Induced by Na···N Interactions for Sodium Batteries. Ind. Eng. Chem. Res. 2023, 62 (1), 51–61. 10.1021/acs.iecr.2c03313. [DOI] [Google Scholar]
- Chen J.; Yang Z.; Xu X.; Qiao Y.; Zhou Z.; Hao Z.; Chen X.; Liu Y.; Wu X.; Zhou X.; Li L.; Chou S. Non-Flammable Succinonitrile-Based Deep Eutectic Electrolyte for Intrinsically Safe High-Voltage Sodium-Ion Batteries. Adv. Mater. 2024, 36 (28), 2400169. 10.1002/adma.202400169. [DOI] [PubMed] [Google Scholar]
- Liu T.; Wu H.; Du X.; Wang J.; Chen Z.; Wang H.; Sun J.; Zhang J.; Niu J.; Yao L.; Zhao J.; Cui G. Water-Locked Eutectic Electrolyte Enables Long-Cycling Aqueous Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14 (29), 33041–33051. 10.1021/acsami.2c04893. [DOI] [PubMed] [Google Scholar]
- Myshakina N. S.; Ahmed Z.; Asher S. A. Dependence of Amide Vibrations on Hydrogen Bonding. J. Phys. Chem. B 2008, 112 (38), 11873–11877. 10.1021/jp8057355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa T.; Shimanouchi T.; Muzushima S. I. Normal Vibrations of N-Methylacetamide. J. Chem. Phys. 2004, 29 (3), 611. 10.1063/1.1744547. [DOI] [Google Scholar]
- Huang J.; Hollenkamp A. F. Thermal Behavior of Ionic Liquids Containing the FSI Anion and the Li + Cation. J. Phys. Chem. C 2010, 114 (49), 21840–21847. 10.1021/jp107740p. [DOI] [Google Scholar]
- Matsumoto K.; Oka T.; Nohira T.; Hagiwara R. Polymorphism of Alkali Bis(Fluorosulfonyl)Amides (M[N(SO 2 F) 2 ], M = Na, K, and Cs). Inorg. Chem. 2013, 52 (2), 568–576. 10.1021/ic3010486. [DOI] [PubMed] [Google Scholar]
- Kerner M.; Plylahan N.; Scheers J.; Johansson P. Ionic Liquid Based Lithium Battery Electrolytes: Fundamental Benefits of Utilising Both TFSI and FSI Anions?. Phys. Chem. Chem. Phys. 2015, 17 (29), 19569–19581. 10.1039/C5CP01891A. [DOI] [PubMed] [Google Scholar]
- Forsyth M.; Yoon H.; Chen F.; Zhu H.; MacFarlane D. R.; Armand M.; Howlett P. C. Novel Na+ Ion Diffusion Mechanism in Mixed Organic-Inorganic Ionic Liquid Electrolyte Leading to High Na+ Transference Number and Stable, High Rate Electrochemical Cycling of Sodium Cells. J. Phys. Chem. C 2016, 120 (8), 4276–4286. 10.1021/acs.jpcc.5b11746. [DOI] [Google Scholar]
- Joos B.; Vranken T.; Marchal W.; Safari M.; Van Bael M. K.; Hardy A. T. Eutectogels: A New Class of Solid Composite Electrolytes for Li/Li-Ion Batteries. Chem. Mater. 2018, 30 (3), 655–662. 10.1021/acs.chemmater.7b03736. [DOI] [Google Scholar]
- Guo M.; Liu C.; Zhang Z.; Zhou J.; Tang Y.; Luo S.; Guo M.; Liu C.; Luo S.; Zhang Z.; Zhou J.; Tang Y. Flexible Ti3C2Tx@Al electrodes with Ultrahigh Areal Capacitance: In Situ Regulation of Interlayer Conductivity and Spacing. Adv. Funct. Mater. 2018, 28, 1803196. 10.1002/adfm.201803196. [DOI] [Google Scholar]
- Dong L.; Xu C.; Yang Q.; Fang J.; Li Y.; Kang F. High-Performance Compressible Supercapacitors Based on Functionally Synergic Multiscale Carbon Composite Textiles. J. Mater. Chem. A Mater. 2015, 3 (8), 4729–4737. 10.1039/C4TA06494A. [DOI] [Google Scholar]
- Tran K. T. T.; Le L. T. M.; Phan A. L. B.; Tran P. H.; Vo T. D.; Truong T. T. T.; Nguyen N. T. B.; Garg A.; Le P. M. L.; Tran M. V. New Deep Eutectic Solvents Based on Ethylene Glycol - LiTFSI and Their Application as an Electrolyte in Electrochemical Double Layer Capacitor (EDLC). J. Mol. Liq. 2020, 320, 114495. 10.1016/j.molliq.2020.114495. [DOI] [Google Scholar]
- Phadke S.; Amara S.; Anouti M. Gas Evolution in Activated-Carbon-Based Supercapacitors with Protic Deep Eutectic Solvent as Electrolyte. ChemPhysChem 2017, 18 (17), 2364–2373. 10.1002/cphc.201700621. [DOI] [PubMed] [Google Scholar]
- Ochai-Ejeh F. O.; Madito M. J.; Momodu D. Y.; Khaleed A. A.; Olaniyan O.; Manyala N. High Performance Hybrid Supercapacitor Device Based on Cobalt Manganese Layered Double Hydroxide and Activated Carbon Derived from Cork (Quercus Suber). Electrochim. Acta 2017, 252, 41–54. 10.1016/j.electacta.2017.08.163. [DOI] [Google Scholar]
- Kavil J.; Anjana P. M.; Periyat P.; Rakhi R. B. Titania Nanotubes Dispersed Graphitic Carbon Nitride Nanosheets as Efficient Electrode Materials for Supercapacitors. J. Mater. Sci.: Mater. Electron. 2018, 29 (19), 16598–16608. 10.1007/s10854-018-9753-1. [DOI] [Google Scholar]
- Chidiac J.; Nikiforidis G.; Timperman L.; Anouti M. Non-Flammable Sodium Asymmetric Imide Salt-Based Deep Eutectic Solvent for Supercapacitor Applications. ChemPhysChem 2022, 23 (19), e202200224 10.1002/cphc.202200224. [DOI] [PubMed] [Google Scholar]
- Zaidi W.; Timperman L.; Anouti M. Deep Eutectic Solvent Based on Sodium Cations as an Electrolyte for Supercapacitor Application. RSC Adv. 2014, 4 (86), 45647–45652. 10.1039/C4RA08178A. [DOI] [Google Scholar]
- Peng J.; Zhang W.; Liu Q.; Wang J.; Chou S.; Liu H.; Dou S. Prussian Blue Analogues for Sodium-Ion Batteries: Past, Present, and Future. Adv. Mater. 2022, 34 (15), 2108384. 10.1002/adma.202108384. [DOI] [PubMed] [Google Scholar]
- Yan G.; Reeves K.; Foix D.; Li Z.; Cometto C.; Mariyappan S.; Salanne M.; Tarascon J.-M. A New Electrolyte Formulation for Securing High Temperature Cycling and Storage Performances of Na-Ion Batteries. Adv. Energy Mater. 2019, 9, 1901431. 10.1002/aenm.201901431. [DOI] [Google Scholar]
- Hou Q.; Li P.; Qi Y.; Wang Y.; Huang M.; Shen C.; Xiang H.; Li N.; Xie K. Temperature-Responsive Solvation of Deep Eutectic Electrolyte Enabling Mesocarbon Microbead Anode for High-Temperature Li-Ion Batteries. ACS Energy Lett. 2023, 8 (9), 3649–3657. 10.1021/acsenergylett.3c01079. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.