Abstract
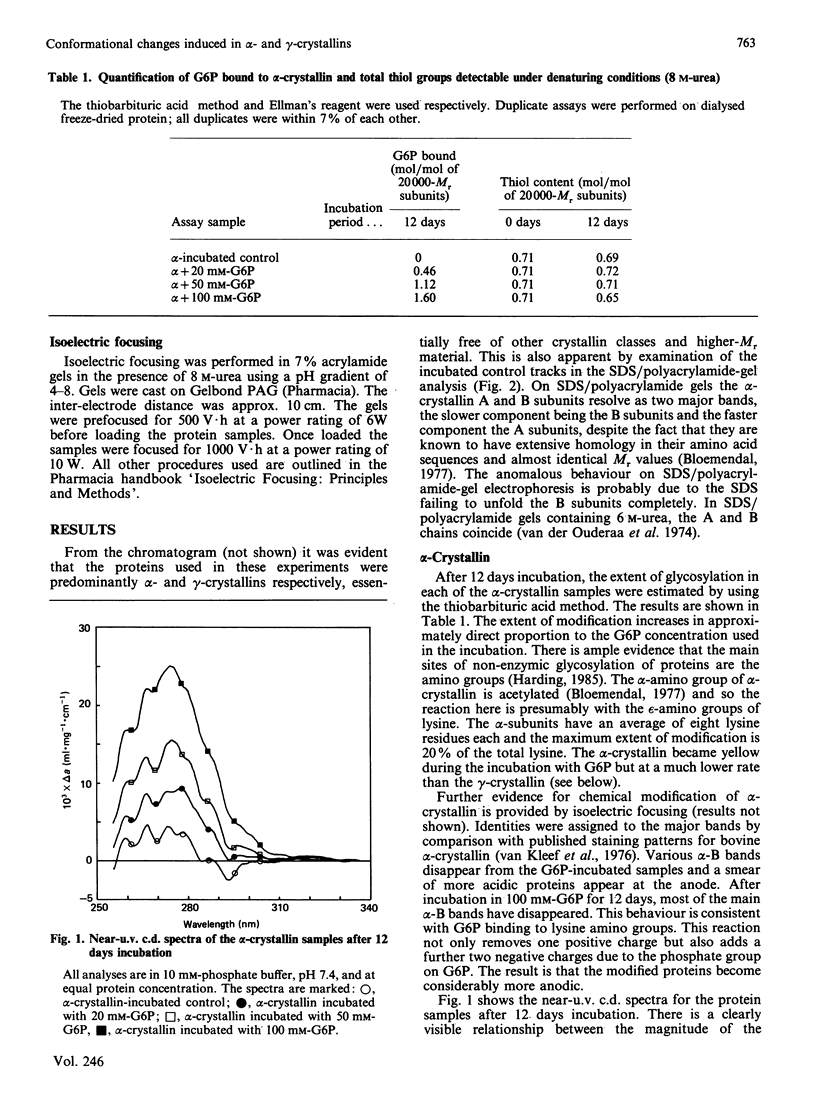
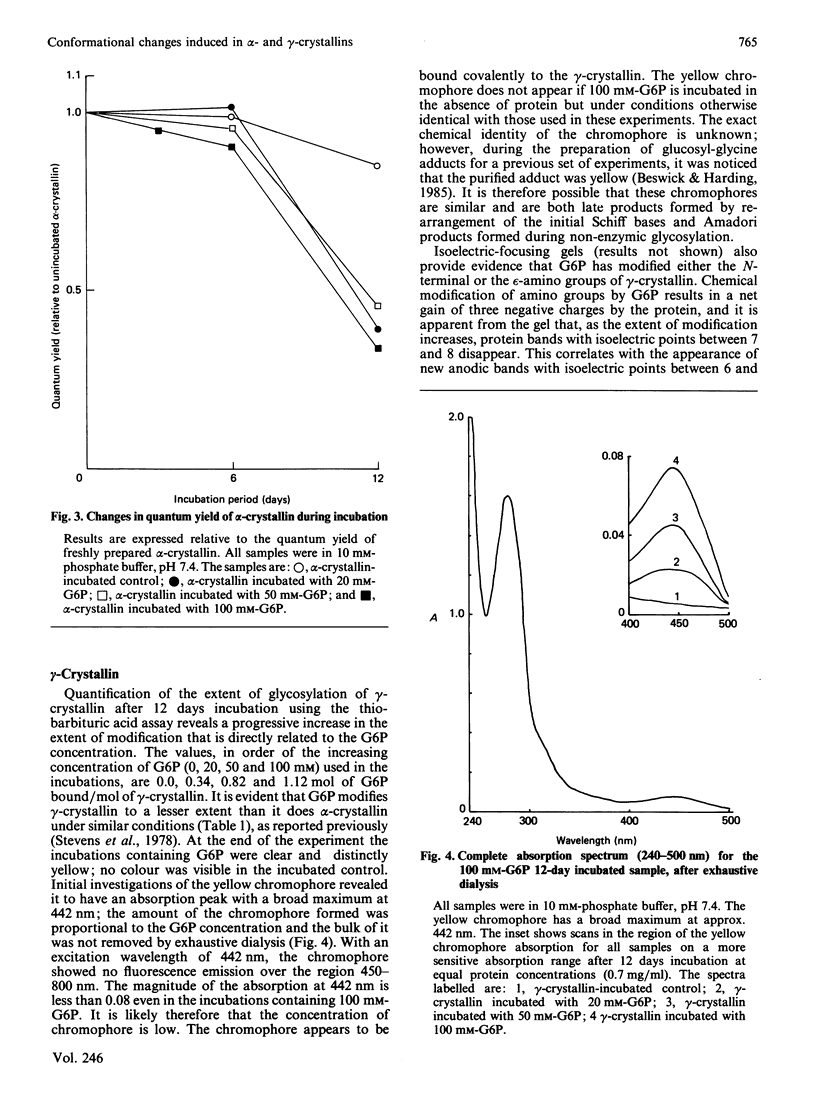
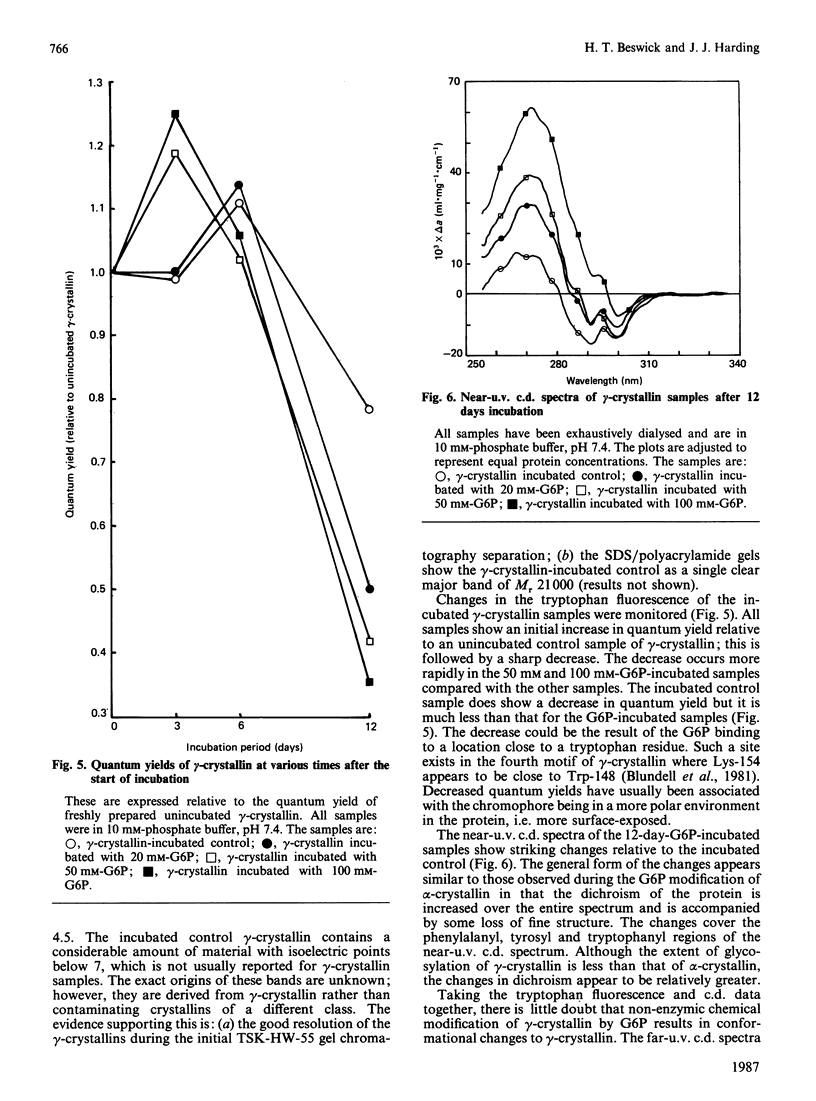
There is good evidence that the non-enzymic chemical modification of proteins plays a role in the aetiology of cataract and diabetic sequelae. This paper presents new evidence that glycosylation of two major lens structural crystallins, alpha- and gamma-crystallins, by glucose 6-phosphate (G6P) induces conformational changes in the proteins. In addition the surface charge on the molecules is altered. These changes would affect protein-protein and protein-water interactions within the lens and could lead to disruption of the short-range order of the lens proteins which is essential for lens transparency. Conformational changes to lens proteins are known to occur in human cataractous lenses but their cause in vivo is not established. Cumulative chemical modification of proteins, over a period of decades, is a strong candidate as a causal agent.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BJORK I. Studies on gamma-crystallin from calf lens. I. Isolation by gel filtration. Exp Eye Res. 1961 Dec;1:145–154. doi: 10.1016/s0014-4835(61)80020-1. [DOI] [PubMed] [Google Scholar]
- Berbers G. A., Hoekman W. A., Bloemendal H., de Jong W. W., Kleinschmidt T., Braunitzer G. Homology between the primary structures of the major bovine beta-crystallin chains. Eur J Biochem. 1984 Mar 15;139(3):467–479. doi: 10.1111/j.1432-1033.1984.tb08029.x. [DOI] [PubMed] [Google Scholar]
- Beswick H. T., Harding J. J. Aldehydes or dicarbonyls in non-enzymic glycosylation of proteins. Biochem J. 1985 Mar 1;226(2):385–389. doi: 10.1042/bj2260385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beswick H. T., Harding J. J. Conformational changes induced in bovine lens alpha-crystallin by carbamylation. Relevance to cataract. Biochem J. 1984 Oct 1;223(1):221–227. doi: 10.1042/bj2230221. [DOI] [PMC free article] [PubMed] [Google Scholar]
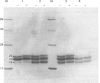
- Bloemendal H. The vertebrate eye lens. Science. 1977 Jul 8;197(4299):127–138. doi: 10.1126/science.877544. [DOI] [PubMed] [Google Scholar]
- Blundell T., Lindley P., Miller L., Moss D., Slingsby C., Tickle I., Turnell B., Wistow G. The molecular structure and stability of the eye lens: x-ray analysis of gamma-crystallin II. Nature. 1981 Feb 26;289(5800):771–777. doi: 10.1038/289771a0. [DOI] [PubMed] [Google Scholar]
- Bours J., Vornhagen R., Herlt M., Rink H. Immunological characterization of calf lens gamma-crystallins, separated by preparative isoelectric focusing. Curr Eye Res. 1981;1(11):651–658. doi: 10.3109/02713688109001869. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984 Oct;101(4):527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- Bucala R., Gallati M., Manabe S., Cotlier E., Cerami A. Glucocorticoid-lens protein adducts in experimentally induced steroid cataracts. Exp Eye Res. 1985 Jun;40(6):853–863. doi: 10.1016/0014-4835(85)90130-7. [DOI] [PubMed] [Google Scholar]
- Day J. F., Thorpe S. R., Baynes J. W. Nonenzymatically glucosylated albumin. In vitro preparation and isolation from normal human serum. J Biol Chem. 1979 Feb 10;254(3):595–597. [PubMed] [Google Scholar]
- Delaye M., Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. 1983 Mar 31-Apr 6Nature. 302(5907):415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Garlick R. L., Mazer J. S. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J Biol Chem. 1983 May 25;258(10):6142–6146. [PubMed] [Google Scholar]
- Gonzalez A. M., Sochor M., McLean P. Effect of experimental diabetes on glycolytic intermediates and regulation of phosphofructokinase in rat lens. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1173–1179. doi: 10.1016/0006-291x(80)91596-x. [DOI] [PubMed] [Google Scholar]
- Harding J. J. Conformational changes in human lens proteins in cataract. Biochem J. 1972 Aug;129(1):97–100. doi: 10.1042/bj1290097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J. J., Dilley K. J. Structural proteins of the mammalian lens: a review with emphasis on changes in development, aging and cataract. Exp Eye Res. 1976 Jan;22(1):1–73. doi: 10.1016/0014-4835(76)90033-6. [DOI] [PubMed] [Google Scholar]
- Harding J. J. Nonenzymatic covalent posttranslational modification of proteins in vivo. Adv Protein Chem. 1985;37:247–334. doi: 10.1016/s0065-3233(08)60066-2. [DOI] [PubMed] [Google Scholar]
- Harding J. J., Rixon K. C. Carbamylation of lens proteins: a possible factor in cataractogenesis in some tropical countries. Exp Eye Res. 1980 Nov;31(5):567–571. doi: 10.1016/s0014-4835(80)80015-7. [DOI] [PubMed] [Google Scholar]
- Horwitz J., Kabasawa I., Kinoshita J. H. Conformation of gamma-crystallins of the calf lens: effects of temperature and denaturing agents. Exp Eye Res. 1977 Aug;25(2):199–208. doi: 10.1016/0014-4835(77)90132-4. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Hamaguchi K. Interaction of alcohols with lysozyme. I. Studies on circular dichroism. J Biochem. 1970 Dec;68(6):785–794. doi: 10.1093/oxfordjournals.jbchem.a129415. [DOI] [PubMed] [Google Scholar]
- Inana G., Piatigorsky J., Norman B., Slingsby C., Blundell T. Gene and protein structure of a beta-crystallin polypeptide in murine lens: relationship of exons and structural motifs. Nature. 1983 Mar 24;302(5906):310–315. doi: 10.1038/302310a0. [DOI] [PubMed] [Google Scholar]
- Kasai K., Nakamura T., Kase N., Hiraoka T., Suzuki R., Kogure F., Shimoda S. I. Increased glycosylation of proteins from cataractous lenses in diabetes. Diabetologia. 1983 Jul;25(1):36–38. doi: 10.1007/BF00251894. [DOI] [PubMed] [Google Scholar]
- Liang J. N., Chakrabarti B. Spectroscopic investigations of bovine lens crystallins. 1. Circular dichroism and intrinsic fluorescence. Biochemistry. 1982 Apr 13;21(8):1847–1852. doi: 10.1021/bi00537a022. [DOI] [PubMed] [Google Scholar]
- Liang J. N., Chylack L. T., Jr Change in the protein tertiary structure with non-enzymatic glycosylation of calf alpha-crystallin. Biochem Biophys Res Commun. 1984 Sep 28;123(3):899–906. [PubMed] [Google Scholar]
- Mok C. C., Waley S. G. N-terminal groups of lens proteins. Exp Eye Res. 1968 Jan;7(1):148–153. doi: 10.1016/s0014-4835(68)80039-9. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Detection of nonenzymatic browning products in the human lens. Biochim Biophys Acta. 1983 Oct 4;760(1):97–103. doi: 10.1016/0304-4165(83)90129-0. [DOI] [PubMed] [Google Scholar]
- Schoenmakers J. G., den Dunnen J. T., Moormann R. J., Jongbloed R., van Leen R. W., Lubsen N. H. The crystallin gene families. Ciba Found Symp. 1984;106:208–218. doi: 10.1002/9780470720875.ch12. [DOI] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968 Oct 24;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Slingsby C., Croft L. R. Structural studies on calf lens gamma-crystallin fraction IV: a comparison of the cysteine-containing tryptic peptides with the corresponding amino acid sequence of gamma-crystallin fraction II. Exp Eye Res. 1978 Mar;26(3):291–304. doi: 10.1016/0014-4835(78)90076-3. [DOI] [PubMed] [Google Scholar]
- Sredy J., Spector A. The phosphorylation of bovine and human lens polypeptides. Exp Eye Res. 1984 Nov;39(5):653–664. doi: 10.1016/0014-4835(84)90064-2. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Witztum J. L. Glucosylation of low-density lipoproteins to an extent comparable to that seen in diabetes slows their catabolism. Diabetes. 1984 Feb;33(2):130–134. doi: 10.2337/diab.33.2.130. [DOI] [PubMed] [Google Scholar]
- Stevens V. J., Rouzer C. A., Monnier V. M., Cerami A. Diabetic cataract formation: potential role of glycosylation of lens crystallins. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2918–2922. doi: 10.1073/pnas.75.6.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam C. V., Radhakrishnamurthy B., Berenson G. S. Photometric determination of glycosylation of hemoglobin in diabetes mellitus. Clin Chem. 1980 Nov;26(12):1683–1687. [PubMed] [Google Scholar]
- Tomarev S. I., Krayev A. S., Skryabin K. G., Bayev A. A., Gause G. G., Jr The nucleotide sequence of a cloned cDNA corresponding to one of the gamma-crystallins from the eye lens of the frog Rana temporaria. FEBS Lett. 1982 Sep 20;146(2):314–318. doi: 10.1016/0014-5793(82)80942-3. [DOI] [PubMed] [Google Scholar]
- Van Kleef S. M., Willems-Thijssen W., Hoenders H. J. Intracellular degradation and deamidation of alpha-crystallin subunits. Eur J Biochem. 1976 Jul 15;66(3):477–483. doi: 10.1111/j.1432-1033.1976.tb10572.x. [DOI] [PubMed] [Google Scholar]
- Van der Ouderaa F. J., de Jong W. W., Bloemendal H. The molecular weight of the basic polypeptide chain alphaB2 of alpha-crystallin. Mol Biol Rep. 1974 Mar;1(6):365–367. doi: 10.1007/BF00309571. [DOI] [PubMed] [Google Scholar]
- Walker J. E. Lysine residue 199 of human serum albumin is modified by acetylsalicyclic acid. FEBS Lett. 1976 Jul 15;66(2):173–175. doi: 10.1016/0014-5793(76)80496-6. [DOI] [PubMed] [Google Scholar]
- Wistow G., Turnell B., Summers L., Slingsby C., Moss D., Miller L., Lindley P., Blundell T. X-ray analysis of the eye lens protein gamma-II crystallin at 1.9 A resolution. J Mol Biol. 1983 Oct 15;170(1):175–202. doi: 10.1016/s0022-2836(83)80232-0. [DOI] [PubMed] [Google Scholar]
- Yu N. T. Raman spectroscopy: a conformational probe in biochemistry. CRC Crit Rev Biochem. 1977;4(3):229–280. doi: 10.3109/10409237709102559. [DOI] [PubMed] [Google Scholar]