Ibrutinib, a first-generation inhibitor of Bruton’s tyrosine kinase (BTK), is an important therapeutic option in chronic lymphocytic leukemia (CLL) (1). In clinical trials, invasive fungal infections (IFI) were uncommon in patients taking ibrutinib, affecting ≤ 1.2% of patients (2), which was comparable to reported IFI incidence in the general CLL population (3). However, there has been subsequent reports of various fungal infections, mainly Cryptococcus, Aspergillus, and Pneumocystis with the use of ibrutinib. Most notably, one study reported 5 cases of Pneumocystis jiroveci in a 96 patient cohort of CLL patients receiving single agent ibrutinib, 4 of which were previously untreated (4). Additionally, atypical localization of fungal infections has been noted. As a result, there has been added scrutiny regarding the risk of IFI with ibrutinib (2, 5).
Recent reports have shown that ibrutinib inhibits macrophage responses, providing a potential mechanism for predisposition to certain IFI; however clinical risk factors have yet to be elucidated (6). To that end, we sought to quantify the incidence and type of IFI in CLL patients treated with ibrutinib. Secondary objectives included the description of offending fungi, IFI sites, and exploratory risk factor identification.
We conducted a retrospective case-control study of patients ≥18 years of age with CLL and treated with ibrutinib (February 2014-August 2018) at The University of Texas MD Anderson Cancer Center. We included patients with ≥6 months of follow up data after starting ibrutinib or deceased within 6 months of starting ibrutinib. We excluded patients with prior stem cell transplantation. Patients receiving ibrutinib therapy were identified through pharmacy prescription records. IFI was defined according to revised 2019 European Organization for Research and Treatment of Cancer/Invasive fungal Infections Cooperative Group guidelines (7). In our institution, patients with CLL do not routinely receive primary antifungal prophylaxis nor do they undergo surveillance (e.g. periodic fungal biomarkers or imaging) for early diagnosis of IFI.
Only patients with proven/probable IFI diagnosed from start date of ibrutinib to 30 days after last dose of ibrutinib were included for analysis. IFIs were identified by electronic health record review. Patients in the control group had a CLL diagnosis and received treatment with ibrutinib but did not meet criteria for proven/probable IFI and had no evidence of other severe infections. Control patients were matched to IFI cases based on date of first ibrutinib dose (+/− 30 days) in a 4:1 ratio and selected for analyses based on unique institutional medical record identifier. Each control patient was only selected once. Established risk factors for infection and pertinent clinical, laboratory and radiological findings of IFI were captured for comparative analysis.
Patient demographics, CLL treatment history, causative fungal organisms, and IFI sites were collected. Potential risk factors including, diabetes, heavily pretreated CLL (≥3 prior lines of CLL therapy), cytotoxic chemotherapy for concomitant malignancy, and prior treatment with alemtuzumab or fludarabine were collected at any time during ibrutinib treatment for IFI cases and controls. Laboratory data including, hypoalbuminemia (< 3 g/dL), hypogammaglobulinemia (IgG < 500 mg/dL), neutropenia (< 500 cells/mm3), lymphopenia (< 1000 cells/mm3), and monocytopenia (< 80 cells/mm3) was included if within 30 days of IFI diagnosis for IFI cases, and at any time during ibrutinib treatment for controls. Glucocorticoid use (≥ 20 mg prednisone equivalents daily for ≥ 14 days) was collected if within 90 days of IFI diagnosis for IFI cases, and at any time during ibrutinib treatment for controls.
IFI incidence was determined by the proportion of proven/probable IFI divided by the total number of included patients. To determine risk factors, conditional logistic regression was used to compare patient-specific factors of IFI cases to controls. Since IFI incidence was rare, multivariable analyses were not performed. Results are reported using odds ratios with 95% confidence intervals (CI). Statistical analyses were preformed using Stata software (Stata version 15.0, StataCorp LP, College Station, TX). This study was approved by the institutional review board with a waiver of informed consent.
Of the 841 patients with CLL included, we identified 21 cases of proven/probable IFI and an incidence of 2.5%. Of the 21 IFIs, the median age at diagnosis was 65 years with 81% being men. Eight (38%) patients received ibrutinib as frontline therapy, while six (29%) patients received ≥3 prior lines of CLL therapy. The median duration of ibrutinib treatment before IFI diagnosis was 4 months (0.5-52.2 months) (suppl table).
The most common organism identified was Aspergillus spp. (n=13) including six cases of Aspergillus fumigatus (Table 1). The remaining cases included Cryptococcus spp. (n=5), Candida spp. (n=2), Fusarium spp. (n=1), Pneumocystis jiroveci (n=1), and Histoplasma spp. (n=1). IFIs were mostly pneumonia (n=20); remaining cases included fungemia (n=3), skin/skin structure (n=1), CNS involvement (n=2), and sinus infections (n=1). Three patients had co-infections with multiple fungal organisms, including one patient with Aspergillus fumigatus and Candida albicans, one patient with Aspergillus terreus and Cryptococcus neoformans, and one patient with Aspergillus spp. and Cryptococcus spp. Four patients were diagnosed with disseminated IFI (Table 1). Ten patients had no classic risk factors for IFI, which included neutropenia within 30 days of infection, glucocorticoid use within 90 days of infection, or heavy pretreatment.
Table 1.
Description of Invasive Fungal Infections (IFI)
| IFI, n (%)α | |
| - Aspergillus spp. | 13 (62) |
| - Candida spp. | 2 (10) |
| - Cryptococcus spp. | 5 (24) |
| - Fusarium spp. | 1 (5) |
| - Histoplasma spp. | 1 (5) |
| - Pneumocystis jiroveci | 1 (5) |
| Site of infection, n (%) | |
| - Blood | 3 (14) |
| - CNS | 2 (10) |
| - Lung | 20 (95) |
| - Sinus | 1 (5) |
| - Skin/skin structure | 1 (5) |
| - Multiple sites of infectionß | 4 (19) |
One patient identified through indirect testing methods (e.g. Beta-D-glucan)
These include: 58-year-old male with Candida albicans in ascitic fluid, blood, and lungs, along with Aspergillus fumigatus in the ascitic fluid who deceased 13 days after IFI diagnosis; 65-year-old male with disseminated Cryptococcus neoformans in cerebrospinal fluid and lungs who resumed ibrutinib two months after IFI diagnosis; 59-year-old male with Cryptococcus spp in his lungs and blood who resumed ibrutinib two weeks after IFI diagnosis; 65-year-old male with Aspergillus spp pneumonia and a CT head concerning for CNS involvement who deceased one day after IFI diagnosis
Importantly, 14 patients continued ibrutinib after IFI diagnosis, with six patients continuing ibrutinib for at least one year, suggesting continuation of ibrutinib after IFI diagnosis is potentially safe. Most patients continued ibrutinib at a decreased dose to account for drug interactions with antifungal agents. At date of last follow-up (≥6 months after IFI for each patient), 10 patients had deceased (48%). Of those patients, 5 deaths were deemed to be IFI-related (24%), 4 of which occurred within 14-days of IFI diagnosis (Figure 1).
Figure 1. Overall survival of CLL patients with IFI on ibrutinib.
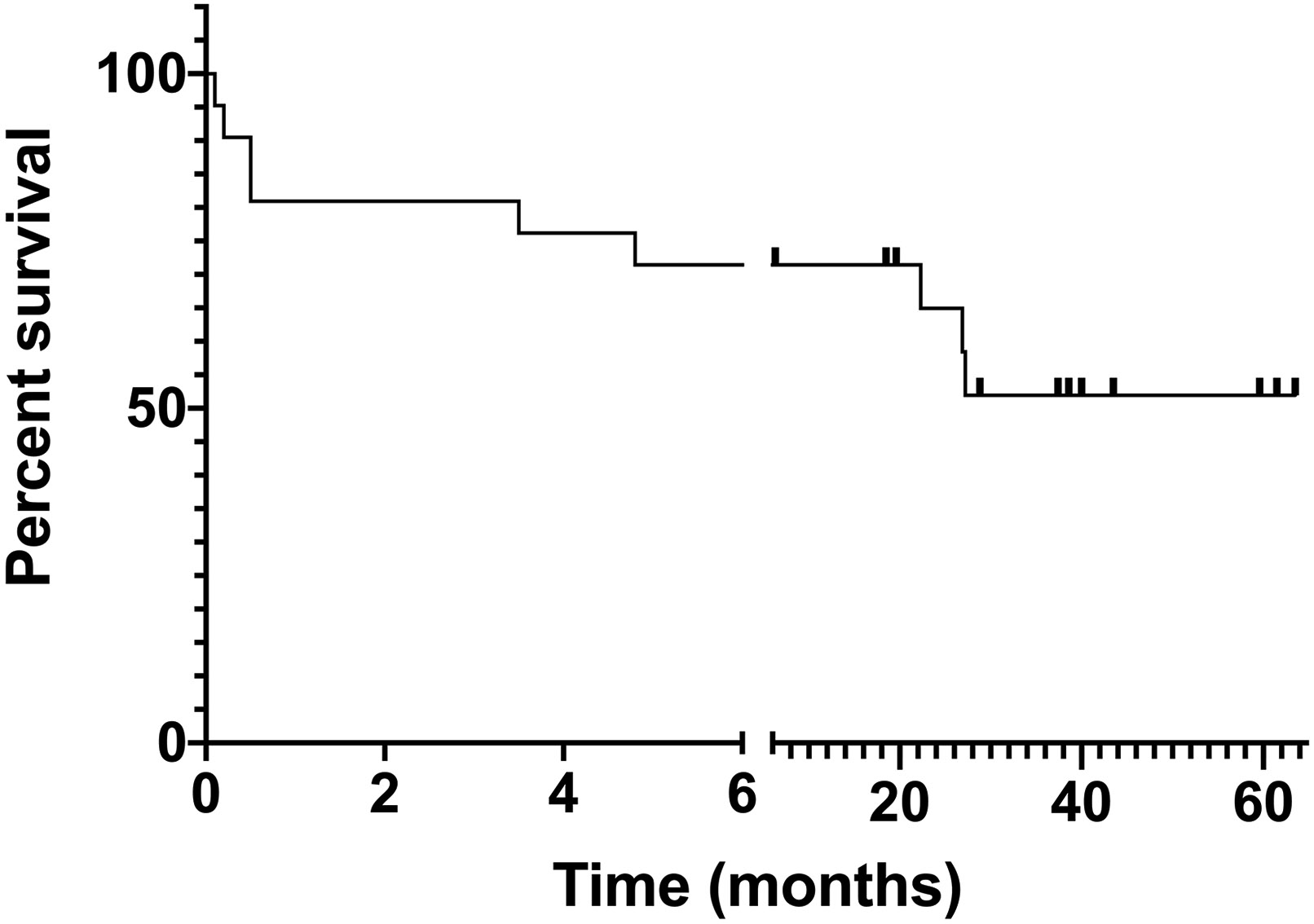
10 of 21 patients were deceased at date of last follow-up (≥ 6 months for each patient; average 36.79 months of follow up). The first 5 deaths (4 in the first month and 1 in the fourth month) were IFI-related, while the other 5 deaths were due to disease progression or other causes.
Twenty IFI cases had data available to conduct a risk factor analysis (Table 2). Prior treatment with ≥3 lines of CLL therapy (OR=5.36, 95% CI 1.25-23.03) and monocytopenia (OR=6.00, 95% CI 1.41-25.45) were significantly associated with IFI in our cohort. While hypoalbuminemia (OR=17.70, 95% CI 3.84-81.46) was also associated with IFI, it was more likely a marker of active infection than a true risk factor for IFI. Other potential risk factors including, systemic glucocorticoid use, neutropenia, and hypogammaglobulinemia were not associated with increased IFI risk.
Table 2.
Analysis of Risk Factors Comparing Patients with IFI to patients without IFI
| Patient Characteristic—n (%) | Proven/Probable IFIΘ |
No IFI N = 80 |
Odds Ratio (95% CI) |
|---|---|---|---|
| ≥ 3 prior lines of therapy | 5 (25) α | 5 (6) | 5.36 (1.25-23.03) |
| Prior alemtuzumab | 1 (5)α | 4 (5) | 1.00 (0.11-8.95) |
| Prior fludarabine | 8 (40) α | 24 (30) | 1.63 (0.56-4.76) |
| Diabetes | 4 (20) α | 11 (14) | 1.50 (0.45-4.96) |
| Glucocorticoid useμ | 2 (10) α | 2 (3) | 4.00 (0.56-28.40) |
| Prior cytotoxic chemotherapyτ | 1 (5) α | 2 (3) | 2.00 (0.18-22.06) |
| Combination therapy for CLL | 9 (45) α | 38 (48) | 0.97 (0.81-1.16) |
| Hypoalbuminemia | 10 (53) δ π | 4 (5) ε | 17.70 (3.84-81.46) |
| Neutropenia | 3 (16)δπ | 10 (13)ε | 1.26 (0.29-5.39) |
| Lymphopenia | 6 (32)δπ | 29 (36)ε | 0.79 (0.27-2.32) |
| Monocytopenia | 5 (26) δ π | 4 (5) ε | 6.00 (1.41-25.45) |
| Hypogammaglobulinemia | 8 (47)Γ | 37 (46)ε | 1.00 (0.35-2.88) |
Hypoalbuminemia = albumin < 3 g/dL; Neutropenia = < 500 cells/m3; Lymphopenia = < 1000 cells/m3; Monocytopenia = < 80 cells/m3
Patients without adequate laboratory values or follow up data within 30 days of IFI diagnosis were not included in the analysis
Defined as use of ≥20 mg/day for ≥ 14 days within 3 months prior to IFI diagnosis
For treatment of prior malignancy other than CLL
N = 20
N = 19
N = 17
Within 30 days of IFI diagnosis
at any time while on ibrutinib therapy
In the largest real-life cohort of CLL patients treated with ibrutinib to date, we report a 2.5% incidence of IFI, which is comparable to the incidence reported in patients with relapsed or refractory CLL treated with ibrutinib (0.5-12%) in recent retrospective studies (2, 5, 8, 9). Half of our analyzable patients (n=10) had no “classic” risk factor such as neutropenia, use of corticosteroids, or heavy pretreatment (≥3 lines of prior therapy). The increased incidence of severe opportunistic infections, not only IFI, in heavily pretreated CLL has been long described (10). Because of the small number of patients in observational studies and low incidence of IFI in CLL, it is difficult to determine whether ibrutinib itself led to excess IFI cases in this population. The association of IFI in heavily pretreated CLL patients on ibrutinib, was seen in some (5, 9) but not all studies (8).
A spectrum of opportunistic fungi, mainly molds, were encountered in our cohort (Table 1) and pneumonia was the predominant clinical manifestation, consistent with prior reports. Of note, a sizeable subset of our patients had polyfungal or disseminated infections, emphasizing the importance of low threshold for suspicion of widely disseminated or uncommon IFI. On the other hand, CNS involvement (3 patients with neuroimaging and 2 patients with evidence of infection) was uncommon in our patients.
Ibrutinib interacts with the immune system in several pathways and can modulate response to infections. BTK is part of a family of kinases known as Tec kinases (11). BTK and Tec are expressed on monocytes and neutrophils and play a role in control of immune functions such as chemotaxis, phagocytosis, and B-cell proliferation (11). Inhibiting BTK also prevents Toll-like receptor 9 activation of calcineurin mediated NFAT activation and reducing TNFα production (12). Relating to fungal infections, BTK inhibition has been shown to negatively affect the phagocytosis of C. albicans and inhibit macrophage responses to A. fumigatus (6, 13). It is possible that ibrutinib’s immunosuppressive effects were additive to monocytopenia, a described risk factor for IFI (14).
There are multiple limitations to our study. Several clinically significant IFIs are missed due to limitations in diagnostic methods (15), combined with our study being retrospective and relied on electronic health record documentation. These factors potentially resulted in underrepresenting the true incidence of IFI. Since this was a single center study, we are unable to capture all patients diagnosed or treated at outside hospitals. Our study focused on patients with CLL and may not be applicable to patients with other lymphoid malignancies treated with ibrutinib. The small number of IFI cases limits the ability to fully assess risk factors and the magnitude of their effect.
In conclusion, our findings support active surveillance of IFI in CLL patients on ibrutinib treatment with ≥3 prior lines of therapy or monocytopenia. Additional studies are required to confirm risk factors for IFI and develop recommendations for appropriate antifungal prophylaxis in this patient population.
Supplementary Material
Footnotes
Disclosures: NJ: Research funding to institution from Pharmacyclics, AbbVie, Genentech, AstraZeneca, BMS, Pfizer, ADC Therapeutics, Incyte, Servier, Cellectis, Verastem, Adaptive Biotechnologies, and Precision Biosciences for clinical studies in which NJ is a principal investigator, advisory board member and honoraria from Pharmacyclics, Janssen, AbbVie, Genentech, AstraZeneca, Verastem, Adaptive Biotechnologies, Servier, and Precision Biosciences; PT: Research funding to institution from Pharmacyclics, AbbVie, Genentech and AstraZeneca, advisory board member and honoraria from Pharmacyclics, AbbVie, Gilead, Genentech, speaking fees from Janssen; MF, SLA, WW, DPK, AJD: no relevant disclosures.
References
- 1.Jain N, O'Brien S. The Shifting Paradigm in Chronic Lymphocytic Leukemia: Is Chemotherapy Still Relevant? Cancer journal (Sudbury, Mass). 2019;25(6):374–7. [DOI] [PubMed] [Google Scholar]
- 2.Chamilos G, Lionakis MS, Kontoyiannis DP. Call for Action: Invasive Fungal Infections Associated With Ibrutinib and Other Small Molecule Kinase Inhibitors Targeting Immune Signaling Pathways. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018;66(1):140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain P, Keating M, Wierda W, Estrov Z, Ferrajoli A, Jain N, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn IE, Jerussi T, Farooqui M, Tian X, Wiestner A, Gea-Banacloche J. Atypical Pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib. Blood. 2016;128(15):1940–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varughese T, Taur Y, Cohen N, Palomba ML, Seo SK, Hohl TM, et al. Serious Infections in Patients Receiving Ibrutinib for Treatment of Lymphoid Cancer. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018;67(5):687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bercusson A, Colley T, Shah A, Warris A, Armstrong-James D. Ibrutinib blocks Btk-dependent NF-kB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis. Blood. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teh BW, Chui W, Handunnetti S, Tam C, Worth LJ, Thursky KA, et al. High rates of proven invasive fungal disease with the use of ibrutinib monotherapy for relapsed or refractory chronic lymphocytic leukemia. Leukemia & lymphoma. 2019;60(6):1572–5. [DOI] [PubMed] [Google Scholar]
- 9.Rogers KA, Mousa L, Zhao Q, Bhat SA, Byrd JC, El Boghdadly Z, et al. Incidence of opportunistic infections during ibrutinib treatment for B-cell malignancies. Leukemia. 2019;33(10):2527–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsiodras S, Samonis G, Keating MJ, Kontoyiannis DP. Infection and immunity in chronic lymphocytic leukemia. Mayo Clinic proceedings. 2000;75(10):1039–54. [DOI] [PubMed] [Google Scholar]
- 11.Jongstra-Bilen J, Puig Cano A, Hasija M, Xiao H, Smith CI, Cybulsky MI. Dual functions of Bruton's tyrosine kinase and Tec kinase during Fcgamma receptor-induced signaling and phagocytosis. Journal of immunology (Baltimore, Md : 1950). 2008;181(1):288–98. [DOI] [PubMed] [Google Scholar]
- 12.Herbst S, Shah A, Mazon Moya M, Marzola V, Jensen B, Reed A, et al. Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus. EMBO molecular medicine. 2015;7(3):240–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strijbis K, Tafesse FG, Fairn GD, Witte MD, Dougan SK, Watson N, et al. Bruton's Tyrosine Kinase (BTK) and Vav1 contribute to Dectin1-dependent phagocytosis of Candida albicans in macrophages. PLoS pathogens. 2013;9(6):e1003446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;47(4):503–9. [DOI] [PubMed] [Google Scholar]
- 15.Multani A, Allard LS, Wangjam T, Sica RA, Epstein DJ, Rezvani AR, et al. Missed diagnosis and misdiagnosis of infectious diseases in hematopoietic cell transplant recipients: an autopsy study. Blood advances. 2019;3(22):3602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


