Abstract
One well-characterized in vitro function of Nef is its ability to remove CD4, the human immunodeficiency virus (HIV) receptor, from the cell surface. Nef accomplishes this by accelerating the internalization and degradation of CD4. Current models propose that Nef promotes CD4 internalization via an increased association of CD4 with clathrin-coated pits (CCP). Here, we investigated the effect of a naturally occurring antiprotozoan antibiotic, ikarugamycin (IKA), on CD4 cell surface expression in human monocytic cells stably expressing HIV type 1 SF2 Nef. IKA was able to efficiently restore CD4 cell surface expression in Nef-expressing cells without affecting either CD4 synthesis or Nef expression. In addition, we demonstrate that IKA is also capable of efficiently blocking CD4 down-modulation in response to phorbol myristate acetate. Our data suggest that IKA may be an efficient and useful inhibitor of CCP-dependent endocytosis.
The nef genes of human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) encode a 27- to 34-kDa myristoylated phosphoprotein (4, 7, 10, 18, 30). For SIV, it has been demonstrated that Nef plays a critical role in the maintenance of high virus load and subsequent development of AIDS in adult macaques (25). The significance of Nef in virus replication and pathogenesis has also been suggested by studies using an SCID-hu mouse model of HIV infection (3, 23). In addition, several long-term nonprogressors have been documented to be infected with nef-defective viruses (12). All of these in vivo findings suggest an important role for Nef in HIV replication and the development of AIDS.
In vitro studies have demonstrated that Nef has multiple functions. These functions include down-modulation of CD4 and major histocompatibility complex class I surface expression, enhancement of viral infectivity, and association with cellular kinases (9, 16, 34, 35, 40, 42, 44, 46, 47). The various functions of Nef appear to be isolate dependent (31), which is not surprising considering that Nef is second only to the envelope protein in its variability. Despite the fact that Nef is a highly variable protein, the ability of Nef to down-modulate CD4 surface expression appears to be highly conserved (5, 8, 33). This suggests that down-modulation of CD4 by Nef may be important to the viability of the virus in vivo. Nef reduces CD4 cell surface expression by dramatically decreasing its half-life. The first step in Nef-induced down-modulation of CD4 has been postulated to be an increase in the rate of formation of clathrin-coated pits (CCPs), which predominantly contain CD4 (14). However, Nef plays a role not only in the preferential internalization of CD4 but also in the transport of CD4 through the endocytic pathway to the lysosome, where it is degraded (2, 5, 6, 8, 13, 16, 26, 38–40, 43).
It has been proposed that Nef accomplishes CD4 down-modulation by acting as a connector between CD4 and host cell proteins that are part of the normal host cell endocytic machinery (17, 28, 38, 39). Based on this model of Nef-mediated CD4 down-modulation, we would expect that an inhibitor of CCP-mediated endocytosis would block an early step in the process of Nef-mediated CD4 downregulation and that this would result in the recovery of CD4 expression on the surfaces of Nef-expressing cells. The antibiotic ikarugamycin (IKA), isolated from cultures of Streptomyces phaeochromogenes sub- sp. ikaruganensis (24), has been previously shown to inhibit the uptake of oxidized low-density lipoprotein (LDL) in a mouse macrophage cell line (19). However, other events in the metabolic pathway of oxidized LDL, such as cell surface binding, transport from endosomes to lysosomes, and lysosomal hydrolysis of oxidized LDL, were not affected by the antibiotic. In this study, we examined the ability of IKA to block Nef-mediated CD4 cell surface downregulation.
PMA-induced downregulation of cell surface CD4 is obstructed by IKA.
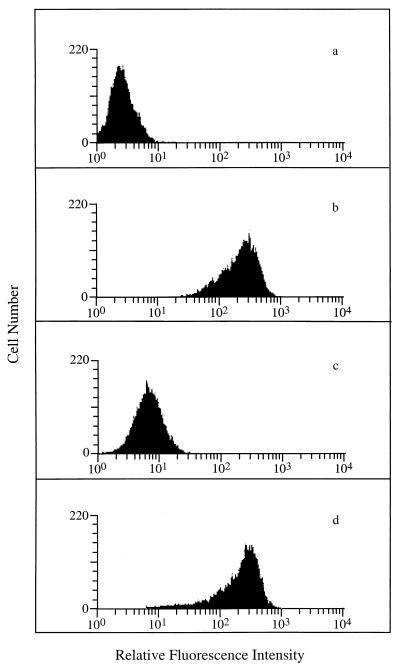
In order to determine whether IKA's effect on endocytosis extended to human monocytic cells, we examined the effect of IKA on the cell surface expression of CD4. CD4 enters the cell through CCP-mediated endocytosis. Under normal tissue culture conditions the rate of internalization of CD4 is relatively slow; however, it has been previously shown that treating cells with phorbol myristate acetate (PMA) induces rapid internalization of CD4 via CCPs (1, 20, 21, 37). In order to determine whether IKA blocked PMA-induced internalization of CD4, U937/LN (control) monocytic cells expressing Neor (16) were treated with PMA in the presence or absence of IKA (2 μM) for 3 h. As shown in Fig. 1c, PMA effectively reduced the steady-state levels of CD4 on the cell surface. However, in the presence of IKA CD4 cell surface expression was unaffected by PMA (Fig. 1d), demonstrating that IKA efficiently blocked CD4 surface down-modulation by PMA.
FIG. 1.
Inhibition of PMA-induced cell surface CD4 downregulation. U937/LN cells were stained with phycoerythrin (PE)-control antibody (a) or PE-CD4 antibody (b) (both from Exalpha, Boston, Mass.). (c and d) U937/LN cells treated with PMA (50 ng/ml) for 3 h in the absence (c) or presence (d) of IKA (2 μM) prior to staining with PE-CD4 antibody. Samples were analyzed on a Becton Dickinson FACScan instrument equipped with LYSYS II software. All fluorescence data were collected in the log mode.
IKA inhibits Nef induced down-modulation of CD4.
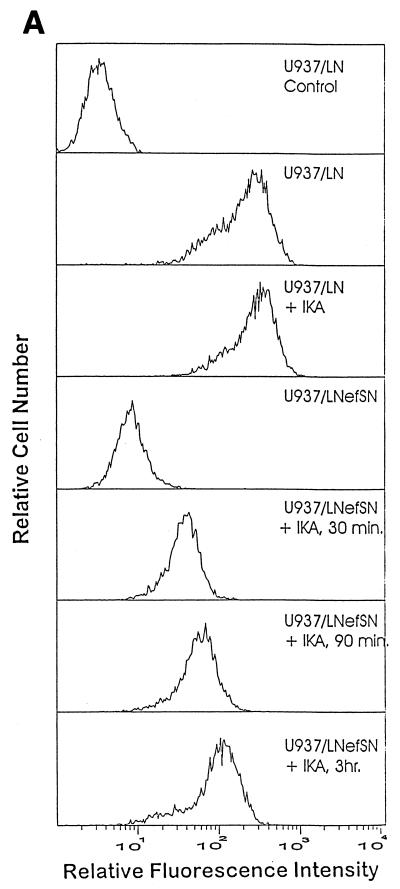
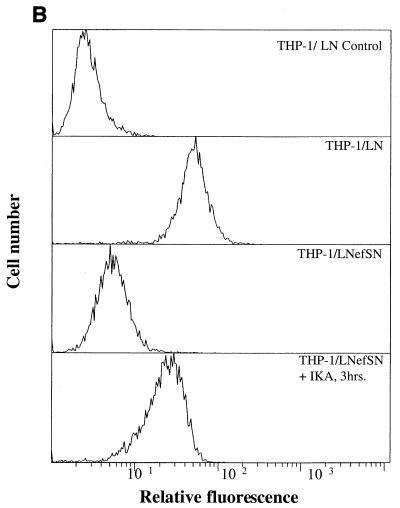
The Nef protein of HIV has also been shown to efficiently down-modulate CD4 cell surface expression; however, the exact mechanism by which Nef down-modulates CD4 has not been fully defined. It has been suggested that Nef induces CD4 cell surface downregulation by enhancing the association of CD4 with CCPs (2, 5, 6, 8, 13, 16, 40, 43). Therefore, we hypothesize that an inhibitor of endocytosis, such as IKA, should block the cell surface CD4 downregulation induced by Nef. To examine the effect of IKA on Nef-induced CD4 cell surface downregulation, U937/LN (control) and U937/LNefSN (Nef-expressing) cells (16) were incubated with or without IKA (2 μM) at 37°C and analyzed for CD4 cell surface expression. In the presence of IKA the surface expression of CD4 increased in a time-dependent manner despite the presence of Nef. After a 30-min incubation with IKA, CD4 levels in U937/LNefSN cells increased fivefold (Fig. 2A). We observed a 13-fold increase in CD4 cell surface levels after cells were incubated for 3 h with IKA (Fig. 2A). Similar results were obtained using a second monocytic cell line (THP-1/LNefSN) stably expressing Nef. Cell surface CD4 levels increased sixfold after treatment with IKA (Fig. 2B). These data show that IKA can efficiently block Nef-induced cell surface down-modulation of CD4. It should be noted that cell viability was not significantly affected after 3 h of incubation with 2 μM IKA as determined by trypan blue exclusion (data not shown).
FIG. 2.
IKA blocks Nef-induced cell surface CD4 downregulation. (A) Flow cytometry analysis of CD4 cell surface expression on U937 cells. U937/LN (control) cells incubated with or without IKA were stained with phycoerythrin (PE)-control antibody or PE-CD4 antibody. U937/LNefSN cells were not treated or were treated with 2 μM IKA for 30 or 90 min or 3 h prior to being stained with PE-CD4 antibody. (B) Flow cytometry analysis of CD4 cell surface expression on THP-1 cells. THP-1/LN (control) cells were stained with PE-control antibody or PE-CD4 antibody. THP-1/LNefSN cells were incubated with or without IKA for 3 h and then stained with PE-CD4 antibody.
IKA increases CD4 half-life in Nef-expressing cells.
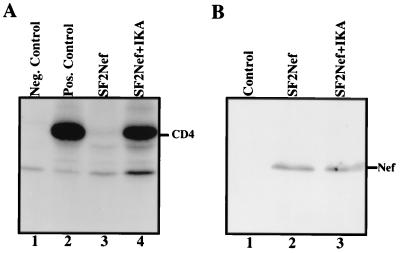
It has been previously shown that the half-life of CD4 is considerably shorter in Nef-expressing cells than in control cells (40). In order to determine whether IKA increased the half-life of CD4, despite the presence of Nef, U937/LNefSN cells were radiolabeled with [35S]methionine and [35S]cysteine and chased in the presence or absence of IKA. Radiolabeled proteins were immunoprecipitated with either a rabbit anti-CD4 antiserum or a normal rabbit serum. In the absence of IKA CD4 was quickly degraded and barely detectable after a 3-h chase (Fig. 3A, lane 3). In contrast, the presence of IKA significantly increased the stability of CD4 (Fig. 3A, compare lanes 3 and 4). Following a 3-h chase, the level of CD4 in Nef-expressing cells in the presence of IKA was approximately 60% of that expressed in U937/LN (control) cells (Fig. 3A, lane 2). These results show that IKA inhibits Nef-induced degradation of CD4 and reinforces the notion that accelerated CD4 endocytosis by Nef is the major event accounting for the down-modulation of CD4 in Nef-expressing cells. To rule out the possibility that IKA affects Nef's stability or otherwise reduces the level of Nef expression, equal amounts of total cellular proteins from U937/LNefSN cells incubated with or without IKA for 3 h were subjected to Western blot analysis for Nef expression. The presence of IKA did not alter the level of Nef protein compared to that in control cells (Fig. 3B). These data confirm that the accumulation of CD4 on the surfaces of cells treated with IKA is not simply due to a lack of Nef expression.
FIG. 3.
IKA increases CD4 half-life in Nef-expressing cells. (A) Analysis of CD4 stability. Control U937/LN cells or U937/LNefSN (Nef-expressing) cells were pulse-labeled for 45 min with [35S]methionine and [35S]cysteine and chased for 3 h in the absence or presence of IKA. Cell lysates were immunoprecipitated with a normal rabbit serum or a rabbit anti-CD4 antiserum. Lane 1, U937/LN cell lysates immunoprecipitated with normal rabbit serum; lane 2, U937/LN cell lysates (without chase) immunoprecipitated with anti-CD4 antiserum; lane 3, U937/LNefSN cell lysates (with chase) immunoprecipitated with anti-CD4 antiserum; lane 4, U937/LNefSN cell lysates (with chase in the presence of IKA) immunoprecipitated with anti-CD4 antiserum. (B) Western blot analysis of Nef expression. Lane 1, U937/LN cells; lane 2, U937/LNefSN cells; lane 3, U937/LNefSN cells incubated with IKA for 3 h.
It is not unusual for a virus to remove its receptor from the cell surface (36). However, HIV has evolved redundant strategies for the elimination of its receptor, CD4, from the cell surface. The envelope protein of HIV has been shown to block transport of CD4 to the cell surface by forming a complex with CD4 that is retained in the endoplasmic reticulum (ER) (11, 22). In addition, Vpu regulates the degradation of newly synthesized CD4 by facilitating transport of CD4 out of the ER and to the proteasome, where it is subsequently degraded (15, 45, 48). Finally, Nef, which is expressed at high levels early after infection, induces rapid internalization of CD4 from the cell surface and subsequent degradation of CD4 in the lysosome. The fact that HIV devotes so much energy to the removal of CD4 from the infected cells suggests that removal of CD4 is important for the overall fitness of the virus. However, the biological relevance of CD4 downregulation in viral pathogenesis remains to be determined.
In recent years there has been much progress in understanding the mechanism by which Nef triggers CD4 internalization. Current models suggest that Nef can act as a specific connector molecule between CD4 and components of CCPs, thereby accelerating the formation CCPs containing CD4. Subsequently CD4 internalization in the presence of Nef is greatly increased (14, 32). CD4 then follows the endocytic pathway to lysosomes, where it is degraded (29). In order to gain a better understanding of how Nef interacts with the endocytic pathway, we began testing numerous agents known to block various stages of the endocytic pathway. Many of these agents inhibited degradation of CD4 in Nef-expressing cells, but this did not result in the recovery of CD4 on the cell surface (29). However, we found that one of the agents we examined, IKA, a macrolide antibiotic, not only increased CD4 half-life in the presence of Nef but also caused a significant recovery of cell surface expression of CD4. The recovery of CD4 was not due to a decrease in Nef levels but rather to an alteration of the transport of CD4 through the endocytic pathway.
There are several theories as to the role that Nef's down-modulation of CD4 may play in replication and fitness of HIV. One study suggested that downregulation of cell surface CD4 by Nef prevents viral superinfection, thus giving rise to a persistent viral infection (8). It has also been suggested that Nef's removal of CD4 from the cell surface results in an increase in the release of budding viral particles from the cell surface and a concomitant increase in virus spread (41). Contrary to the latter hypothesis, Lama et al. (27) have suggested that high levels of CD4 do not block viral budding but rather prevent incorporation of the envelope protein into the newly formed particles and thereby render the particles noninfectious. In either case Nef would aid in the formation of infectious particles by reducing CD4 surface expression. The fact that IKA is capable of restoring CD4 expression in Nef-expressing cells raises the possibility that IKA may have some therapeutic value. Restored levels of CD4 on the surfaces of infected cells may inhibit the formation of infectious HIV particles and thus decrease the rate of spread of HIV in an infected individual. In addition, restoration of cell surface CD4 expression may also restore some normal functions lost as a result of a lack of CD4 expression in infected T cells. However, the current lack of specificity of this drug limits this approach. The fact that IKA blocks PMA-induced CD4 down-modulation and inhibits the uptake of oxidized LDL (19) suggests that IKA may be a general inhibitor of CCP-mediated endocytosis. Our results suggest that IKA is a useful agent for studying the fundamental process of endocytosis, as well as being useful for studying the effects of Nef on cell surface expression of CD4.
Acknowledgments
Tianci Luo and Brenda L. Fredericksen contributed equally to this work.
We thank R. Munford for critical comments on the manuscript and R. Koup, R. Gaynor, and D. Foster for continued support of this work.
This research was supported by National Institutes of Health grant AI33331.
REFERENCES
- 1.Acres R B, Conlon P J, Mochizuki D Y, Gallis B. Rapid phosphorylation and modulation of the T4 antigen on cloned helper T cells induced by phorbol myristate acetate or antigen. J Biol Chem. 1986;261:16210–16224. [PubMed] [Google Scholar]
- 2.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 3.Aldrovandi G M, Zack J A. Replication and pathogenicity of human immunodeficiency virus type 1 accessory gene mutants in SCID-hu mice. J Virol. 1996;70:1505–1511. doi: 10.1128/jvi.70.3.1505-1511.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allan J S, Coligan J E, Lee T H, McLane M F, Kanki P J, Groopman J E, Essex M. A new HTLV-III/LAV encoded antigen detected by antibodies from AIDS patients. Science. 1985;230:810–813. doi: 10.1126/science.2997921. [DOI] [PubMed] [Google Scholar]
- 5.Anderson S, Shugars D C, Swanstrom R, Garcia J V. Nef from primary isolates of human immunodeficiency virus type 1 suppresses surface CD4 expression in human and mouse T cells. J Virol. 1993;67:4923–4931. doi: 10.1128/jvi.67.8.4923-4931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson S J, Lenburg M, Landau N R, Garcia J V. The cytoplasmic domain of CD4 is sufficient for its down-regulation from the cell surface by human immunodeficiency virus type 1 Nef. J Virol. 1994;68:3092–3101. doi: 10.1128/jvi.68.5.3092-3101.1994. . (Erratum, 68:4705.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandres J C, Luria S, Ratner L. Regulation of human immunodeficiency virus Nef protein by phosphorylation. Virology. 1994;201:157–161. doi: 10.1006/viro.1994.1278. [DOI] [PubMed] [Google Scholar]
- 8.Benson R E, Sanfridson A, Ottinger J S, Doyle C, Cullen B R. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus Nef prevents viral super infection. J Exp Med. 1993;177:1561–1566. doi: 10.1084/jem.177.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowers M Y, Spina C A, Kwoh T J, Fitch N J, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombini S, Arya S K, Reitz M S, Jagodzinski L, Beaver B, Wong-Staal F. Structure of simian immunodeficiency virus regulatory genes. Proc Natl Acad Sci USA. 1989;86:4813–4817. doi: 10.1073/pnas.86.13.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crise B, Buonocore L, Rose J K. CD4 is retained in the endoplasmic reticulum by the human immunodeficiency virus type 1 glycoprotein precursor. J Virol. 1990;64:5585–5593. doi: 10.1128/jvi.64.11.5585-5593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 13.Foster J L, Anderson S J, Frazier A L, Garcia J V. Specific suppression of human CD4 surface expression by Nef from the pathogenic simian immunodeficiency virus SIVmac239open. Virology. 1994;201:373–379. doi: 10.1006/viro.1994.1303. [DOI] [PubMed] [Google Scholar]
- 14.Foti M, Mangasarian A, Piguet V, Lew D P, Krause K H, Trono D, Carpentier J L. Nef-mediated clathrin-coated pit formation. J Cell Biol. 1997;139:37–47. doi: 10.1083/jcb.139.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita K, Omura S, Silver J. Rapid degradation of CD4 in cells expressing human immunodeficiency virus type 1 Env and Vpu is blocked by proteasome inhibitors. J Gen Virol. 1997;78:619–625. doi: 10.1099/0022-1317-78-3-619. . (Erratum, 78:2129–2130.) [DOI] [PubMed] [Google Scholar]
- 16.Garcia J V, Miller A D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nat Cell Biol. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg M E, Bronson S, Lock M, Neumann M, Pavlakis G N, Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guy B, Kieny M P, Riviere Y, Le Peuch C, Dott K, Girard M, Montagnier L, Lecocq J P. HIV F/3′ orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature. 1987;330:266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- 19.Hasumi K, Shinohara C, Naganuma S, Endo A. Inhibition of the uptake of oxidized low-density lipoprotein in macrophage J774 by the antibiotic ikarugamycin. Eur J Biochem. 1992;205:841–846. doi: 10.1111/j.1432-1033.1992.tb16848.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoxie J A, Matthews D M, Callahan K J, Cassel D L, Cooper R A. Transient modulation and internalization of T4 antigen induced by phorbol esters. J Immunol. 1986;137:1194–1201. [PubMed] [Google Scholar]
- 21.Hurley T R, Luo K, Sefton B M. Activators of protein kinase C induce dissociation of CD4, but not CD8, from p561ck. Science. 1989;245:407–409. doi: 10.1126/science.2787934. [DOI] [PubMed] [Google Scholar]
- 22.Jabbar M A, Nayak D P. Intracellular interaction of human immunodeficiency virus type 1 (ARV-2) envelope glycoprotein gp160 with CD4 blocks the movement and maturation of CD4 to the plasma membrane. J Virol. 1990;64:6297–6304. doi: 10.1128/jvi.64.12.6297-6304.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamieson B D, Aldrovandi G M, Planelles V, Jowett J B, Gao L, Bloch L M, Chen I S, Zack J A. Requirement of human immunodeficiency virus type 1 nef for in vivo replication and pathogenicity. J Virol. 1994;68:3478–3485. doi: 10.1128/jvi.68.6.3478-3485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jomon K, Kuroda Y, Ajisaka M, Sakai H. A new antibiotic, ikarugamycin. J Antibiot (Tokyo) 1972;25:271–280. doi: 10.7164/antibiotics.25.271. [DOI] [PubMed] [Google Scholar]
- 25.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y H, Chang S H, Kwon J H, Rhee S S. HIV-1 Nef plays an essential role in two independent processes in CD4 down-regulation: dissociation of the CD4–p56(lck) complex and targeting of CD4 to lysosomes. Virology. 1999;257:208–219. doi: 10.1006/viro.1999.9642. [DOI] [PubMed] [Google Scholar]
- 27.Lama J, Mangasarian A, Trono D. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr Biol. 1999;9:622–631. doi: 10.1016/s0960-9822(99)80284-x. [DOI] [PubMed] [Google Scholar]
- 28.Lu X, Yu H, Liu S H, Brodsky F M, Peterlin B M. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- 29.Luo T, Anderson S J, Garcia J V. Inhibition of Nef- and phorbol ester-induced CD4 degradation by macrolide antibiotics. J Virol. 1996;70:1527–1534. doi: 10.1128/jvi.70.3.1527-1534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo T, Downing J R, Garcia J V. Induction of phosphorylation of human immunodeficiency virus type 1 Nef and enhancement of CD4 downregulation by phorbol myristate acetate. J Virol. 1997;71:2535–2539. doi: 10.1128/jvi.71.3.2535-2539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo T, Garcia J V. The association of Nef with a cellular serine/threonine kinase and its enhancement of infectivity are viral isolate dependent. J Virol. 1996;70:6493–6496. doi: 10.1128/jvi.70.9.6493-6496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangasarian A, Foti M, Aiken C, Chin D, Carpentier J L, Trono D. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity. 1997;6:67–77. doi: 10.1016/s1074-7613(00)80243-5. [DOI] [PubMed] [Google Scholar]
- 33.Mariani R, Skowronski J. CD4 down-regulation by nef alleles isolated from human immunodeficiency virus type 1-infected individuals. Proc Natl Acad Sci USA. 1993;90:5549–5553. doi: 10.1073/pnas.90.12.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller M D, Feinberg M B, Greene W C. The HIV-1 nef gene acts as a positive viral infectivity factor. Trends Microbiol. 1994;2:294–298. doi: 10.1016/0966-842x(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 35.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palese P, Tobita K, Ueda M, Compans R W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974;61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 37.Pelchen-Matthews A, Parsons I J, Marsh M. Phorbol ester-induced downregulation of CD4 is a multistep process involving dissociation from p561ck, increased association with clathrin-coated pits, and altered endosomal sorting. J Exp Med. 1993;178:1209–1222. doi: 10.1084/jem.178.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piguet V, Chen Y L, Mangasarian A, Foti M, Carpentier J L, Trono D. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 1998;17:2472–2481. doi: 10.1093/emboj/17.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piguet V, Gu F, Foti M, Demaurex N, Gruenberg J, Carpentier J L, Trono D. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell. 1999;97:63–73. doi: 10.1016/s0092-8674(00)80715-1. [DOI] [PubMed] [Google Scholar]
- 40.Rhee S S, Marsh J W. Human immunodeficiency virus type 1 Nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J Virol. 1994;68:5156–5163. doi: 10.1128/jvi.68.8.5156-5163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross T M, Oran A E, Cullen B R. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr Biol. 1999;9:613–621. doi: 10.1016/s0960-9822(99)80283-8. [DOI] [PubMed] [Google Scholar]
- 42.Saksela K. HIV-1 Nef and host cell protein kinases. Front Biosci. 1997;2:d606–d618. doi: 10.2741/a217. [DOI] [PubMed] [Google Scholar]
- 43.Sanfridson A, Hester S, Doyle C. Nef proteins encoded by human and simian immunodeficiency viruses induce the accumulation of endosomes and lysosomes in human T cells. Proc Natl Acad Sci USA. 1997;94:873–878. doi: 10.1073/pnas.94.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawai E T, Baur A, Struble H, Peterlin B M, Levy J A, Cheng-Mayer C. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc Natl Acad Sci USA. 1994;91:1539–1543. doi: 10.1073/pnas.91.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schubert U, Anton L C, Bacik I, Cox J H, Bour S, Bennink J R, Orlowski M, Strebel K, Yewdell J W. CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway. J Virol. 1998;72:2280–2288. doi: 10.1128/jvi.72.3.2280-2288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 47.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willey R L, Maldarelli F, Martin M A, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol. 1992;66:7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]