ABSTRACT
Background
This paper compares the most recent data on the incidence and prevalence of kidney replacement therapy (KRT), kidney transplantation rates, and mortality on KRT from Europe to those from the United States (US), including comparisons of treatment modalities (haemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation (KTx)).
Methods
Data were derived from the annual reports of the European Renal Association (ERA) Registry and the United States Renal Data System (USRDS). The European data include information from national and regional renal registries providing the ERA Registry with individual patient data. Additional analyses were performed to present results for all participating European countries together.
Results
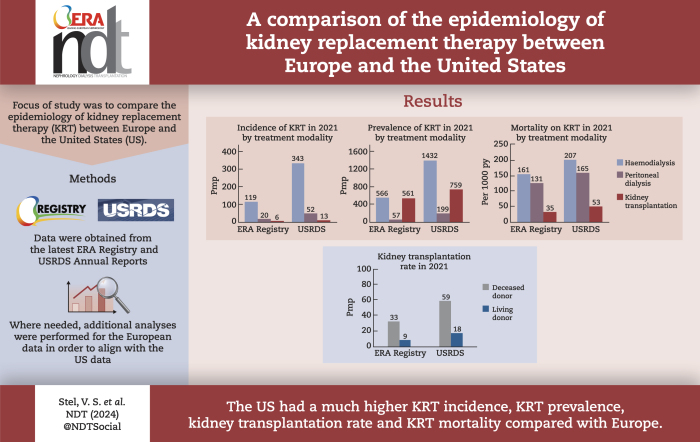
In 2021, the KRT incidence in the US (409.7 per million population (pmp)) was almost 3-fold higher than in Europe (144.4 pmp). Despite the substantial difference in KRT incidence, approximately the same proportion of patients initiated HD (Europe: 82%, US: 84%), PD (14%; 13%, respectively), or underwent pre-emptive KTx (4%; 3%, respectively). The KRT prevalence in the US (2436.1 pmp) was 2-fold higher than in Europe (1187.8 pmp). Within Europe, approximately half of all prevalent patients were living with a functioning graft (47%), while in the US, this was one third (32%). The number of kidney transplantations performed was almost twice as high in the US (77.0 pmp) compared to Europe (41.6 pmp). The mortality of patients receiving KRT was 1.6-fold higher in the US (157.3 per 1000 patient years) compared to Europe (98.7 per 1000 patient years).
Conclusions
The US had a much higher KRT incidence, prevalence, and mortality compared to Europe, and despite a higher kidney transplantation rate, a lower proportion of prevalent patients with a functioning graft.
Keywords: epidemiology, incidence, kidney replacement therapy, mortality, prevalence
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Annual reports from the European Renal Association (ERA) Registry and the Unites States Renal Data System (USRDS) show large international differences in the epidemiology of KRT.
The ERA Registry annual reports mainly focus on differences within Europe, while the USRDS annual reports compare data from the United States (US) with other countries.
This study adds:
This study compares the epidemiology of KRT between Europe and the US using the most recent data from 2021, in which participating European countries are presented aggregately.
In 2021, the US had an almost 3-fold higher KRT incidence, a 2-fold higher KRT prevalence, an almost 2-fold higher kidney transplantation rate, and a 1.6-fold higher KRT mortality compared to Europe.
Despite the much higher kidney transplantation rate in the US, approximately half of all European prevalent patients were living with a functioning graft, while in the US this was one third.
Potential impact:
Renal registries are valuable in providing an annual update on the frequency and outcomes of KRT and may therefore play an important role in reducing inequalities in kidney care.
INTRODUCTION
Both the European Renal Association (ERA) Registry and the Unites States Renal Data System (USRDS) publish annual reports and scientific articles on the epidemiology of kidney replacement therapy (KRT), including the incidence and prevalence of KRT, kidney transplantation rates, and patient and graft survival [1, 2]. To date, the most recently published annual reports from the ERA Registry and USRDS were published in the summer and fall of 2023 and include data from 2021. These reports show large international differences in the epidemiology of KRT, with the ERA Registry mainly focusing on differences within Europe and the USRDS comparing data from the United States (US) to other countries. However, little attention has been paid to specific differences between Europe and the US.
Therefore, in this paper, we compared the most recent data on the incidence and prevalence of KRT, kidney transplantation rates, and mortality on KRT from Europe to those from the US. In addition, we made comparisons with respect to treatment modalities (haemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation (KTx)).
MATERIALS AND METHODS
Study population and data collection
This article is based on data on the epidemiology of KRT derived from the annual report of the ERA Registry [1] and from reference tables in sections D, E, H, and M from the USRDS report [2]. For the European data, we performed additional analyses in order to present the results of the participating European countries as a whole.
The European data include information from national and regional renal registries providing the ERA Registry with individual patient data [1]. These include individual patient data from 35 national or regional registries in the following 18 countries: Austria, Belgium, Bosnia and Herzegovina, Denmark, Estonia, Finland, France, Greece, Iceland, Montenegro, The Netherlands, Norway, Romania, Serbia, Spain, Sweden, Switzerland, and the United Kingdom. Data on paediatric patients were unavailable for the registries of Dutch-speaking and French-speaking Belgium, Montenegro, and several Spanish regions (Cantabria, Castile and Léon, Castile-La Mancha, and Navarre). Participating renal registries provided data on age at KRT initiation, sex, primary renal disease (PRD), changes in KRT treatment modality, and date and cause of death. Informed consent was obtained by each registry in accordance with national and/or regional regulations. Compliance with ethical standards was confirmed by the medical ethical committee of the Amsterdam Medical Centre (W21_123 No. 21.136). Population data were obtained from the statistical office of the European Union (Eurostat) [3] or via national statistics agencies. More information on the study population and data collection can be found in the ERA Registry and USRDS annual reports [1, 2].
Analyses
For the annual reports of the ERA Registry and the USRDS, the incidence, prevalence, and transplantation rate calculations were performed in a similar manner. KRT incidence was defined as the number of patients starting KRT in a given year, and calculated per million population (pmp) or per million age-related population (pmarp) using the mid-year general population. KRT prevalence was defined as the number of patients receiving KRT on 31 December of a given year, and the kidney transplantation rate as the number of patients receiving a kidney transplant in a year, both calculated per million population. For the ERA Registry data, age categories were adapted to align with the US data.
Mortality data is not presented as such in the ERA Registry reports and were therefore additionally calculated using the same methods as the USRDS. The number of patients who died in a year while receiving KRT was divided by the total time at risk of death for all patients on KRT in the same year and presented per 1000 patient years. The total time-at-risk-of-death for an individual was the time between 1 January (for prevalent patients in the previous year) or the date of KRT initiation (for incident patients) until the date of death, date of loss to follow-up, 90 days after recovery of function, or 31 December. In addition, mortality was calculated by treatment modality, so time at risk for dialysis patients also ended at the date of kidney transplantation.
As the COVID-19 pandemic has influenced the epidemiology of KRT [4], time trends, presented as average annual % change, were calculated separately for the time periods 2012–2019 and 2019–2021.
RESULTS
Incidence of kidney replacement therapy
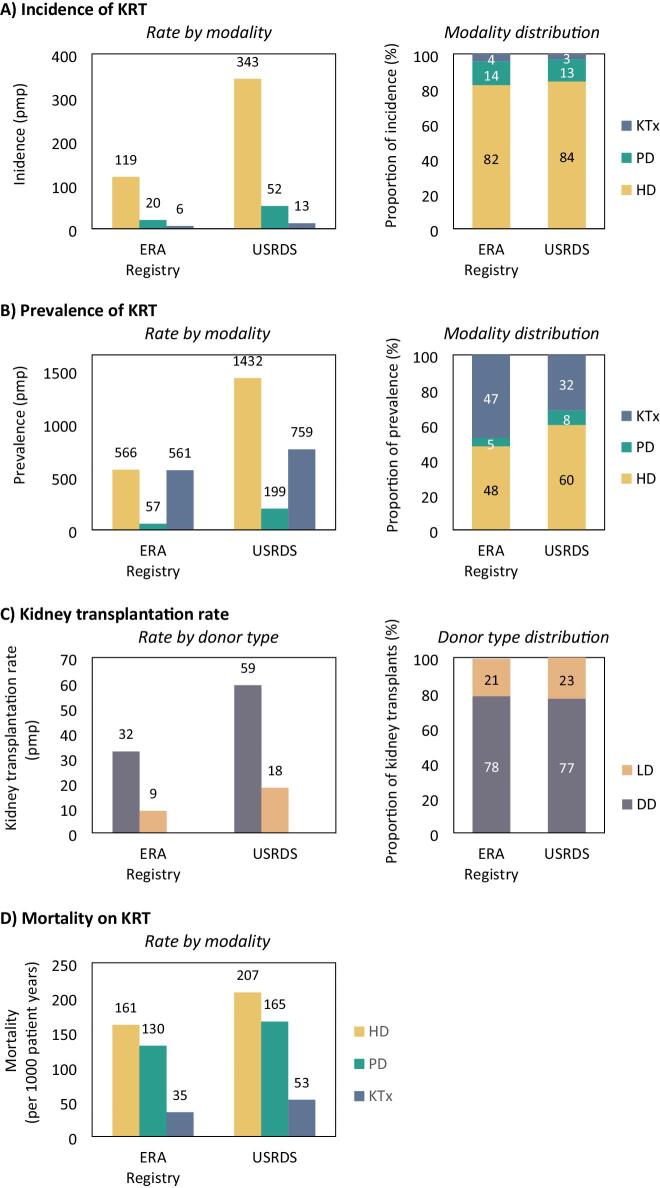
In Europe, 42 391 patients from a population of 294 million Europeans initiated KRT in 2021, corresponding to a KRT incidence of 144.4 pmp (Table 1). In the US, as many as 135 972 patients from a population of 332 million inhabitants initiated KRT in 2021, corresponding to a KRT incidence of 409.7 pmp (Table 1, Fig. 1A), making KRT incidence in the US almost three times higher than in Europe. Despite the substantial difference in KRT incidence between Europe and the US, the distribution of treatment modalities was comparable for Europe and the US for patients initiating HD (82% in Europe and 84% in the US, Fig. 2A), PD (14% and 13%, respectively), and undergoing pre-emptive KTx (4% and 3%, respectively). However, the incidence pmp for HD as initial treatment modality was almost three times higher in the US than in Europe, 2.6 times higher for PD, and two times higher for pre-emptive kidney transplantation.
Table 1:
Trends in the incidence, prevalence, and mortality of KRT, and trends in the kidney transplantation rates for the ERA Registry and the USRDS.
| Yearly rate | Average annualpercent change | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012– | 2019– | ||||||||||||
| Type | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2019 | 2021 | |
| Incidence of KRT (pmp) | |||||||||||||
| ERA Registry | All | 135.0 | 136.6 | 140.2 | 142.2 | 144.9 | 146.4 | 146.2 | 147.8 | 138.3 | 144.4 | +1.3 | −1.0 |
| HD | 110.2 | 110.9 | 113.8 | 115.7 | 117.7 | 118.5 | 119.0 | 120.4 | 113.2 | 118.6 | +1.3 | −0.6 | |
| PD | 18.1 | 19.0 | 19.3 | 19.3 | 20.0 | 20.0 | 19.9 | 19.7 | 19.3 | 19.7 | +1.3 | 0.0 | |
| KTx | 5.9 | 6.3 | 6.7 | 6.8 | 7.0 | 7.8 | 7.2 | 7.5 | 5.7 | 6.1 | +3.6 | −8.8 | |
| USRDS | All | 370.8 | 379.3 | 386.9 | 398.4 | 398.3 | 394.6 | 401.4 | 410.7 | 396.7 | 409.7 | +1.5 | −0.1 |
| HD | 330.0 | 335.0 | 340.7 | 350.5 | 349.4 | 344.1 | 346.1 | 350.9 | 333.6 | 343.0 | +0.9 | −1.1 | |
| PD | 30.8 | 34.4 | 35.9 | 37.9 | 38.5 | 39.7 | 43.7 | 47.0 | 50.3 | 51.9 | +6.3 | +5.1 | |
| KTx | 8.9 | 9.1 | 9.3 | 9.0 | 9.8 | 10.3 | 11.0 | 12.0 | 11.8 | 12.5 | +4.5 | +2.2 | |
| Prevalence of KRT (pmp) | |||||||||||||
| ERA Registry | All | 990.4 | 1015.1 | 1047.3 | 1075.1 | 1099.1 | 1128.5 | 1151.2 | 1172.2 | 1173.8 | 1187.8 | +2.4 | +0.7 |
| HD | 485.6 | 495.7 | 511.1 | 521.6 | 530.4 | 541.6 | 551.2 | 559.9 | 558.5 | 566.8 | +2.1 | +0.6 | |
| PD | 57.0 | 57.6 | 57.6 | 57.4 | 56.9 | 57.2 | 56.8 | 56.5 | 57.1 | 57.0 | −0.1 | +0.4 | |
| KTx | 442.2 | 456.7 | 473.4 | 491.3 | 506.7 | 525.2 | 539.0 | 552.3 | 555.1 | 561.5 | +3.2 | +0.8 | |
| USRDS | All | 2029.2 | 2092.5 | 2156.2 | 2221.3 | 2279.1 | 2331.3 | 2391.0 | 2457.7 | 2445.1 | 2436.1 | +2.8 | −0.4 |
| HD | 1296.2 | 1333.1 | 1373.9 | 1415.3 | 1453.3 | 1481.0 | 1506.1 | 1530.1 | 1489.2 | 1431.6 | +2.4 | −3.3 | |
| PD | 126.9 | 139.2 | 147.5 | 155.4 | 160.2 | 164.7 | 175.1 | 189.2 | 198.5 | 199.4 | +5.9 | +2.7 | |
| KTx | 600.9 | 615.2 | 628.4 | 642.4 | 658.9 | 677.7 | 700.1 | 727.4 | 745.1 | 759.2 | +2.8 | +2.2 | |
| Kidney transplantation rate (pmp) | |||||||||||||
| ERA Registry | All | 42.3 | 43.3 | 44.1 | 45.1 | 46.4 | 48.5 | 47.8 | 48.1 | 37.3 | 41.6 | +1.9 | −5.5 |
| DD | 31.1 | 31.6 | 32.0 | 34.2 | 35.6 | 37.5 | 37.4 | 37.7 | 29.9 | 32.5 | +2.8 | −6.1 | |
| LD | 9.7 | 10.3 | 10.6 | 10.7 | 10.6 | 10.9 | 10.3 | 10.2 | 7.3 | 8.8 | +0.8 | −3.8 | |
| USRDS | All | 55.3 | 56.1 | 56.1 | 58.1 | 61.7 | 63.6 | 67.4 | 74.0 | 71.8 | 77.0 | +4.3 | +2.1 |
| DD | 37.3 | 37.8 | 38.6 | 40.5 | 44.2 | 45.7 | 47.7 | 53.1 | 55.9 | 59.0 | +5.2 | +5.4 | |
| LD | 17.9 | 18.2 | 17.4 | 17.6 | 17.5 | 17.9 | 19.7 | 20.9 | 15.9 | 18.0 | +2.3 | −5.3 | |
| Mortality of KRT (per 1000 py) | |||||||||||||
| ERA Registry | All | 93.1 | 94.7 | 92.2 | 94.3 | 88.7 | 86.2 | 91.2 | 91.8 | 102.3 | 98.7 | −0.1 | +3.9 |
| HD | 156.7 | 156.6 | 152.5 | 155.7 | 146.9 | 141.9 | 152.8 | 153.9 | 171.0 | 160.6 | −0.2 | +2.5 | |
| PD | 137.7 | 135.5 | 133.9 | 137.1 | 131.8 | 132.2 | 130.4 | 132.5 | 133.8 | 130.5 | −0.5 | −0.7 | |
| KTx | 23.8 | 24.6 | 24.2 | 25.4 | 24.3 | 24.8 | 26.1 | 26.6 | 31.5 | 34.9 | +1.6 | +14.6 | |
| USRDS | All | 138.9 | 136.3 | 135.3 | 136.4 | 135.2 | 136.0 | 134.6 | 132.2 | 156.2 | 157.3 | −0.7 | +9.4 |
| HD | 188.0 | 184.3 | 182.1 | 183.0 | 180.7 | 181.7 | 180.3 | 177.0 | 208.3 | 207.3 | −0.9 | +8.6 | |
| PD | 131.7 | 131.1 | 131.1 | 132.0 | 128.6 | 132.1 | 128.9 | 130.2 | 158.0 | 165.3 | −0.1 | +13.0 | |
| KTx | 31.0 | 30.7 | 31.7 | 32.4 | 33.0 | 33.4 | 33.2 | 33.4 | 44.4 | 52.8 | +1.1 | +25.9 | |
HD: haemodialysis; KTx: kidney transplantation; PD: peritoneal dialysis.
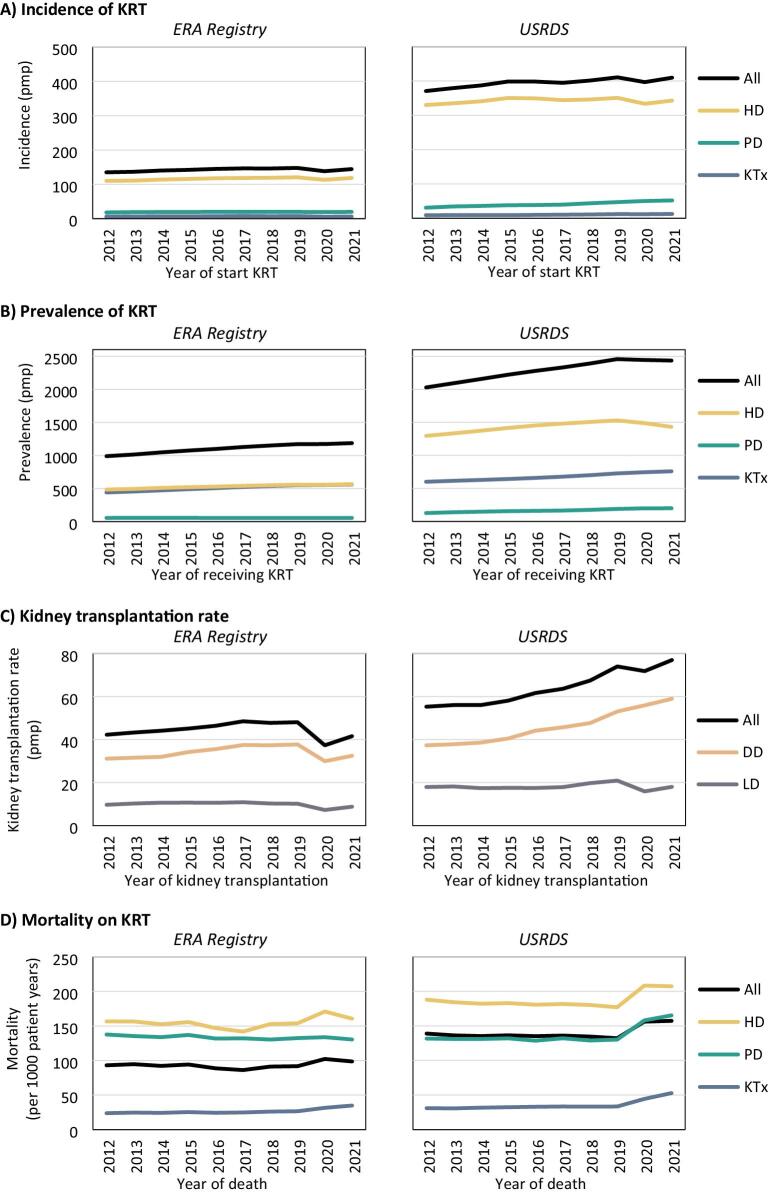
Figure 1:
Trends over time in (A) the incidence of KRT, (B) the prevalence of KRT on 31 December, and (C) the kidney transplantation rate per million population, and (D) in the mortality on KRT per 1000 patient years, for the ERA Registry and the USRDS, overall and by treatment modality or donor type. DD: deceased donor; HD: haemodialysis; KTx: kidney transplantation; LD: living donor; PD: peritoneal dialysis; pmp: per million population.
Figure 2:
(A) The incidence of KRT, (B) the prevalence of KRT on 31 December, (C) the kidney transplantation, and (D) in the mortality on KRT in 2021, as rate per million population or per 1000 patient years and as proportion of all patients, by registry. DD: deceased donor; HD: haemodialysis; KTx: kidney transplantation; LD: living donor; PD: peritoneal dialysis; pmp: per million population.
Between 2012 and 2019, the overall KRT incidence increased both in Europe and the US (+1.3% and +1.5% annually, Table 1, Fig. 1A). After 2019, the incidence decreased in Europe (−1.0%), while it remained almost stable in the US. Interestingly, both in Europe and the US, pre-emptive kidney transplantation increased between 2012 and 2019 (+3.6% and +4.5%, respectively), while in the US, the use of PD as initial treatment modality also increased substantially (+6.3%). During the COVID-19 pandemic, the incidence of HD as initial KRT modality decreased in both Europe and the US (−0.6% and −1.1%, respectively), while the incidence of PD as first KRT modality remained stable in Europe and increased annually by +5.1% in the US. Additionally, pre-emptive kidney transplantation was reduced substantially in Europe (−8.8%), while it continued to increase in the US (+2.2%), albeit at a slower pace than before 2019 (+4.5%).
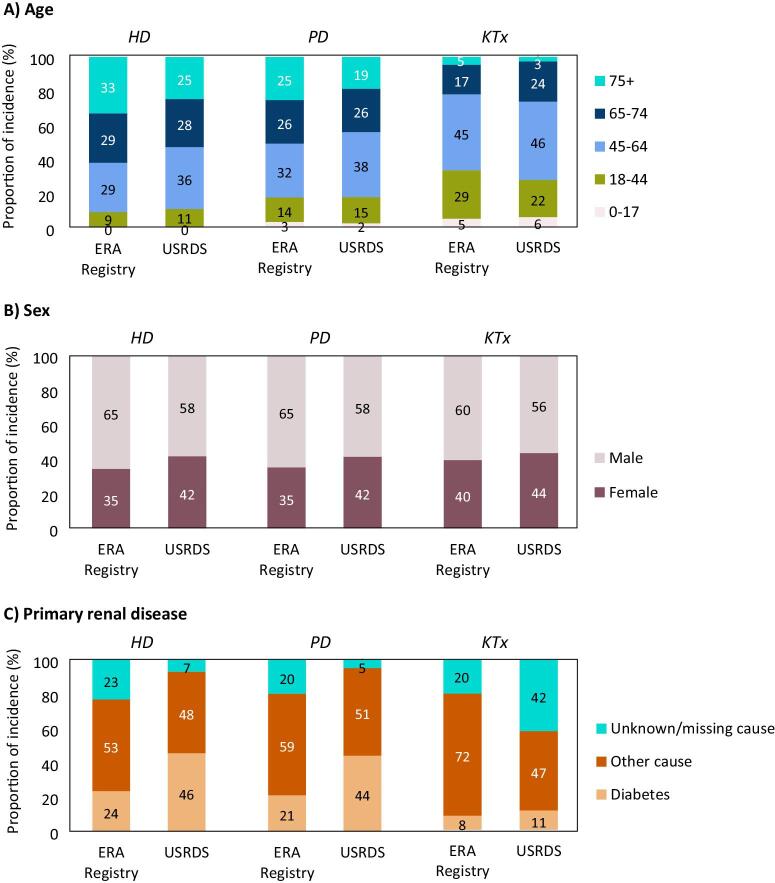
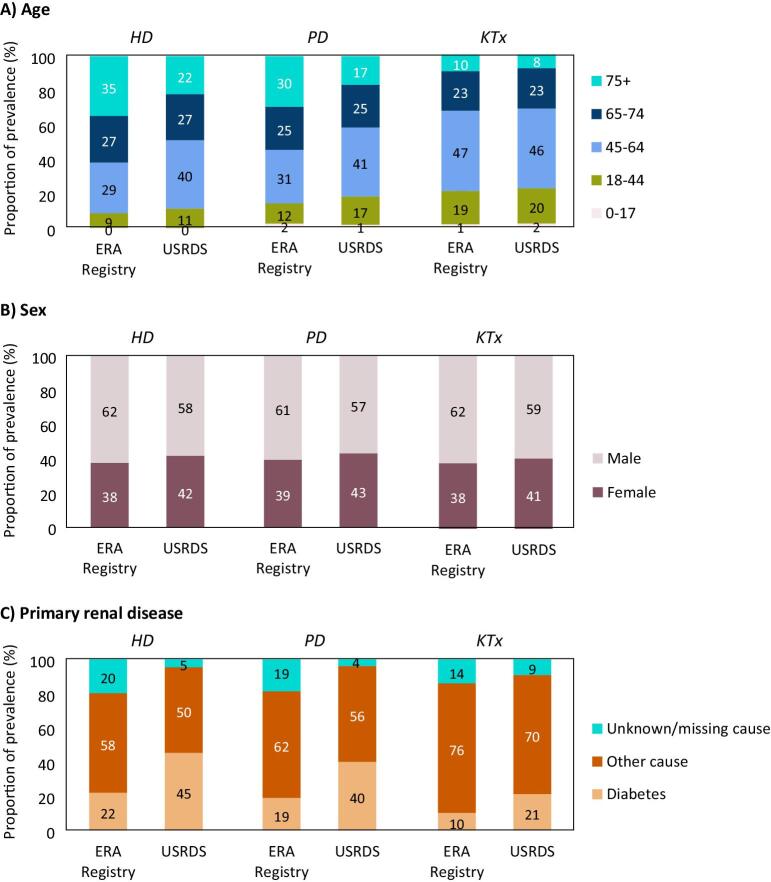
In 2021, despite the much higher KRT incidence in the US than in Europe, the proportion of older (≥65 years) incident dialysis patients was higher in Europe (HD: 62% in Europe and 53% in the US; PD: 51% in Europe and 45% in the US, Fig. 3A). In contrast, the proportion of patients receiving a pre-emptive kidney transplant after the age of 65 years was lower in Europe (22%) than in the US (27%). Of note, for all three initial treatment modalities the proportion of female patients starting KRT was lower in Europe (HD and PD: 35%, KTx: 40%) than in the US (HD and PD: 42%, KTx: 44%, Fig. 3B). Furthermore, the proportion of incident KRT patients on dialysis with diabetes as primary renal disease in Europe (HD: 24%, PD: 21%) was roughly half of that in the US (HD: 46%, PD 44%, Fig. 3C). For patients who received a pre-emptive kidney transplant, this difference was less pronounced (8% in Europe and 11% in the US).
Figure 3:
Distribution of (A) age, (B) sex, and (C) diabetes as primary renal disease among patients starting KRT in 2021, by initial treatment modality and registry. HD: haemodialysis; KTx: kidney transplantation; PD: peritoneal dialysis.
Prevalence of kidney replacement therapy
In the participating European countries, 349 504 patients were receiving KRT on 31 December 2021, corresponding to a KRT prevalence of 1187.8 pmp (Table 1, Fig. 1B). In the US, 808 536 were receiving KRT on 31 December 2021, corresponding to a KRT prevalence of 2436.1 pmp, making the US KRT prevalence twice as high as in Europe. Furthermore, in the US, the prevalence of PD per million population was 3.5 times higher, the prevalence of HD 2.5 times higher, and the prevalence of kidney transplantation was 1.4 times higher than in Europe. Within Europe, almost half of all prevalent patients were living with a functioning graft (47%), while in the US, this was only one third (32%, Fig. 2B).
In Europe, the overall KRT prevalence increased from 990.4 pmp in 2012 to 1172.2 pmp in 2019 (+2.4% annually) and increased further to 1187.8 pmp in 2021 (+0.7%, Table 1, Fig. 1B). In the US, the KRT prevalence was 2029.2 pmp in 2012, and increased annually by +2.8% to 2457.7 pmp in 2019, but decreased slightly thereafter to 2436.1 pmp in 2021. Stratified by treatment modality, the prevalence of HD and KTx increased both in Europe (HD: +2.1%, KTx: +3.2%) and the US (HD: +2.4%, KTx: +2.8%), while the prevalence of PD only increased in the US (+5.9%).
In 2021, despite the much higher KRT prevalence in the US compared to Europe, the proportion of older (≥65 years) prevalent dialysis patients was higher in Europe (HD: 62%, PD: 55%) than in the US (HD: 49%, PD: 42%, Fig. 4A), while the proportion of older kidney transplant recipients was comparable (Europe: 33%, US: 31%). For all treatment modalities, the proportion of female prevalent KRT patients was lower in Europe (∼38%) than in the US (∼42%, Fig. 4B), while the proportion of prevalent KRT patients with diabetes as primary renal disease in Europe (HD: 22%, PD: 19%, KTx: 10%) was about half that in the US (HD: 45%, PD 40% KTx: 21%, Fig. 4C).
Figure 4:
Distribution of (A) age, (B) sex, and (C) diabetes as primary renal disease among patients receiving KRT on 31 December 2021, by treatment modality and registry. HD: haemodialysis; KTx: kidney transplantation; PD: peritoneal dialysis.
Kidney transplantation
In the participating countries in the ERA Registry, 12 244 kidney transplantations were performed in 2021, corresponding to a kidney transplantation rate of 41.6 pmp. In the US, 17 357 kidney transplantations were performed, corresponding to a kidney transplantation rate of 77.0 pmp (Table 1, Fig. 1C). These findings indicate that the number of kidney transplantations performed per million population was almost twice as high in the US compared to Europe. Of note, although the US has a much higher kidney transplantation rate, only a third of prevalent KRT patients in the US are living with a functioning graft, compared to approximately half of all patients with KRT in Europe (Fig. 4). In both Europe and the US, the majority of kidney transplants were from deceased donors (DD, 78% and 77%, respectively, Fig. 2C).
Between 2012 and 2019, the kidney transplantation rate increased at a lower rate in Europe (+1.9% annually) than in the US (+4.3%, Table 1, Fig. 1C) for both DD transplantation (Europe: +2.8%, US: +5.2%) and living donor (LD) transplantation (Europe +0.8%, US: +2.3%). After 2019, kidney transplantation rates decreased in Europe (DD: −6.1%, LD: −3.8), while in the US, DD kidney transplantation continued to increase with +5.4% annually and LD kidney transplantation decreased (−5.3%).
Mortality on kidney replacement therapy
In Europe, 34 484 patients died while receiving KRT in 2021, corresponding to a mortality rate of 98.7 per 1000 patient years at risk. In the US, 131 744 patients died while receiving KRT in 2021, corresponding to a 1.6-fold higher mortality of 157.3 per 1000 patient years (Table 1, Fig. 1D). Stratified by treatment modality, in Europe, the mortality rate for HD and PD patients was approximately 20% lower, and for kidney transplant recipients 30% lower when compared to the US (Fig. 2D).
Interestingly, between 2012 and 2019, mortality rates slightly decreased for patients receiving dialysis, and increased for kidney transplant recipients, both in Europe (HD: −0.2%, PD: −0.5%, KTx: +1.6%, Table 1, Fig. 1D) and in the US (HD: −0.9%, PD: −0.1%, KTx: +1.1%). During the COVID-19 pandemic, mortality increased to a lower extent in Europe (HD: +2.5%, PD: −0.7%, KTx: +14.6%) than in the US (HD: +8.6%, PD: +13.0%, KTx: +25.9%).
DISCUSSION
This study is based on data on the epidemiology of KRT derived from annual reports of the ERA Registry [1] and USRDS [2] up to and including 2021, supplemented with results from additional analyses on European data to present results of participating European countries in aggregated form. The findings show substantial differences in the epidemiology of KRT between Europe and the US. Below we will briefly discuss the results, in particular the results on treatment modality.
Incidence of kidney replacement therapy
In 2021, the incidence of KRT in the US was nearly 3-fold higher than in Europe. This difference in KRT incidence could be explained by several factors. First, there may be a greater demand of KRT in the US due to a higher prevalence of chronic kidney disease (CKD) in the general population [5, 6]. In the US [5] the prevalence was estimated to be 13.9% for CKD stages 1–5 and 5.5% for CKD stages 3–5, while in a selected group of European countries the prevalence has been estimated at 3.3% to 17.3% for CKD stages 1–5 and 1% to 5.9% for CKD stages 3–5 [6]. However, for many (participating) European countries the prevalence of CKD is unknown [6], which hampers the comparison with the US. The prevalence of CKD may, in turn, be influenced by the prevalence of major risk factors for CKD, such as diabetes mellitus and obesity, which may be more prevalent in the US compared to most participating European countries [7, 8]. Second, a higher rate of progression from CKD to end-stage kidney disease (ESKD) in the US may cause a higher KRT incidence [9]. However, a comparison between the progression of CKD to ESKD between the US and Europe is challenging as one should also take into account the competing risk of mortality and literature on this subject is limited [6, 10, 11]. Third, better access to KRT and an earlier start of KRT in the US as well as wider treatment availability may also lead to a higher KRT incidence. Yet, it is unclear whether the US has a more liberal policy accepting patients to KRT [12]. For example, the proportion of patients aged 75 years and over when initiating KRT was lower in the US than in Europe, which may indicate that in the US they were less inclined to treat older patients with KRT compared to Europe. On the other hand, the KRT incidence for ESKD due to diabetes mellitus was twice as high in the US (46%) as in Europe (24%), although this could also be due to the aforementioned potential higher prevalence of CKD and diabetes mellitus in the US general population. In the US in 2021, the mean estimated glomerular filtration rate (eGFR) at initiation of KRT was 9.9 mL/min/1.73 m2 [2], but less is known about the eGFR at KRT initiation in Europe [13, 14]. Also, the use of comprehensive conservative management as an alternative to KRT may influence the KRT incidence; however, data on the frequency of comprehensive conservative management in the US and Europe are scarce [15, 16].
Despite the higher incidence of KRT in the US compared to participating European countries, for both populations, the vast majority of patients started KRT with HD (>80%), and approximately the same percentage of patients started with PD (13–14%) or underwent pre-emptive kidney transplantation (3–4%). Pre-emptive kidney transplantation rates increased between 2012 and 2019 both in the US and in Europe. The use of PD as an initial KRT modality also increased in both populations, but to a much larger extent in the US (+6.3% annually) than in Europe (+1.3% annually). This substantial increase in PD use in the US may have been the result of the introduction of a new payment system for dialysis treatment in 2011 [17].
Prevalence of kidney replacement therapy
In 2021, the prevalence of KRT was more than twice as high in the US as in Europe. Nearly half of European prevalent patients were receiving HD (48%) and the other half was living with a functioning graft (47%), while in the US, a majority of patients were receiving HD (60%) and only about one third of patients were living with a functioning graft (32%). This will be further discussed in the section on kidney transplantation. In the US, the prevalence of PD increased between 2012 and 2019, and was likely influenced by the aforementioned payment system for dialysis treatment [17].
Kidney transplantation
In 2021, the overall KTx rate was almost twice as high in the US as in Europe (41.6 pmp vs 77.0 pmp), which was the case for both living and deceased donor KTx. Despite this, the number of KTx per 1000 dialysis patients was lower in the US than in most European countries [2], which is the consequence of the much higher number of patients receiving dialysis in the US. This largely explains why, despite the higher KTx rate in the US when compared to Europe, the proportion of prevalent KRT patients living with a functioning graft was lower in the US. Possibly, in the US a lower proportion of dialysis patients were considered suitable for a kidney transplant. The higher mortality in both dialysis patients and kidney transplant recipients in the US is further discussed in the section on mortality. Of note, over time both living and deceased donor KTx rates increased to a larger extent in the US than in Europe.
Mortality on kidney replacement therapy
In 2021, the mortality on KRT was 1.6 times higher in the US than in Europe. Additionally, the mortality on HD was 1.3 times higher and the mortality on PD was 1.6 times higher in the US than in Europe. An explanation for the higher mortality in the US may be that patients have a greater number of comorbidities and a less favorable prognosis at the start of KRT. Another possible explanation may lie in practice patterns, such as higher catheter use in incident dialysis patients in the US compared to European countries, which may be associated with higher mortality on dialysis than the use of arteriovenous fistulas [18, 19].
In 2021, the mortality rate for kidney transplant recipients is approximately one third higher in the US compared to Europe and the gap seemed to increase during and after the pandemic. Previously published articles suggest that the differences in particular in long-term survival in kidney transplant recipients may at least partly caused by a difference in insurance coverage after transplantation, where in the US there may more barriers in free access to immunosuppressive drugs that can lead to medication non-adherence and late allograft loss [20, 21]. Also, transplant recipients in the US may suffer from more co-morbidities than in Europe [20–22]. Furthermore, the Eurotransplant Senior Programme (ESP) was launched back in 1999 to improve longevity matching of deceased donor organs in several European countries, while maintaining allograft and patient survival. In the US, they have also implemented a deceased donation kidney allocation system to improve transplant outcomes, but much later in 2014 [20].
Strength and limitations
The main strength of this study is that it includes data of almost all dialysis and kidney transplant patients in a large number of European countries and the US. We were able to include data of the majority of Western and some Eastern European countries. The results may, however, not be generalizable to non-participating European countries and across individual European countries and individual States in the US. Moreover, we only present unadjusted results in this article. In both the ERA Registry and USRDS annual reports age and sex standardized incidence, prevalence and mortality rates can be found; however, these are using different reference values. Last, the epidemiology of KRT may have been influenced by the pandemic, but this is mainly discussed elsewhere [23–26].
Conclusion
The findings of this study show that, in 2021, the incidence of KRT in the US was nearly 3-fold higher than in Europe. In both the US and Europe, approximately 80% of the patients started on HD and only 3–4% of patients received a pre-emptive KTx. Despite the almost 2-fold higher KTx rate in the US, approximately half of the prevalent European KRT patients were living with a functioning graft compared with only about one-third in the US. Furthermore, the mortality on KRT was 1.6-fold higher in the US compared to Europe. Renal registries are valuable in providing an annual update on the frequency and outcomes of KRT and may therefore play an important role in reducing inequalities in kidney care.
ACKNOWLEDGEMENTS
We would like to thank the patients and the staff of the dialysis and transplant units for contributing the data via their national and regional renal registries. Furthermore, we gratefully acknowledge the following registries and persons for their contribution of the data: Austrian Dialysis and Transplant Registry [OEDTR] (D. Kaiser-Feistmantl, L. Buchwinkler and G. Mayer); Dutch speaking Belgian Society of Nephrology [NBVN] (L. Heylen, V. De Meyer and J. De Meester); French speaking Belgian Society of Nephrology [GNFB] (JM. des Grottes and F. Collart); Renal Registry Bosnia and Herzegovina (B. Jakovljevic and Z. Kelava); Danish Nephrology Registry [DNS]; Estonian Society of Nephrology (Ü. Pechter, M. Ots-Rosenberg, and K. Lilienthal); Finnish Registry for Kidney Diseases (J. Helve); France: The Epidemiology and Information Network in Nephrology [REIN] (C. Couchoud); Hellenic Renal Registry; Icelandic End-Stage Renal Disease Registry (R. Palsson); Montenegro Renal Registry (M. Ratkovic and F. Tomović); Norwegian Renal Registry (A.V. Reisæter and A. Åsberg); Romanian Renal Registry [RRR] (G. Mircescu, L. Garneata, and E. Podgoreanu); Renal Registry in Serbia (M Kravljaca and all dialysis units in Serbia); Swedish Renal Registry [SRR] (K.G. Prütz, M. Stendahl, M. Evans, T. Lundgren, H. Rydell and M. Segelmark); Swiss Dialysis Registry (R. Guidotti); Dutch Renal Registry [RENINE] (P. Verschoor and L. Heuveling); UK Renal Registry (all the staff of the UK Renal Registry and of the renal units submitting data); Scottish Renal Registry [SRR] (all of the Scottish renal units); and the regional registries of Andalusia [SICATA] (P. Castro de la Nuez (on behalf of all users of SICATA)), Aragon (F. Arribas Monzón), Asturias (M.R. Camblor, J.R. Quirós, and RERCA working group), Basque country [UNIPAR] (Á. Magaz, J. Aranzabal, M. Rodrigo, and I. Moina), Canary Islands (C. García Cantón and D. Marrero Miranda), Cantabria (J.C. Ruiz San Millán), Castile and León (M. Prieto Velasco and M.Á. Palencia García), Castile-La Mancha (G. Gutiérrez Ávila and I. Moreno Alía), Catalonia [RMRC] (J Comas and M Vázquez), Community of Madrid (M.I. Aparicio de Madre and F Tornero Molina), Extremadura (all the renal units (Nephrology and Dialysis) from Extremadura), Galicia (E. Bouzas-Caamaño), La Rioja (E. Huarte Loza and H. Hernández Vargas), Murcia (I. Marín Sánchez and C. Santiuste de Pablos), Navarre (J. Manrique Escola), and Valencian region (O. Zurriaga); and A. Weerstra in the AMC Registry office for data collection and management.
Contributor Information
Vianda S Stel, ERA Registry, Department of Medical Informatics, Amsterdam UMC Location, University of Amsterdam, Amsterdam, the Netherlands; Amsterdam Public Health Research Institute, Quality of Care, Amsterdam, the Netherlands.
Rianne Boenink, ERA Registry, Department of Medical Informatics, Amsterdam UMC Location, University of Amsterdam, Amsterdam, the Netherlands; Amsterdam Public Health Research Institute, Quality of Care, Amsterdam, the Netherlands.
Megan E Astley, ERA Registry, Department of Medical Informatics, Amsterdam UMC Location, University of Amsterdam, Amsterdam, the Netherlands; Amsterdam Public Health Research Institute, Quality of Care, Amsterdam, the Netherlands.
Brittany A Boerstra, ERA Registry, Department of Medical Informatics, Amsterdam UMC Location, University of Amsterdam, Amsterdam, the Netherlands; Amsterdam Public Health Research Institute, Quality of Care, Amsterdam, the Netherlands.
Danilo Radunovic, Clinical Center of Montenegro, Clinic for Nephrology, Podgorica, Montenegro.
Rannveig Skrunes, Department of Medicine, Haukeland University Hospital, Bergen, Norway; Department of Clinical Medicine, University of Bergen, Bergen, Norway.
Juan C Ruiz San Millán, Nephrology Department, Hospital Universitario Marqués de Valdecilla, University of Cantabria, IDIVAL, Santander, Cantabria, Spain.
Maria F Slon Roblero, Nephrology Department, Hospital Universitario de Navarra, Pamplona, Navarre, Spain.
Samira Bell, Scottish Renal Registry, Public Health Scotland, Meridian Court, Glasgow, UK; Division of Population Health and Genomics, University of Dundee, Dundee, UK.
Pablo Ucio Mingo, Coordinación Autonómica de Trasplantes de Castilla y León, Dirección General de Asistencia Sanitaria y Humanización, Gerencia Regional de Salud de Castilla y León, Valladolid, Castilla y León, Spain.
Marc A G J ten Dam, Dutch Registry RENINE, Nefrovisie, Utrecht, the Netherlands.
Patrice M Ambühl, Division of Nephrology, Stadtspital Zürich, Zurich, Switzerland.
Halima Resic, Society for Nephrology, Dialysis and Transplantation of Bosnia and Herzegovina, Sarajevo, Bosnia and Herzegovina.
Olga Lucia Rodríguez Arévalo, Registry of Kidney Patients of the Valencian Community, General Directorate of Public Health, Ministry of Health, Valencia, Spain; Health and Well-being Technologies Program, Polytechnic University of Valencia, Valencia, Spain.
Nuria Aresté-Fosalba, Nephrology Department, Virgen Macarena Hospital, Seville, Andalusia, Spain; Information System of Andalusian Transplant Coordination (SICATA), Seville, Andalusia, Spain.
Jaume Tort i Bardolet, Catalan Transplant Organization (OCATT), Catalan Health Service, Department of Health, Barcelona, Spain.
Mathilde Lassalle, REIN Registry (Renal Epidemiology and Information Network), Paris, France.
Sara Trujillo-Alemán, Health Quality Assessment and Information System Service, Dirección General de Programas Asistenciales, Servicio Canario de la Salud, Las Palmas de Gran Canaria, Spain.
Olafur S Indridason, Division of Nephrology, Landspitali-The National University Hospital of Iceland, Reykjavik, Iceland.
Marta Artamendi, Nephrology Department, Hospital San Pedro, Logroño, La Rioja, Spain.
Patrik Finne, Department of Nephrology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Marta Rodríguez Camblor, Public Health Directorate, Asturias, Spain.
Dorothea Nitsch, Department of Non-Communicable Disease Epidemiology, London School of Hygiene & Tropical Medicine, Renal Unit, Royal Free London NHS Foundation Trust, UK Kidney Association, Bristol, UK.
Kristine Hommel, Department of Nephrology, Holbaek Hospital, Holbaek, Denmark.
George Moustakas, Nephrology Department, General Hospital of Athens ‘G.Gennimatas’, Athens, Greece.
Julia Kerschbaum, Austrian Dialysis and Transplant Registry, Department of Internal Medicine IV – Nephrology and Hypertension, Medical University Innsbruck, Innsbruck, Austria.
Mirjana Lausevic, School of Medicine, University of Belgrade, Belgrade, Serbia; Clinic of Nephrology, University Clinical Centre of Serbia, Belgrade, Serbia.
Kitty J Jager, ERA Registry, Department of Medical Informatics, Amsterdam UMC Location, University of Amsterdam, Amsterdam, the Netherlands; Amsterdam Public Health Research Institute, Quality of Care, Amsterdam, the Netherlands.
Alberto Ortiz, Department of Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain; Department of Medicine, Universidad Autonoma de Madrid, Madrid, Spain.
Anneke Kramer, ERA Registry, Department of Medical Informatics, Amsterdam UMC Location, University of Amsterdam, Amsterdam, the Netherlands; Amsterdam Public Health Research Institute, Quality of Care, Amsterdam, the Netherlands.
FUNDING
The ERA Registry is funded by the European Renal Association (ERA). This article was written by A. Kramer et al. on behalf of the ERA Registry which is an official body of the ERA (European Renal Association).
AUTHORS’ CONTRIBUTIONS
Conceptualization: A.K., V.S.S., A.O.; Methodology: A.K., R.B., V.S.S.; Formal analysis: A.K.; Writing–original draft: A.K., V.S.S., M.E.A., B.A.B., A.O. and K.J.J.; Writing–review & editing: D.R., R.S., J.C.R.S.M., M.F.S.R., S.B., P.U.M., M.A.G.J.D., M.L., H.R., O.L.R.A., N.A.-F., J.T.B., M.L., S.T.-A., O.S.I., M.A., P.F., M.R.C., D.N., K.H., G.M., J.K., and P.M.A. All authors read and approved the final manuscript.
DATA AVAILABILITY STATEMENT
Most of the data underlying this article have been published in the ERA Registry Annual Report 2021 and in the USRDS 2023 Annual Report. The remaining data cannot be shared with any third party because the national and regional registries that provided data to the ERA Registry remain the owners of the data.
CONFLICT OF INTEREST STATEMENT
V.S.S. reports research funding from the European Renal Association, outside the submitted work. R.S. reports travel support from Chiesi, Amicus, Sanofi Genzyme and Takeda, outside the submitted work. M.F.S.R. reports consulting fees from Baxter, Fresenius and Nipro; lecture fees from Baxter and Fresenius; travel grants from Vifor, Fresenius and Palex; and membership of the Fresenius European Home Dialysis Advisory Board, outside the submitted work. S.B. reports consulting fees from GSK and Astra Zeneca, outside the submitted work. M.L. reports membership of the Serbian Society of Nephrology board, outside the submitted work. H.R. reports membership of BANTAO, MKS and EAPE boards, outside the submitted work. N.A.-F. reports lecture fees from Astra-Zeneca, Vifor Pharma, Boehringer-Ingelheim and Bayer; travel grants from Vifor Pharma; and membership of Astra Zeneca and the Andalusian Nephrology Society boards, all outside the submitted work. P.F. reports grants from Finska läkaresällskapet and Liv och Hälsa and consulting fees from Baxter, Astra Zeneca, GSK and Astellas, and membership of a Baxter board, outside the submitted work. D.N. reports board membership of the UK Kidney Association, outside the submitted work. K.J.J. reports research funding from the European Renal Association and the European Society for Paediatric Nephrology; and board membership from the SHare RR working group (ISN), outside the submitted work. A.O. reports grants from Sanofi, consultancy or speaker fees or travel support from Advicciene, Astellas, Astrazeneca, Amicus, Amgen, Boehringer Ingelheim, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Lilly, Alexion, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex, and Vifor Fresenius Medical Care Renal Pharma; Directorship of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra Astrazeneca-UAM of chronic kidney disease and electrolytes; membership of the European Renal Association board; and stock options from Telara Farma, all outside the submitted work. R.B., M.E.A., B.A.B., D.R., J.C.R.S.M., P.U.M., M.A.G.J.D., O.L.R.A., J.T.B., M.L., S.T.A., O.S.I., M.A., M.R.C., K.H., G.M., J.K., P.M.A., and A.K. declare that they have no relevant conflicts of interests.
REFERENCES
- 1.Boerstra BA, Boenink R, Astley MEet al. The ERA Registry Annual Report 2021: a summary. Clin Kidney J 2024;17:sfad281. 10.1093/ckj/sfad281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System . 2023 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2023. Available from: https://usrds-adr.niddk.nih.gov/2023. [Google Scholar]
- 3.European Statistical Office (Eurostat) . Database—Population and Social Conditions. https://ec.europa.eu/eurostat/data/database (12 January 2023, date last accessed). [Google Scholar]
- 4.Carriazo S, Aparicio-Madre MI, Tornero-Molina Fet al. Impact of different COVID-19 waves on kidney replacement therapy epidemiology and mortality: REMER 2020. Nephrol Dial Transplant 2022;37:2253–63. 10.1093/ndt/gfac234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . Kidney disease surveillance system: trends in prevalence of CKD stages among U.S. Adults. Available from: https://nccd.cdc.gov/ckd/detail.aspx?Qnum=Q372 (5 January 2023, date last accessed).
- 6.Brück K, Stel VS, Gambaro Get al. CKD prevalence varies across the European general population. J Am Soc Nephrol 2016;27:2135–47. 10.1681/ASN.2015050542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBD 2021 Diabetes Collaborators . Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023;402:203–34. 10.1016/S0140-6736(23)01301-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . Noncommunicable disease surveillance, monitoring and reporting. Available from: https://www.who.int/teams/noncommunicable-diseases/surveillance/data/diabetes-profiles (5 January 2023, date last accessed).
- 9.Hallan SI, Coresh J, Astor BCet al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 2006;17:2275–84. 10.1681/ASN.2005121273 [DOI] [PubMed] [Google Scholar]
- 10.Swartling O, Rydell H, Stendahl Met al. CKD progression and mortality among men and women: a nationwide study in Sweden. Am J Kidney Dis 2021;78:190–9.e1. 10.1053/j.ajkd.2020.11.026 [DOI] [PubMed] [Google Scholar]
- 11.Borrelli S, Leonardis D, Minutolo Ret al. Epidemiology of CKD regression in patients under nephrology care. PLoS One 2015;10:e0140138. 10.1371/journal.pone.0140138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thurlow JS, Joshi M, Yan Get al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am J Nephrol 2021;52:98–107. 10.1159/000514550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UK Renal Registry . UK Renal Registry 25th Annual Report—data to 31/12/2021. Bristol, UK. Available from: https://ukkidney.org/audit-research/annual-report.
- 14.Finnish Registry for Kidney Diseases . Finnish Registry for Kidney Diseases Report 2021. Available from: https://www.muma.fi/liitto/suomen_munuaistautirekisteri/finnish_registry_for_kidney_diseases.
- 15.Stel VS, de Jong RW, Kramer Aet al. Supplemented ERA-EDTA Registry data evaluated the frequency of dialysis, kidney transplantation, and comprehensive conservative management for patients with kidney failure in Europe. Kidney Int 2021;100:182–95. 10.1016/j.kint.2020.12.010 [DOI] [PubMed] [Google Scholar]
- 16.Bello AK, Okpechi IG, Levin Aet al. International Society of Nephrology—Global Kidney Health Atlas: a report by the International Society of Nephrology: an assessment of Global kidney health Care Status focussing on capacity, availability, accessibility, affordability and outcomes of Kidney disease. Available from: https://www.theisn.org/initiatives/global-kidney-health-atlas.
- 17.Sloan CE, Coffman CJ, Sanders LLet al. Trends in peritoneal dialysis use in the United States after Medicare payment reform. Clin J Am Soc Nephrol 2019;14:1763–72. 10.2215/CJN.05910519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson BM, Akizawa T, Jager KJet al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 2016;388:294–306. 10.1016/S0140-6736(16)30448-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pisoni RL, Arrington CJ, Albert JMet al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis 2009;53:475–91. 10.1053/j.ajkd.2008.10.043 [DOI] [PubMed] [Google Scholar]
- 20.Wang JH, Skeans MA, Israni AK.. Current status of kidney transplant outcomes: dying to survive. Adv Chronic Kidney Dis 2016;23 :281–6. 10.1053/j.ackd.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 21.Gondos A, Döhler B, Brenner Het al. Kidney graft survival in Europe and the United States: strikingly different long-term outcomes. Transplantation 2013;95:267–74. 10.1097/TP.0b013e3182708ea8 [DOI] [PubMed] [Google Scholar]
- 22.Ojo AO, Morales JM, González-Molina Met al. Comparison of the long-term outcomes of kidney transplantation: USA versus Spain. Nephrol Dial Transplant 2013;28:213–20. 10.1093/ndt/gfs287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jager KJ, Kramer A, Chesnaye NCet al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 2020;98:1540–8. 10.1016/j.kint.2020.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United States Renal Data System . 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2022; Available from: https://usrds-adr.niddk.nih.gov/2022. [Google Scholar]
- 25.Aubert O, Yoo D, Zielinski Det al. COVID-19 pandemic and worldwide organ transplantation: a population-based study. Lancet Public Health 2021;6:e709–19. 10.1016/S2468-2667(21)00200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kute VB, Tullius SG, Rane Het al. Global impact of the COVID-19 Pandemic on solid organ transplant. Transplant Proc 2022;54:1412–6. 10.1016/j.transproceed.2022.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Most of the data underlying this article have been published in the ERA Registry Annual Report 2021 and in the USRDS 2023 Annual Report. The remaining data cannot be shared with any third party because the national and regional registries that provided data to the ERA Registry remain the owners of the data.