Abstract
Memory impairment is one of the main characteristics of postoperative cognitive dysfunction. It remains elusive how postoperative pathological changes of the brain link to the memory impairment. The clinical setting of perioperation was mimicked via partial hepatectomy under sevoflurane anaesthesia together with preoperative restraint stress (Hep‐Sev‐stress) in mice. Memory changes were assessed with fear conditioning. The medial prefrontal cortex (mPFC)‐dorsal hippocampus connectivity was evaluated with injecting neurotracer 28 days before surgery. Astrocytic activation was limited via injecting AAV‐GFAP‐hM4Di‐eGFP into the mPFC. Astrocytic and microglial phagocytosis of synapses were visualised with co‐labelling hippocampal neuronal axon terminals with PSD‐95 and S100β or Iba1. Neuroinflammation and oxidative stress status were also detected. Hep‐Sev‐stress impaired the memory consolidation (mean [standard error], 49.91 [2.55]% vs. 35.40 [3.97]% in the contextual memory, p = 0.007; 40.72 [2.78]% vs. 27.77 [2.22]% in cued memory, p = 0.002) and the cued memory retrieval (39.00 [3.08]% vs. 24.11 [2.06]%, p = 0.001) in mice when compared with these in the naïve controls. Hep‐Sev‐stress damaged the connectivity from the dorsal hippocampus to mPFC but not from the mPFC to the dorsal hippocampus and increased the astrocytic but not microglial phagocytosis of hippocampal neuronal axon terminals in the mPFC. The intervention also induced neuroinflammation and oxidative stress in the dorsal hippocampus and the mPFC in a regional‐dependent manner. Limiting astrocyte activation in the mPFC alleviated memory consolidation impairment induced by Hep‐Sev‐stress. Postoperative memory consolidation was impaired due to astrocytic phagocytosis‐induced connectivity injury from the dorsal hippocampus to the medial prefrontal cortex.
Keywords: astrocytic phagocytosis, cognitive dysfunction, connectivity, memory consolidation, microglial phagocytosis
Anaesthesia and surgery activated astrocytic engulfment of hippocampal neuronal axon terminals in the mPFC and caused connectivity damage from dorsal hippocampus to mPFC and consequently impaired memory consolidation (created with bioRender.com).
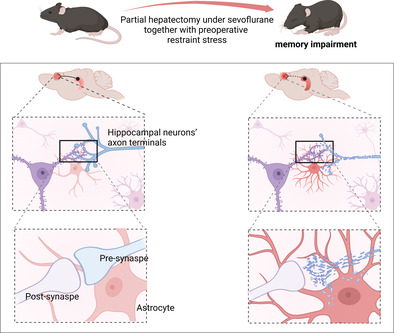
1. INTRODUCTION
Postoperative cognitive dysfunction (POCD), also namely perioperative neurocognitive disorder, characterised as memory impairment occurs frequently in the elderly following surgery. This surgical complication affects the quality of patients' life and increases the medical and social burdens [1, 2]. Accumulating evidence suggested that neuroinflammation, oxidative stress, synaptic dysfunction and blood–brain barrier dis‐integrity all were considered to be the pathogenesis of POCD [3, 4]. However, how these pathological changes induce the memory impairment remains elusive.
Learning and memory is considered a process including information acquisition, consolidation, storage, retrieval, long‐term maintenance and fading [5]. During these processes, hippocampus‐medial prefrontal cortex circuit (HPC‐mPFC) plays an important role [6]. Hippocampus rapidly encodes new information, then forms short‐term memory, which is then conveyed to mPFC to be consolidated and stored. The mPFC exerts top–down control over hippocampus to retrieve memories [7]. Glial cells (mainly including microglia and astrocytes) actively participate in this process via pruning synapses [8, 9, 10]. Uncontrolled synaptic pruning by glial cells damages inter‐neuronal connectivity and, hence, leads to memory impairment [11, 12, 13]. Our previous study demonstrated that POCD is associated with the damage of HPC‐mPFC connectivity [14], but underlying mechanisms remain unknown. Here, we further investigate memory processing damage after surgery and the role of glial activation related to pruning synapses mediated the HPC‐mPFC connectivity changes.
2. METHODS AND MATERIALS
2.1. Animals
All experiments were performed in accordance with the guidelines for experimental animal use of the Central South University, Changsha, China, and the ARRIVE guideline. The protocol (CSU‐2022‐0010) was approved by the Ethics committee of the Central South University. C57BL/6 male mice (8 weeks, 20–25 g) were purchased from the Central South University. All mice were housed in pathogen‐free cages with free access to food and water in the unit for which the temperature (24 ± 0.5), humidity (60%) and 12 h dark–light cycle were tightly controlled.
2.2. Perioperative scenario model
To simulate the clinical settings of patients with preoperative fasting and concerns about the anaesthesia and surgery, mice were subjected to restraint stress in transparent tubes without food and water from 6:00 PM to 9:00 AM 1 day before partial hepatectomy under 2%–2.5% sevoflurane anaesthesia reported previously. Briefly, transverse incision was made below the xyphoid. The left lobe of the liver was securely ligated and removed, and the incision was then sutured with sterile sutures. A total of 2.5% lidocaine cream was applied in the surgical wound area once every 8 h within 48 h after surgery to relieve postoperative pain.
2.3. Fear conditioning test
This test consisted of three phases: fear conditioning, contextual memory test (hippocampus‐dependent) and cued memory test (hippocampus‐independent) [15] on the day after surgery or on 1st, 3rd, 7th and 14th days after surgery. During fear conditioning, mice were placed in a conditioning chamber (context A). After 120 s of habitation, they were exposed to three tone‐shock pairings consisting of a 20 s tone (2800 Hz, 80 dB) that co‐terminated with a 2 s foot shock (0.5 mA), which were delivered with a 60 s interval and they were then returned to their home cages. In contextual memory test, mice were placed back to context A for 3 min without tone and shock and the freezing time was calculated as the percentage of freezing time of these 3‐min period. Two hours later, mice were placed in a new test chamber (context B) to assess cued memory and received three cycles of the 20 s tone alone with a 60 s inter‐cycle interval (total 3 min). In the fear conditioning and the cued fear test, the relative freezing time was calculated as the percentage of freezing time during three cycles of the tone. The inside of the unit was cleaned with 70% alcohol between each test [16]. All data were analysed through offline video recordings by a researcher who was blinded to the experimental design.
2.4. Open field test
Open field test was performed in some cohorts to evaluate locomotor activities 6 h after surgery. Mice were placed in the centre of the open field box with floor divided into 25 squares and were recorded for 5 min using a digital camera. The total squares crossed were calculated off‐line.
2.5. Viral injections
Some cohorts received viral injection into the hippocampus or the mPFC bilaterally under sodium pentobarbital anaesthesia (50 mg kg−1, i.p. injection) as reported previously [10] before stress challenge and surgery. For tracing postsynaptic projection of neurons in the hippocampus towards the mPFC, mice were micro‐injected in the hippocampus (anterior/posterior: −2.18 from bregma; medial/lateral: ±1.60 from bregma; dorsal/ventral: −2.20 to −1.40 mm; two injections per hemisphere) with pAAV‐CAG‐eGFP (TOS272, Cax9X, Suzhou, China) and in the mPFC (anterior/posterior: +0.30; medial/lateral: ±0.26; dorsal/ventral: −1.50 to −1.38 mm; two injections per hemisphere) with pAAV5‐CAG‐PSD95‐mCherry (Vigene biosciences, Shandong, China). To label the synapses formed from the mPFC to hippocampus, viruses pAAV‐CAG‐eGFP were injected into the mPFC, and pAAV5‐CAG‐PSD95‐mCherry were injected into the hippocampus with co‐ordinations as above. Four weeks later, they received with or without (control) preoperative restraint stress and partial hepatectomy. To quantify the connectivity from the hippocampus to the mPFC, the density of synapses co‐labelled with eGFP and PSD‐95 in the mPFC (per μm) were counted [17]. To quantify the connectivity from mPFC to the hippocampus, the number of synapses co‐labelled with eGFP and PSD‐95 in the lower half of CA3 pyramidal layer were also counted [18].
To limit astrocytes' activity, some cohorts received Adeno‐associated virus (neurotracer) (AAV)‐GFAP‐hM4Di‐eGFP (Sunbio Medical Biotechnology, Shanghai, China) injected into the mPFC 28 days before surgery or pAAV‐GFAP‐AB(mC1.1‐1)D‐eGFP (pMT467, Sunbio Medical Biotechnology, Shanghai, China) (negative control) injected in the mPFC of the control and surgery groups. In addition, Clozapine‐N‐oxide (CNO; 0.5 mg/kg, dissolved in 0.9%saline, i.p.; Medhem Express, NJ, USA) was injected to limit astrocyte activation once a day on the day of surgery and 1 day after surgery (30 min before surgery and fear memory test).
2.6. Immunofluorescent staining
One day after surgery or 28 days after viral injection, mice were euthanized, and brain samples were collected. The frozen sections were washed with 0.01 M phosphate‐buffered saline (PBS) 10 min each time for three times, then incubated with blocking solution (5% albumin serum in 0.01 M PBS) for 1 h at room temperature. Next, they were incubated in primary antibodies (rabbit anti‐Iba1, 1:1000, 019–19741, Wako, Tokyo, Japan; rabbit anti‐S100β, 1:200, 15146‐1‐AP, Proteintech, Wuhan, China) overnight at 4°C. The sections were then washed with 0·01 M PBS three times and then incubated in secondary antibody (1:200, 111‐295‐144, 111‐546‐045, Jackson ImmunoResearch, PA, USA; 1:200, RS3111, Immunoway, TX, USA) for 2 h at room temperature. After three washes in PBS, the sections were coverslipped with Vectamount mounting medium with DAPI (H‐1000, Vector labs, Burlingame, CA, USA). All images were acquired with LSM800 confocal microscope and Zen2009 image acquisition software (Carl Zeiss, Jane, Germany). The stacks were overlaid by 10 images (1 μm per images).
2.7. Quantitation of TNF‐α mRNA and protein
One day after surgery, both hippocampal and mPFC tissues were harvested for TNF‐α mRNA and protein measurements. The level of TNF‐α mRNA was detected by quantitative real‐time polymerase chain reaction with the reported probe sequences (GAPDH: F′‐AGGTCGGTGTGAACGGATTTG, R′‐TGTAGACCATGTAGTTGAGG‐TCA; TNF‐α: F′‐CCCTCACACTCAGATCATCTTCT, R′‐GCTACGACGTGGGCTACAG) [14]. The level of TNF‐α protein in the hippocampal and mPFC was detected according to the protocol of TNF‐α Elisa kit (H002‐1‐2, NJJCBIO, Nanjing, China).
2.8. Reactive oxygen species detection
Hippocampal and mPFC frozen sections were incubated with dihydroethidium (1:500, No.7008, Sigma, CA, USA) at 37°C for 30 min in the dark. Then the sections were coverslipped with Vecta mounting medium with DAPI (Vector labs). Images were acquired using LSM800 confocal microscope and labelled reactive oxygen species (ROS) positive neurons were quantified [19].
2.9. Statistical analyses
The sample size was calculated based on our preliminary data that stress and surgery caused learning and memory ability decreased by 30%. With a desired power of 80% and type I error set at 0.05 [14], then minimal n = 3–6/group was required and used for subsequent formal experiments. Data were presented as mean (SEM) or median (inter‐quartile range [IQR]) as appropriate. Student's unpaired two‐tailed t‐test or one‐way or two‐way ANOVA followed by Tukey's test for comparison or Mann–Whitney U test for comparisons wherever appropriate. The statistical significance was set at p < 0.05. All data statistical analyses were performed with SPSS software (version 25.0, SPSS, Chicago, USA) or Prism 5 (Graphpad).
3. RESULTS
3.1. Memory consolidation and cued memory retrieval were impaired after preoperative restraint stress followed by partial hepatectomy under sevoflurane anaesthesia in mice
To investigate postoperative memory changes, mice were divided into the control group and surgery group (treated with preoperative restraint stress followed by sevoflurane anaesthesia and partial hepatectomy). They then received fear conditioning test (Figure 1A). The mice in the surgery group showed no difference in freezing levels during fear conditioning compared with the controls (Figure 1B), indicating that memory acquisition was not significantly affected. In contrast, during the testing phase, the freezing levels of both the context‐ (30.93 [2.03]% vs. 21.51 [1.29]%, p = 0.0010) and tone‐dependent memories (55.15 [2.78]% vs. 39.97 [2.91]%, p = 0.0014) in the surgery group were significantly reduced compared with the controls, suggesting a memory impairment occurred after surgery (Figure 1C,D).
FIGURE 1.
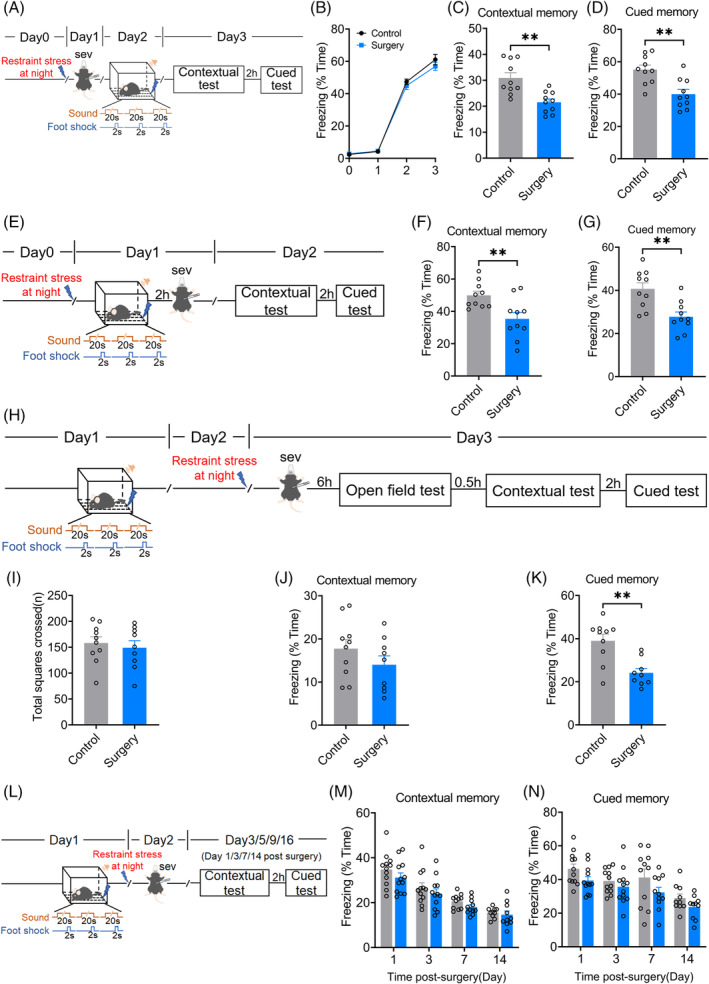
Memory consolidation and cued memory retrieval were impaired after preoperative restraint stress followed by sevoflurane anaesthesia and partial hepatectomy in mice. (A) Time schedule for surgery, perioperative stress, fear conditioning and tests. (B) Freezing levels in fear conditioning. Freezing levels in contextual (C) and cued (D) fear tests. (E) Time schedule for surgery, perioperative stress, fear conditioning and tests of memory consolidation. Freezing levels in contextual (F) and cued (G) fear tests. (H) Time schedule for perioperative stress, surgery, open field, fear conditioning and tests of memory retrieval. (I) Total distance of mice 6 h after surgery. Freezing levels in contextual (J) and cued (K) fear tests. (L) Time schedule for perioperative stress, surgery, fear conditioning and tests at 1, 3, 7, 14 days after surgery. Freezing levels in contextual (M) and cued (N) fear tests. Data are represented as mean ± SEM (n = 9–10) and analysed with Student t‐test (for sub‐figure C, D, F, G, I–K) or two‐way ANOVA followed by Tukey's test for comparison (for sub‐figure B, M and N) where appropriate; **p < 0.01.
Memory consolidation is the process by which acquired information is to become persistent long‐term memory (≥24 h) [20]. If a drug/treatment does not affect information acquisition, but prevents the development to long‐term memory, it is considered to affect memory consolidation [21]. Thus, we performed the surgery 2 h after conditioning in another cohort and they were then tested for memory consolidation 24 h after fear conditioning (Figure 1E). The context‐ (49.91 [2.55]% vs. 35.40 [3.97]%, p = 0.0065) and tone‐dependent memories (40.72 [2.78]% vs. 27.77 [2.22]%, p = 0.0019) of the surgery group were significantly lower than that of the normal control group (Figure 1F,G), suggesting memory consolidation impairment after surgery.
Memory retrieval is to recall memory encoded and stored previously in the brain [22]. To differentiate the effect of surgery on memory retrieval, mice received surgery 2 days after conditioning and were subjected to memory test 6 h later (Figure 1H). There was no significant difference between groups in the total moving distance in open field (158.20 [11.90] vs. 149.10 [13.55], p = 0.6192) (Figure 1I). No significant difference in contextual memory (17.73 [2.12]% vs. 14.03 [2.05]%, p = 0.2280) but significant difference in cued memory tested on the same day after surgery (39.00 [3.08]% in the control vs. 24.11 [2.06]% in the surgery group, p = 0.0011) was found between two groups (Figure 1J,K), suggesting an impairment of cued memory retrieval after surgery.
To test the long‐term memory maintenance after surgery, surgery was given 1 day after fear conditioning, and their contextual and cued memory were tested on 1, 3, 7 and 14 days after surgery (Figure 1L). There was no significant difference between two groups (F (3, 82) = 0.1891, p = 0.9035 of contextual memory; F (3, 82) = 0.3039, p = 0.8225 of the cued memory) (Figure 1M,N).
3.2. The connectivity from the dorsal hippocampus to the medial prefrontal cortex was damaged after preoperative restraint stress followed by sevoflurane anaesthesia and partial hepatectomy in mice
The connectivity between the hippocampus and the medial prefrontal cortex (mPFC) is closely involved in memory consolidation [23]. The pAAV‐CAG‐eGFP anterograde tracer virus was injected into the hippocampus of mice to label the axon terminals of hippocampal neurons and the pAAV5‐CAG‐PSD95‐mcherry virus was injected into the cingulate cortex of mPFC to label the postsynaptic part of mPFC's neurons (Figure 2A,B). The co‐labelling synapses in the mPFC were significantly decreased in surgery group (0.33 [0.02] vs. 0.17 [0.02], p < 0.0001) (Figure 2C,D). To assess the hippocampal synapses projected from the mPFC, the pAAV‐CAG‐eGFP anterograde tracer virus was injected into the mPFC cingulate cortex to label the axon terminals of mPFC's neurons, and the pAAV5‐CAG‐PSD95‐mcherry virus was injected into the hippocampus of mice to label the postsynaptic part of hippocampal neurons (Figure 2E,F). No significant change of the co‐labelling synapse numbers in the hippocampus was found in the surgery group (14.17 [1.20] vs. 13.83 [2.01], p = 0.8894) (Figure 2G,H). These findings indicated that the connectivity from the hippocampus to mPFC was damaged, but not vice versa.
FIGURE 2.
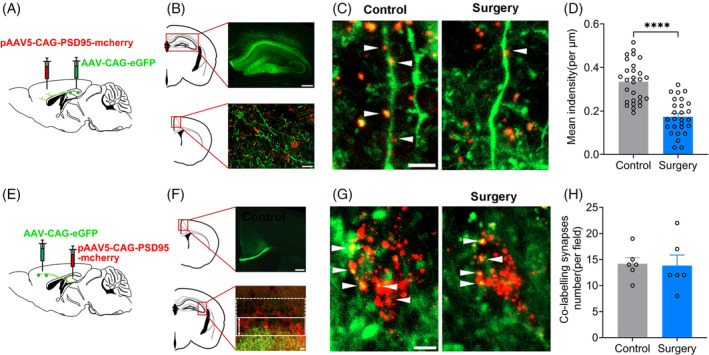
The co‐labelling synapses in medial prefrontal cortex (mPFC) were damaged after preoperative restraint stress followed by sevoflurane anaesthesia and partial hepatectomy in mice. (A and B) Schematic and confocal images of coronal sections to show virus injection sites and the connection from hippocampus to mPFC. Scale bar: 200 μm (upper panel), 20 μm (lower panel). (C) Confocal images of eGFP (green) and PSD‐95 (red) in mPFC. Scale bar: 5 μm. (D) Quantification of co‐labelling synapses (eGFP+PSD‐95) in density using ZEN and Image J. n = 4 mice, 6–9 dendrites from each mouse were counted. Student t‐test. (E and F) Schematic and confocal images of coronal sections to show virus injection sites and the connection from mPFC to CA3 of hippocampus. Scale bar: 100 μm (upper panel), 20 μm (lower panel). (G) Confocal images of eGFP (green) and PSD‐95 (red) in CA3. (G) Confocal images of eGFP (green) and PSD‐95 (red) in CA3. Scale bar: 5 μm. (H)Quantification of co‐labelling synapses (eGFP+PSD‐95) numbers. Data are represented as mean ± SEM (n = 6) and analysed with Student t‐test. ****p < 0.0001.
3.3. The astrocytic phagocytosis of dorsal hippocampal neuronal axon terminals in the medial prefrontal cortex was increased after preoperative restraint stress followed by sevoflurane anaesthesia and partial hepatectomy in mice
Astrocytes and microglia modulate brain function and the connectivity of interbrain regions by pruning synapses [24, 25]. Astrocytes and microglia were activated in the mPFC following surgery (p < 0.0001, respectively) (Figure 3A,B; Figure 4A,B). The number of the hippocampal neurons' axon terminals engulfed in each astrocyte (median [IQR], 2 [2] vs. 3 [2], p = 0.01) and the percentage of astrocytes engulfing hippocampal neurons' axon terminals (37.94 [4.08]% vs. 61.80 [5.52]%, p = 0.0037) was significantly increased in the surgery group compared with the controls (Figure 3C,D,F). No significant inter‐group difference in the astrocyte phagocytosis of PSD‐95 (a marker of postsynaptic structure) was detected (Figure 3C,E,F). No significant inter‐group difference was also detected in microglial phagocytosis of hippocampal neurons' axon terminals and PSD‐95 of mPFC (Figure 4C–F). These suggested that astrocyte's phagocytosis of hippocampal neuronal axon terminals in the mPFC contributed to the connectivity damage from the hippocampus to mPFC after surgery.
FIGURE 3.
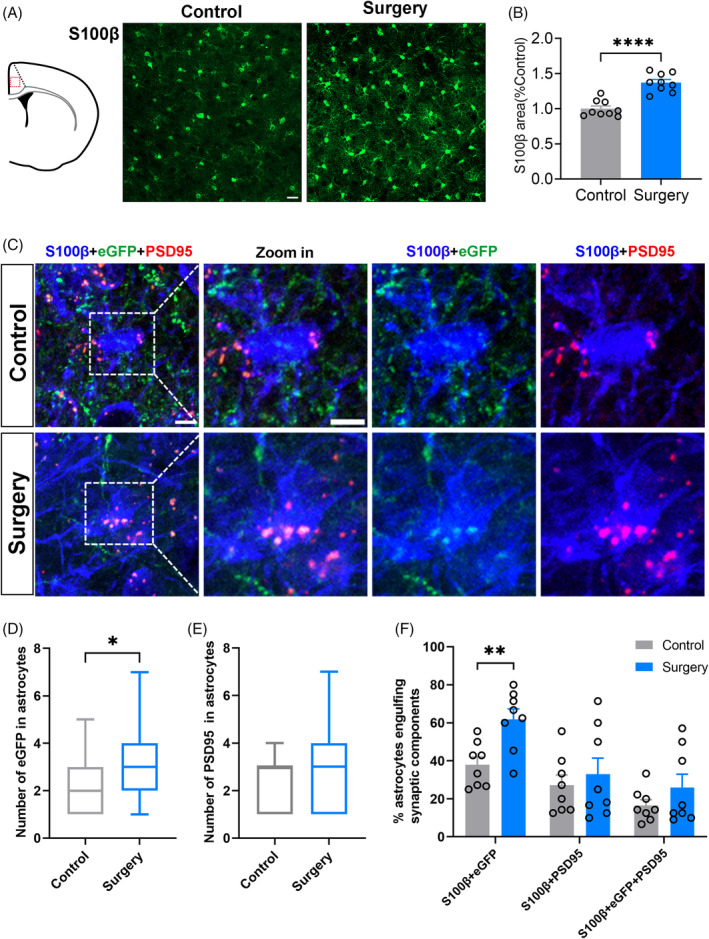
Preoperative restraint stress followed by sevoflurane anaesthesia and partial hepatectomy increased the astrocytic phagocytosis of dorsal hippocampal neuronal axon terminals in the medial prefrontal cortex (mPFC). (A) Confocal images of S100β (green) staining (scale bars: 20 μm). (B) Positive area percentage of astrocytes in the mPFC 1 day after surgery (n = 3 mice, 3 images from each mouse were counted). (C) Representative confocal images of engulfed eGFP (green) and PSD‐95 (red) in astrocytes (blue) in mPFC of control and surgery group. Scale bars: 5 μm. Quantification of engulfed eGFP (D) and engulfed PSD‐95 (E) in astrocytes (n = 8 mice, 3–6 cells from each mouse were counted). (F) The percentage of astrocytes engulfing eGFP and (or) PSD‐95 in fields (160 μm × 160 μm) using ZEN and ImageJ (n = 8). Data are represented as mean ± SEM or median (1–99 percentile) and analysed with Student t‐test or Mann–Whitney U test where appropriate. *p < 0.05, **p < 0.01, ****p < 0.0001.
FIGURE 4.
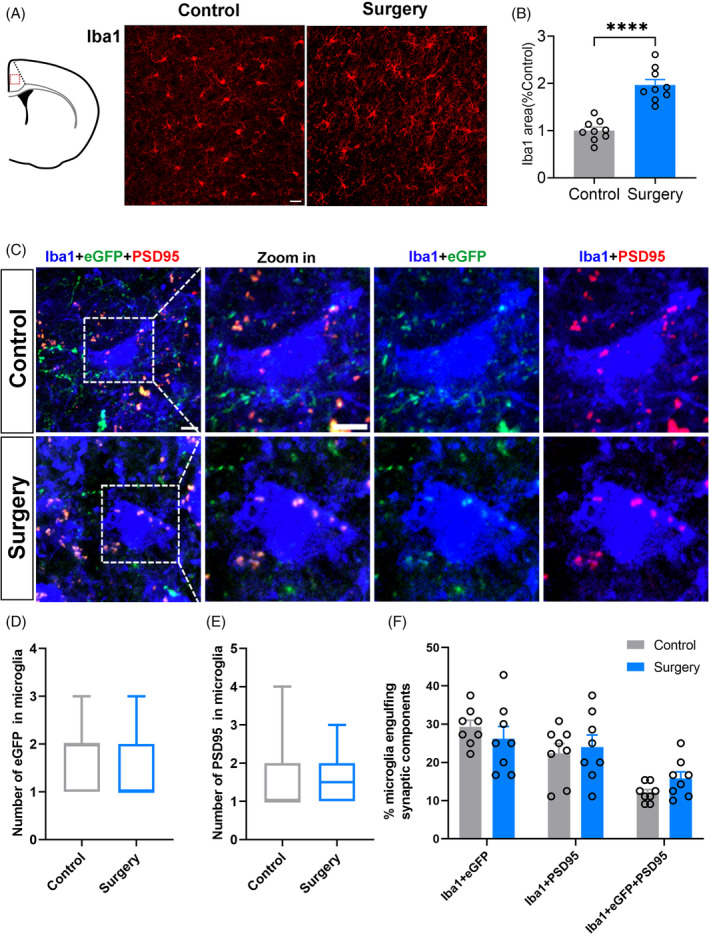
Sevoflurane anaesthesia combined with partial hepatectomy and preoperative restraint stress did not change the microglial phagocytosis in medial prefrontal cortex (mPFC). (A) Confocal images of Iba1 (red) staining. (B) Positive area percentage of microglial cells in the mPFC 1 day after surgery (scale bars: 20 μm, n = 3 mice, 3 images from each mouse were counted). (C)Representative confocal images of engulfed eGFP (green) and PSD‐95 (red) in microglia (blue) in mPFC. Scale bars: 5 μm. Quantification of engulfed eGFP (D) and engulfed PSD‐95 (E) in microglia (n = 8 mice, 3–6 cells from each mouse were counted. (F) Quantifying the percentage of microglia engulfing eGFP and (or) PSD‐95 in fields (160 μm × 160 μm) using ZEN and ImageJ (n = 8). Data are represented as mean ± SEM or median (1–99 percentile). Data are represented as mean ± SEM or median (1–99 percentile) and analysed with Student t‐test or Mann–Whitney U test where appropriate. ****p < 0.0001.
3.4. Neuroinflammatory response and oxidative stress in the dorsal hippocampus and the medial prefrontal cortex were induced after surgery in a region‐dependent manner
Neuroinflammation and oxidative stress play an important role in POCD pathogenesis [26]. Thus, the neuroinflammatory response and oxidative stress in the hippocampus and mPFC after surgery were investigated to see whether they were related to the connectivity damage from the hippocampus to mPFC after surgery. TNF‐α and ROS in the hippocampus and mPFC were significantly increased after surgery (Figure 5). The increase of inflammatory factors in the mPFC was greater than those of the hippocampus (TNFα mRNA: p < 0.0001 in mPFC and p < 0.0001 in hippocampus, Figure 5A; TNFα protein: p = 0.0020 in mPFC and p = 0.0178 in hippocampus, Figure 5B). In contrast, the increase of ROS level in the mPFC was lower than that of hippocampus (p < 0.0001 in mPFC and p = 0.0170 in hippocampus, Figure 5C,D).
FIGURE 5.
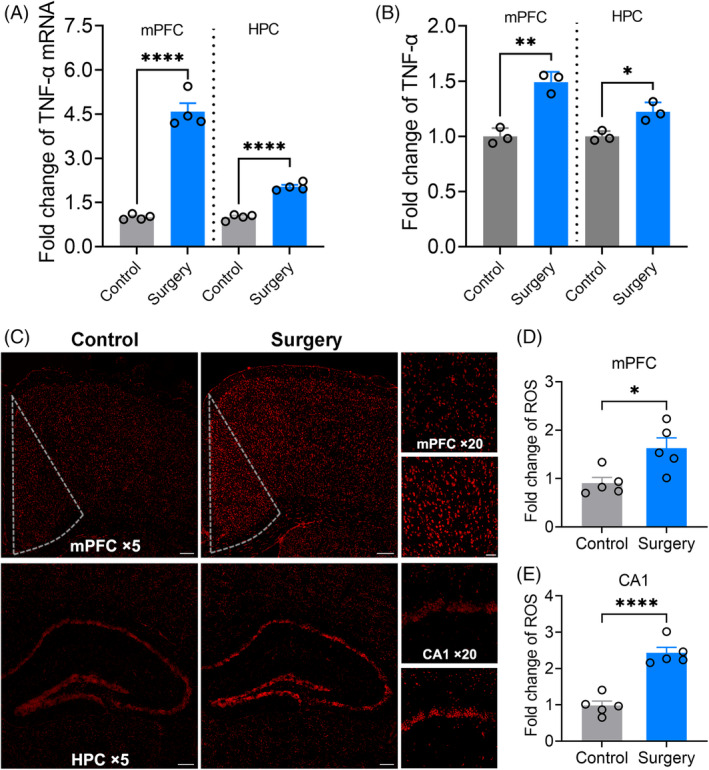
Neuroinflammatory reaction and oxidative stress in dorsal hippocampus and medial prefrontal cortex (mPFC) were induced after surgery in a region dependent manner. (A) and (B) The levels of TNF‐α mRNA and protein in the mPFC and hippocampus. (C) Confocal images of ROS in mPFC and hippocampus. Scale bars: ×5: 200 μm; ×20: 50 μm. (D) The fold change of ROS level in the mPFC. (E) The fold change of ROS level in the CA1. Data are represented as mean ± SEM (n = 4–5) and analysed with Student t‐test. *p < 0.05, ***p < 0.001, ****p < 0.0001.
3.5. Limiting astrocyte activation in the medial prefrontal cortex alleviated contextual memory consolidation impairment induced by sevoflurane anaesthesia combined with partial hepatectomy and preoperative restraint stress
To further investigate the role of astrocyte's phagocytosis of the mPFC on postoperative memory consolidation impairment, pAAV‐GFAP‐hM4Di‐eGFP was injected into mPFC 28 days before partial hepatectomy and fear memory test (Figure 6A). No significant difference was detected in freezing level between the Controls + Vehicle, Surgery + Vehicle and Surgery + hM4Di groups during fear conditioning (all p > 0.05) (Figure 6B). However, there were significant differences of freezing time between three groups in fear memory test (contextual memory: F (2, 32) = 17.85, p < 0.0001; cued memory: F (2, 31) = 3.969, p = 0.0292). The freezing time of the Control + Vehicle group was significantly higher than that of the Surgery + Vehicle group in the contextual memory test (34.01 [2.37]% vs. 15.55 [1.37]%, p < 0.0001) and cued memory test (55.99 [3.93]% vs. 45.1 [1.82]%, p = 0.040) (Figure 6C,D), and the freezing time in the contextual memory test (28.18 [2.57]% vs. 15.55 [1.37]%, p = 0.001) in the Surgery + hM4Di group was significantly higher than that of the Surgery + Vehicle group (Figure 6C), indicating that limiting astrocyte activation ameliorated postoperative contextual memory consolidation impairment.
FIGURE 6.
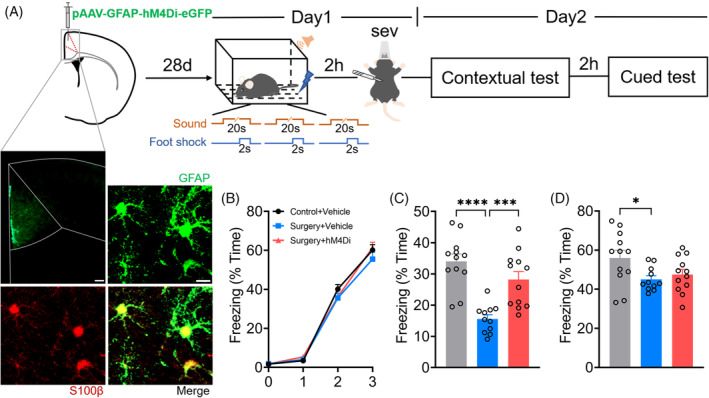
Inhibition of astrocyte activation in the medial prefrontal cortex (mPFC) alleviated contextual memory consolidation impairment induced by preoperative restraint stress followed by sevoflurane anaesthesia and partial hepatectomy. (A) Timelines and pAAV‐GFAP‐hM4Di labelling. pAAV‐GFAP‐hM4Di was injected in mPFC 28 days before fear memory behaviour for memory consolidation test. Co‐localization of eGFP labelling with the S100+ astrocytes. Scale bar: 100 μm (upper left panel); 10 μm (upper right panel). (B) Freezing levels in fear conditioning. (C) Freezing levels in contextual tests. (D) Freezing levels in cued tests. Data are represented as mean ± SEM (n = 11–12) and analysed with one‐way or two‐way ANOVA followed by Tukey's test for comparison where appropriate. *p < 0.05, ***p < 0.005, ****p < 0.0001.
4. DISCUSSION
In the current study, we found that anaesthesia and surgery together impaired memory consolidation and cued memory retrieval. However, the contextual memory retrieval as well as memory acquisition, storage and long‐term maintenance were not affected.
Contextual memory consolidation was reported to be dependent on the connectivity from the dorsal hippocampus to the mPFC [5] whilst contextual memory retrieval depends on the connectivity from the mPFC to the dorsal hippocampus [18]. Moreover, cued memory consolidation and retrieval likely depend on the connectivity from the mPFC to amygdala [27]. In this study, we found that anaesthesia and surgery damaged the connectivity from the dorsal hippocampus to mPFC, but not from the mPFC to dorsal hippocampus, corresponding to the impairment of memory consolidation reported here. The underlying mechanisms of this discrepancy are unknown. However, interestingly, an increase in ROS level of the hippocampus was significantly higher than that of the mPFC. Thus, it is possible that ROS induced more injury to vulnerable neurons in the hippocampus and hence caused the relatively more damage to the ‘dynamic’ projections from the dorsal hippocampus to mPFC but this warrants further study.
The synaptic remodelling modulated by glial cells has well been recognised in physiological and pathological conditions [24]; In particular, microglial cells were considered to play a main role of synaptic remodelling as a sole mediator of synapse elimination [28].For instance, microglia mediated the synaptic loss in Alzheimer's disease [12]; the phagocytosis of microglia led to depressive disorder [29]. Recent studies showed that astrocytes also were able to eliminate synapses in the developing and mature brain [11]. In this study, anaesthesia and surgery induced a significant increase of astrocytic engulfment of hippocampal neuronal axon terminals in the mPFC, but not microglial engulfment. Limiting astrocyte activation in the mPFC also alleviated contextual memory consolidation impairment induced by anaesthesia and surgery. This is in line with the findings from the previous studies of POCD and ischemic stroke [13, 30, 31]. Hippocampal microglial activation triggered a neurotoxic‐specific astrocyte response during POCD development [30] and limiting neuroinflammation through inhibiting astrocytic activation attenuated postoperative cognitive impairment in aged mice [31]. In ischemic stroke, microglial synapse engulfment was evident during the early phase within the ischemic core region, whereas phagocytic astrocytes were also found within the ischemic perifocal region at day 7 after ischemic stroke [13], suggesting that a damage magnitude may depend on the characteristics of astrocytic and microglial engulfment related to injurious nature of brain cell. Thus, it is possible that brain injury induced by anaesthesia and surgery is enough to activate astrocytic phagocytosis, but not enough to activate microglial phagocytosis reported herein per se.
Our study had some limitations. First, anaesthesia alone group was not included in the current study and which part, either anaesthesia or surgery, caused more changes found in this currently is unknown. However, both anaesthesia and surgery are always parallel together in clinical settings and, hence, our data are valid simulating clinical practice improvement. Second, our study only focused on the changes in the hippocampus‐dependent memory and how both anaesthesia and surgery affect hippocampus‐independent memory and associated neural circuit changes remains elusive and warrants further study. Third, GFAP, a classic marker of astrocytes, was not used to detect astrocyte phagocytosis, because it labelled too few astrocytes in the mPFC of mice for objective quantification of astrocyte phagocytosis. In contrast, S100β, which was used in our study, is widely used to detect astrocytes in mice brain [32]. Fourth, interestingly, we found that cued memory was impaired immediately after surgery (Figure 1K) but this was not the case 1 day after surgery (Figure 1N). This was due to the transit effect of anaesthesia and surgery or due to its quick recovery from damage is open to a question. Finally, this study was only focused on the impact of anaesthesia/surgery on the memory processing and the underlying mechanisms including neuroinflammation. Systemic inflammatory biomarkers, for example, blood cytokines, following treatments were not measured but the correlation between peripheral and central inflammation in this area of study has been well established [31].
In conclusion, our work demonstrated that anaesthesia and surgery impaired both consolidation and retrieval of memory. Mechanistically, anaesthesia and surgery activated astrocytic engulfment, but not microglial engulfment, of hippocampal neuronal axon terminals in the mPFC and caused connectivity damage from the dorsal hippocampus to mPFC and consequently impaired memory consolidation. Our work, for the first time, demonstrated that both anaesthesia and surgery caused astrocyte phagocytosis, further resulted in the dorsal hippocampus to mPFC neural circuit damage and, consequently, impaired both consolidation and retrieval of memory. Protecting astrocytes to avoid their phagocytosis may become new therapeutic target for developing strategy in preventing and treating POCD development.
AUTHOR CONTRIBUTIONS
Conceptualization: JT, DM, XM. Methodology: JT, DM, XM, YL. Investigation: XM, LH, JT, CL. Funding acquisition: JT, WO. Supervision: JT, DM. Writing of original draft: JT, DM, WO, XM. Writing, review, and editing: JT, DM, WO, XM.
FUNDING INFORMATION
This work was supported by the by National Natural Science Foundation of China (No. 81870861).
CONFLICT OF INTEREST STATEMENT
The authors have declared that no conflict of interest exists.
Ma X, Le Y, Hu L, Ouyang W, Li C, Ma D, et al. Astrocytic phagocytosis in the medial prefrontal cortex jeopardises postoperative memory consolidation in mice. Brain Pathology. 2024;34(6):e13253. 10.1111/bpa.13253
Contributor Information
Daqing Ma, Email: d.ma@imperial.ac.uk.
Jianbin Tong, Email: jianbintong@csu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
REFERENCES
- 1. Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. [DOI] [PubMed] [Google Scholar]
- 2. Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery. Anesth Analg. 2018;127(2):496–505. [DOI] [PubMed] [Google Scholar]
- 3. Zhu Y, Zhou M, Jia X, Zhang W, Shi Y, Bai S, et al. Inflammation disrupts the brain network of executive function after cardiac surgery. Ann Surg. 2023;277(3):e689–e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Netto MB, de Oliveira Junior AN, Goldim M, Mathias K, Fileti ME, da Rosa N, et al. Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain Behav Immun. 2018;73:661–669. [DOI] [PubMed] [Google Scholar]
- 5. Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23(17):R764–R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eichenbaum H. A cortical‐hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1(1):41–50. [DOI] [PubMed] [Google Scholar]
- 7. Eichenbaum H. Prefrontal‐hippocampal interactions in episodic memory. Nat Rev Neurosci. 2017;18(9):547–558. [DOI] [PubMed] [Google Scholar]
- 8. Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504(7480):394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement‐dependent manner. Neuron. 2012;74(4):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kol A, Adamsky A, Groysman M, Kreisel T, London M, Goshen I. Astrocytes contribute to remote memory formation by modulating hippocampal‐cortical communication during learning. Nat Neurosci. 2020;23(10):1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee JH, Kim JY, Noh S, Lee H, Lee SY, Mun JY, et al. Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature. 2021;590(7847):612–617. [DOI] [PubMed] [Google Scholar]
- 12. Hong S, Beja‐Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352(6286):712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morizawa YM, Hirayama Y, Ohno N, Shibata S, Shigetomi E, Sui Y, et al. Reactive astrocytes function as phagocytes after brain ischemia via ABCA1‐mediated pathway. Nat Commun. 2017;8(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen J, Liu S, Wang X, Huang J, Phillips J, Ma D, et al. HDAC6 inhibition alleviates anesthesia and surgery‐induced less medial prefrontal‐dorsal hippocampus connectivity and cognitive impairment in aged rats. Mol Neurobiol. 2022;59(10):6158–6169. [DOI] [PubMed] [Google Scholar]
- 15. Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11(2):180–187. [DOI] [PubMed] [Google Scholar]
- 16. Cui X, Zhou S, Xia G, Chen J, Jiang L, Huang J, et al. A multispecies probiotic accelerates fear extinction and inhibits relapse in mice: role of microglia. Neuropharmacology. 2021;193:108613. [DOI] [PubMed] [Google Scholar]
- 17. Yang L, Liu C, Li W, Ma Y, Huo S, Ozathaley A, et al. Depression‐like behavior associated with E/I imbalance of mPFC and amygdala without TRPC channels in mice of knockout IL‐10 from microglia. Brain Behav Immun. 2021;97:68–78. [DOI] [PubMed] [Google Scholar]
- 18. Rajasethupathy P, Sankaran S, Marshel JH, Kim CK, Ferenczi E, Lee SY, et al. Projections from neocortex mediate top‐down control of memory retrieval. Nature. 2015;526(7575):653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Su WJ, Chen Y, Wu TY, Gong H, Shen XL, et al. Effects of hydrogen‐rich water on depressive‐like behavior in mice. Sci Rep. 2016;6:23742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147(3):509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44(1):75–91. [DOI] [PubMed] [Google Scholar]
- 22. Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157(1):163–186. [DOI] [PubMed] [Google Scholar]
- 23. Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, et al. Engrams and circuits crucial for systems consolidation of a memory. Science. 2017;356(6333):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Allen NJ, Lyons DA. Glia as architects of central nervous system formation and function. Science. 2018;362(6411):181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun. 2018;9(1):1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alam A, Hana Z, Jin Z, Suen KC, Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cho JH, Deisseroth K, Bolshakov VY. Synaptic encoding of fear extinction in mPFC‐amygdala circuits. Neuron. 2013;80(6):1491–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frost JL, Schafer DP. Microglia: architects of the developing nervous system. Trends Cell Biol. 2016;26(8):587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse‐related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18(9):1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li D, Chen M, Meng T, Fei J. Hippocampal microglial activation triggers a neurotoxic‐specific astrocyte response and mediates etomidate‐induced long‐term synaptic inhibition. J Neuroinflammation. 2020;17(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, et al. Role of interleukin‐1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68(3):360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mederos S, Sanchez‐Puelles C, Esparza J, Valero M, Ponomarenko A, Perea G. GABAergic signaling to astrocytes in the prefrontal cortex sustains goal‐directed behaviors. Nat Neurosci. 2021;24(1):82–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.


